Abstract
Mechanical forces influence homeostasis in virtually every tissue [1–2]. Tendon, constantly exposed to variable mechanical force, is an excellent model in which to study the conversion of mechanical stimuli into a biochemical response [3–5]. Here we show in a mouse model of acute tendon injury and in vitro that physical forces regulate the release of active transforming growth factor (TGF)-β from the extracellular matrix (ECM). The quantity of active TGF-β detected in tissue exposed to various levels of tensile loading correlates directly with the extent of physical forces. At physiological levels, mechanical forces maintain, through TGF-β/Smad2/3-mediated signaling, the expression of Scleraxis (Scx), a transcription factor specific for tenocytes and their progenitors. The gradual and temporary loss of tensile loading causes reversible loss of Scx expression, whereas sudden interruption, such as in transection tendon injury, destabilizes the structural organization of the ECM and leads to excessive release of active TGF-β and massive tenocyte death, which can be prevented by the TGF-β type I receptor inhibitor SD208. Our findings demonstrate a critical role for mechanical force in adult tendon homeostasis. Furthermore, this mechanism could translate physical force into biochemical signals in much broader variety of tissues or systems in the body.
Keywords: Scleraxis, Tendon, Tensile loading, Mechanical force, TGF-β, Smad2/3
Results
Tendon is a fibrous connective tissue made of specialized fibroblasts called “tenocytes” and an abundant ECM, whose physiology and pathology depends heavily on mechanical stimuli [3–5]. Tendon transfers contraction forces (tensile loading) from skeletal muscle to bone and consequently possesses high tensile stiffness and strength. Despite numerous studies, it is still largely unknown at molecular levels how transmittal forces play a role in the regeneration and maintenance of adult tendon.
During embryonic development, tenocytes originate from mesodermal compartments, as do skeletal myoblasts, chondrocytes, and osteoblasts [6]. The basic helix-loop-helix transcription factor Scx is found to be essential for tendon development: Scx-deficient mice show a complete loss of major force-transmitting and intermuscular tendons [7]. Although multipotent mesenchymal progenitors might express Scx upon specific lineage commitment, only tendon progenitor cells and tenocytes retain its expression, making Scx a highly specific marker of tenogenic (precursor) cells and mature differentiated tenocytes [8–9].
ScxGFP expression in tenocytes of adult tendon
To understand the role that continuous transmittal force from skeletal muscle to bone plays in adult tendon homeostasis, we utilized a transgenic mouse strain that expresses the Scx promoter-driven GFP marker (ScxGFP, Figure 1A and 1B)[9]. Robust ScxGFP expression in a majority of tenocytes (94.0±3.4%) clearly distinguished them from adjacent skeletal muscle cells in the myotendinous junction and from adjacent chondrocytes in cartilage (Figure 1B). ScxGFP expression did not affect the tendon ECM composition, the expression of collagen type I or type III, fibronectin, tenascin-C, small leucine-rich proteoglycan fibromodulin or cartilage oligomeric matrix protein (COMP or thrombospondin 5) (Figure S1A, data not shown). Scx expression specificity was also confirmed in vitro. Primary tenocytes isolated from 10-wk-old ScxGFP mice expressed high levels of GFP (Figure 1C), whereas primary chondrocytes and osteoblasts isolated from newborn ScxGFP mouse ribs and calvaria were negative (Figure 1C). As demonstrated with RT-PCR, adult tenocytes expressed Scx and the mature tenocyte marker tenomodulin [10], the major tendon ECM component collagen type I, and the collagen receptors integrin alpha1 and integrin alpha11 [11–12]. In contrast, primary dermal fibroblasts isolated from the same mice expressed neither Scx nor tenomodulin, showing phenotypic differences between tenocytes and non-tendon fibroblasts in the adult. Neither cell type expressed the chondrogenic master gene Sox9 [6] (Figure 1D). The ScxGFP transgene can therefore be used to specifically identify and study adult tenocytes.
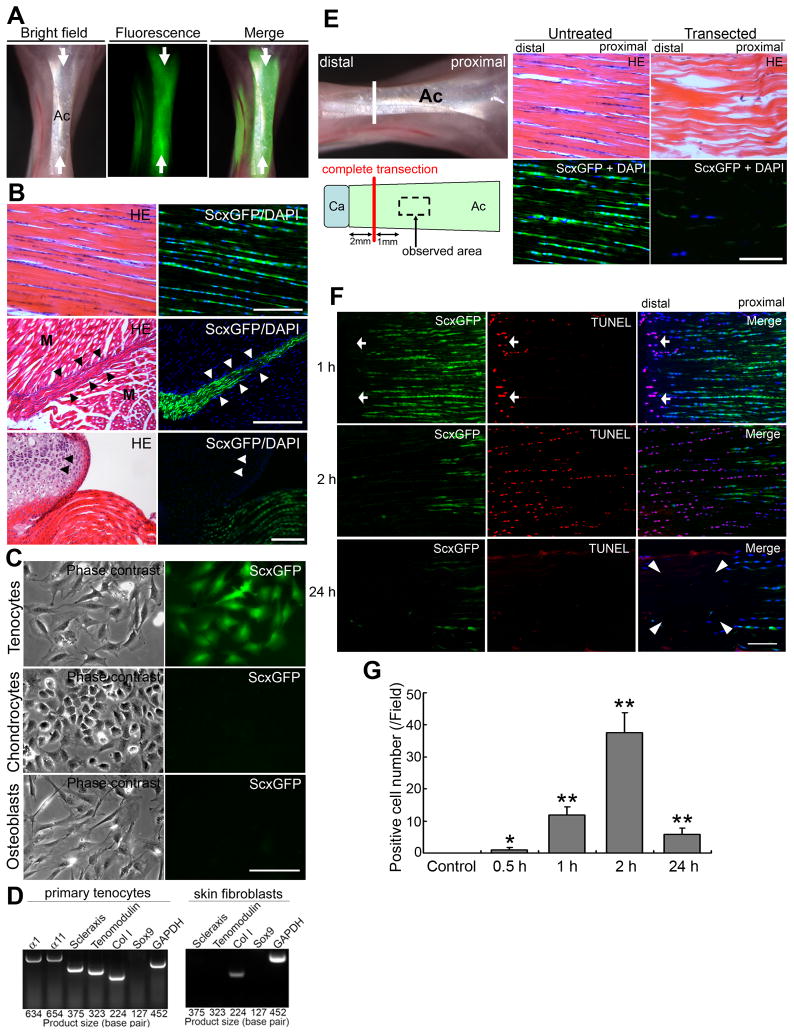
Figure 1. Loss of tensile loading causes tenocyte cell death in adult Achilles tendons.
(A) – (D) Characterization of adult ScxGFP transgenic mice.
(A) Achilles tendons (Ac, arrows) in 10-wk-old ScxGFP transgenic mice express a robust ScxGFP signal (green) under fluorescence stereomicroscopy.
(B) Histological analysis of Achilles tendons in 10-wk-old ScxGFP transgenic mice. HE-stained sections (left panels) and the same areas with GFP/UV filters (right panels: green, ScxGFP; blue, DAPI [cell nuclei]). Upper panels: adult Achilles tendon. Note that aligned tenocytes express ScxGFP. Middle panels: Myotendinous junction at proximal Achilles tendon. ScxGFP is expressed only in tenocytes (arrow heads); myocytes (M) are completely negative for ScxGFP. Lower panels: Distal insertion of Achilles tendon. ScxGFP is expressed only in tenocytes. Chondrocytes (arrow heads) at the calcaneus are completely negative for ScxGFP. Bars = 50 μm.
(C) Only primary tenocytes from ScxGFP transgenic mice express ScxGFP in vitro. Left panels: Phase contrast micrographs of primary tenocytes, chondrocytes and osteoblasts. Right panels: Fluorescence micrographs of the same area with a GFP filter. Bar = 100 μm.
(D) RT-PCR analysis of gene expression profiles related to tendon and cartilage in primary adult wild-type mouse tenocytes and dermal skin fibroblasts. Tenocyte markers Scx and tenomodulin and chondocyte marker Sox9 are not expressed in skin fibroblasts. α1, α1 integrin; α11, α11 integrin; Col I, collagen type I; GAPDH, glyceraldehyde 3-phosphate dehydrogenase (as a control gene).
(E) – (G) Acute tensile loading-loss model by complete transection of adult Achilles tendons.
(E) Left panels: Illustrations of the complete transection model. Achilles tendon (Ac) was completely transected at 2 mm proximal to the calcaneus (Ca) (white line in picture and red line in drawing, respectively). Middle and right panels: Histological analysis of intact (untreated) and transected tendon tissues at 3 d after operation. HE-stained sections and the same areas with GFP/UV filters (green, ScxGFP; blue, DAPI). Sections were prepared approximately 3 mm from calcaneus in both transected and intact tendons as shown in left panels. Note that very few cells retain ScxGFP expression in comparison with normal intact adult tendon. Bar = 50 μm.
(F) Analysis of cell death. Expression of ScxGFP (green; left panels), TUNEL staining (red; middle panels) and merged images (right panels) with DAPI (blue) at 1, 2, and 24 h after complete transection. Upper panels: 1 h after transection. ScxGFP expression is diminished, and TUNEL-positive cells appear at the edge of the tendon (arrows). Middle panels: 2 h after transection. ScxGFP expression is significantly diminished, and TUNEL-positive cells expand to the proximal side of the Achilles tendon. Lower panels: 24 h after transection. Extensive cell death results in the formation of an acellular region (arrowheads). Bar = 100 μm.
(G) Analysis of TUNEL-positive cells in non-transected (Control) and transected tendons. Error bars represent standard deviation (n = 4; field = 0.07 mm2). *, P < 0.05; **, P < 0.001: significantly different compared to the number of positive cells in control non-transected tendons.
Effects of acute loss of tensile loading on ScxGFP expression and tenocytes
The complete transection model was chosen to study tendon injury because this model best mimics clinically acute tendon injuries (i.e., definite interruption of tendon continuity and immediate loss of tensile loading) (Table S1) [13–14]. Almost 70% fewer tenocytes were found at 3 d post-transection (17.5±2.5 cells/field in transected vs. 56.8±3.3 in sham-operated tendons [n = 4; field = 0.037 mm2, P = 0.0003]), and only a small portion of the remaining cells (11.7%) retained low ScxGFP expression post-transection (Figure 1E). Acute loss of tensile loading correlated with the loss of tenocyte viability by as early as 0.5 h after transection. ScxGFP loss and gain of positive TUNEL gradually spread to the proximal region of the transected Achilles tendon with time (Figure 1F and 1G). Therefore a sudden loss of continuous transmittal force from skeletal muscles leads to massive death of tenocytes. This finding could explain why tendon injuries very rarely heal. No obvious massive bleeding or inflammatory response and no significant change in microvascularity for at least up to 2 h within tendon tissues after complete transection was confirmed (Figure S1B–S1D).
Gradual loss of tensile loading allows reversible Scx expression but has profound effects on the mechanical properties of adult tendons
Loss or attenuation of ScxGFP expression even in surviving tenocytes suggested that ScxGFP expression might depend on mechanical signaling. To test that, we experimentally induced a reversible gradual loss of continuous transmittal force from skeletal muscles using botulinum toxin A. This toxin blocks the release of acetylcholine specifically from presynaptic motor nerve terminals and induces a gradual but reversible skeletal muscle weakness, resulting in decreased muscle force – 25% of normal at 3 d post injection, returning to normal after 24 wks [15–19]. A single dose of botulinum toxin A (6 U kg−1 body weight) injected into the triceps muscle of 10- to 12-wk-old ScxGFP mice (Figure 2A) did not cause any cell death (TUNEL-positive cells: 2 of 206 = 0.97% at 24 h; 1 of 237 = 0.42% at 4 d). However, at 1 wk post injection the number of tenocytes expressing ScxGFP decreased by ~79% and the level of expression was significantly lower compared to that of saline-injected controls (Table S2 and Figure 2A and 2B). Immunohistochemical analysis also showed a significant decrease in the abundance of collagen type I fibrils and COMP expression (Figure 2A and 2B). Control saline-injected tendons showed no phenotypic changes (data not shown).
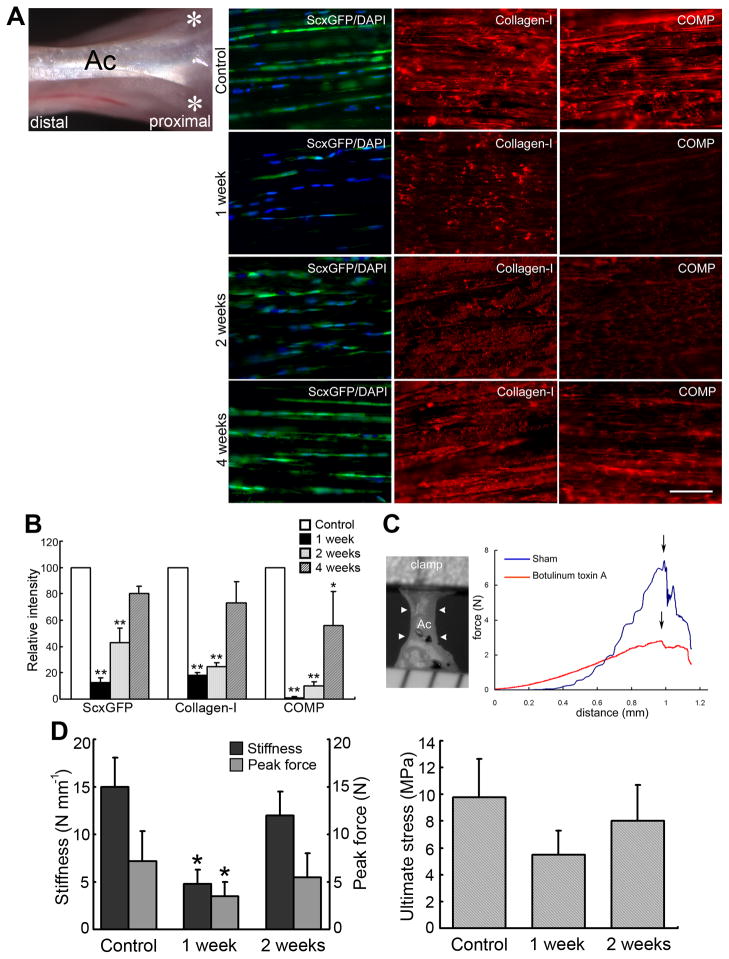
Figure 2. A gradual tensile loading-loss model by local injection of botulinum toxin A into adult Achilles tendons.
(A) Left panel: Illustration of the botulinum toxin treatment. The toxin (6 U kg−1 weight) was injected intramuscularly into medial and lateral sides of the gastrocnemius muscle (asterisks). Ac, Achilles tendon. Right panels: Time course of fluorescence micrographs in toxin-treated Achilles tendons of ScxGFP mice (green, ScxGFP; blue, DAPI; red, collagen type I and COMP). Bar = 50 μm.
(B) Analysis of ScxGFP, collagen type I and COMP intensities shown in Figure 2A. Relative fluorescence intensities are shown relative to each control value of 100 (control tendon) (n = 5 for each group). Error bars represent standard deviation. *, P < 0.05; **, P < 0.01: significantly different compared to controls.
(C) Biomechanical analysis of toxin-treated tendon tissues. Left panel: Resected Achilles tendon (Ac, arrowheads) for biomechanical experiments. Right panel: Typical force-distance curves in toxin-treated (botulinum toxin A, red line) and saline-treated (sham, blue line) tendon tissue at 1 wk post injection. Arrows indicate peak force.
(D) Stiffness, peak force, and ultimate stress in untreated tendons (Control) and at 1 and 2 wks after toxin treatment. Error bars represent standard deviation (n = 5). Note that the toxin-treated Achilles tendons exhibit significantly reduced stiffness and peak force compared to untreated controls at 1 wk post treatment although ultimate stress is not significantly different. *, P < 0.05.
Since Scx regulates the expression of pro-α1(I) collagen (Col1a1) in tenocytes [20], the profound impact of tensile loading on tenocyte cell viability and ScxGFP expression indicated that even a gradual decrease in tensile loading could affect the mechanical properties of adult tendons. Indeed, at 1 wk post injection, tendons in toxin-injected mice showed a significant decrease in stiffness (>3-fold) and peak force (>2.5-fold) in comparison with control mice (P < 0.05, Figure 2C and 2D). Ultimate stress also decreased considerably at 1 wk post injection although without significance (P = 0.08; Figure 2D). At 2 wks post injection, tendons from toxin-injected mice began to recover: the number of ScxGFP-expressing tenocytes and the deposition of collagen type-I and COMP increased (Figure 2A and 2B), and the difference in stiffness and peak force compared to controls was diminished (Figure 2D). Transmittal force from skeletal muscles therefore has a major impact on adult tendon homeostasis.
Mechanical force regulating ScxGFP can be reversibly operated in vitro
To determine how mechanical force regulates tenocyte function at the molecular level, primary tenocyte cultures were isolated from adult ScxGFP mouse Achilles tendon (Figure 1C, upper panels). ScxGFP was initially expressed in all primary tenocytes, but expression decreased to <30% after only 48 h and <5% by day 6 (Figure S2A and S2B). To quantify the force required to retain ScxGFP expression, populations of primary cultured ScxGFP tenocytes were simultaneously exposed to different levels of mechanical force using a microfluidic chamber system composed of multiple bifurcating networks [21]. This fluid-flow device is a model system that applies shear stress to the tenocytes and mimics the response to mechanical stimuli [22–24]. The level of mechanical force depended on the location of cells in the chamber subdivisions, designated I to IV (Figure 3A). Shear stress values for these locations in the chamber were obtained by solving Navier-Stokes (N-S) equations using computational fluid dynamics software (COMSOL). The validity and applicability of equations and the usefulness of software was verified by our group in separate experiments involving microbeads [21]. There was agreement between the velocity profiles predicted by the N-S equations and experimental results using the technique of particle image velocimetry. The fluid flow regime through the device was laminar. As such, surface roughness or ECM accumulation had negligible effects on wall shear stress and overall frictional resistance. Only under turbulent flow conditions would ECM accumulation and other factors that lead to changes in surface “roughness” have a significant effect [25]. Using an inlet flow rate of 1 ml h−1 that resulted in an estimated shear stress range of 0–0.60 dyne cm−2, it was found that a stress of 0.14 dyne cm−2 (area II) was optimal for retaining ScxGFP expression in the primary tenocytes (Figure 3B and 3C). To determine whether the decreased expression of ScxGFP after 6 d of culture was a reversible or an irreversible response, primary tenocytes were then exposed to mechanical strain on day 7. Tenocytes exposed to shear stress of 0.14 dyne cm−2 in area II indeed showed the highest induction of ScxGFP (Figure S2C).
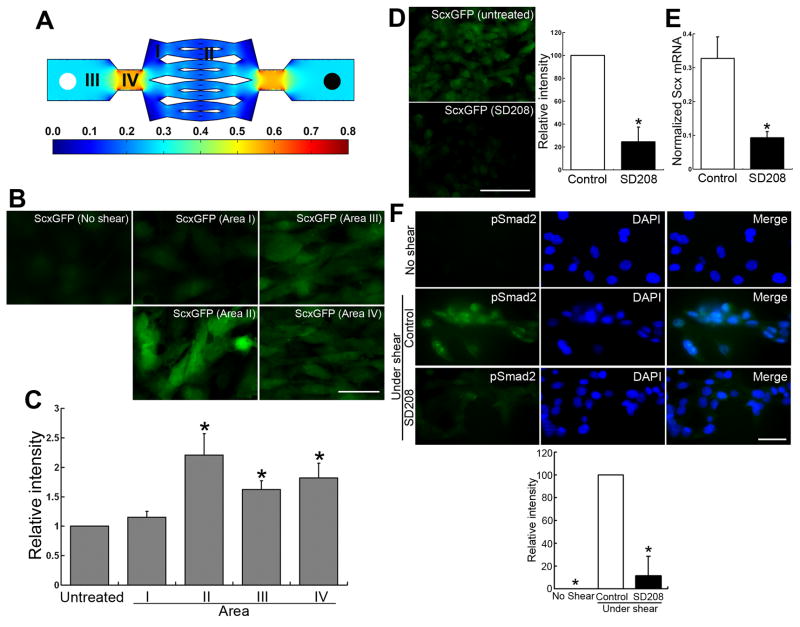
Figure 3. Mechanical force and Smad2-mediated signaling is required for maintenance of Scx expression in adult tenocytes in vitro.
(A) Diagram of microfluidic chamber and regions. The composition is a bifurcating network of several generations with areas (I–IV) of different shear stresses (dyne cm−2), as indicated by the color legend. A steady flow of cell culture medium from the input tube (left white circle) to the output tube (right black circle) was supplied by a syringe pump. For a flow rate of 1 ml h−1, the wall mechanical strain in each area was about 0.1 (area I), 0.14 (area II), 0.30 (area III), and 0.60 (area IV) dyne cm−2, respectively.
(B) ScxGFP expression in primary tenocytes at 7 d under different mechanical forces (flow rate, 1 ml hr−1). Note that tenocytes in areas II (0.14 dyne cm−2), III (0.30 dyne cm−2) and IV (0.60 dyne cm−2) retain ScxGFP expression, whereas tenocytes in untreated controls and area I (0.1 dyne cm−2) show a marked decrease in ScxGFP levels. Bar = 50 μm.
(C) Analysis of ScxGFP intensities in primary tenocytes at 7 d under the different mechanical forces shown in Figure 3B. GFP intensity of untreated tenocytes at 7 d after culture (No shear) was set to 1.0. Relative GFP intensities of tenocytes determined in each area are shown relative to untreated control. Results are the mean of measurements for 100 cells in each area. Error bars represent standard deviation. ScxGFP levels in areas II, III and IV are significantly higher than those in untreated controls. *, P < 0.05.
(D) Left panels: ScxGFP in primary tenocytes at 7 d under 0.14 dyne cm−2 mechanical force with or without 1.0 μM TGF-β type-I receptor inhibitor SD208. Note that SD208 markedly inhibits mechanical force-mediated ScxGFP induction. Bar = 100 μm. Right panel: Analysis of ScxGFP intensities. GFP intensity of tenocytes is shown relative to the control value of 100 (percent of control). Error bars represent standard deviation. Note that SD208 significantly (75.6%) inhibits mechanical force-mediated ScxGFP induction. *, P < 0.001.
(E) Real-time PCR analysis of endogenous Scx mRNA expression in primary tenocytes at 7 d under 0.14 dyne cm−2 mechanical force with or without (Control) 1.0 μM SD208. The intensity of Scx signals were normalized to that of 18S rRNA signals. Error bars represent standard deviation obtained by error propagation (n = 3). Note that SD208 significantly (72.0%) downregulates mechanical force-mediated endogenous Scx mRNA expression, and this downregulation correlates with ScxGFP levels shown in Figure 3D. *, P < 0.001.
(F) Effect of SD208 on pSmad2 levels in primary tenocytes from wild-type mice at 7 d under mechanical force with or without (Control) 1.0 μM SD208 or without shear stress. Upper panels: Expression of pSmad2 (green; left panels), nuclear staining with DAPI (blue; middle panels) and merged images (right panels). Bar = 25 μm. Lower panel: Analysis of pSmad2 intensities. The intensity is shown relative to the control value of 100 (Control tenocytes under shear stress). Error bars represent standard deviation. *, P < 0.001.
TGF-β and mechanical force function to maintain Scx expression, and Smad2/3 plays an essential role in both mechanical and biochemical signaling pathways that regulate Scx expression
Next, we examined 11 candidate cytokines/growth factors for their role in regulating Scx expression in adult tenocytes [26–32]. TGF-β1, -2 and -3 were the most potent and induced similar levels of ScxGFP expression. GDF8 (myostatin, another member of the TGF-β superfamily and a negative regulator of skeletal muscle mass [33]) was ~4-fold less potent than the TGF-βs. Neither the osteoinductive cytokine BMP2 [34], used as a negative control, nor any other cytokines/growth factors examined had any effect. With each of these, the level of ScxGFP expression dropped to that of untreated controls by 7 d of culture (Figure S2D).
In TGF-β superfamily-mediated signaling, all TGF-β ligands, including TGF-β1, -2, and -3 and GDF8, bind to cell surface TGF-β type I (designated as activin receptor-like kinases [ALKs]) and type II receptors. Upon ligand-binding, both receptor types form heterotetrameric complexes, which initiate downstream Smad-signaling pathways [35–36]. ScxGFP levels in tenocytes treated with 1.0 μM of the TGF-β type I receptor inhibitor SD208 [37] were significantly lower after 7 d of culture (24.4±13.0%, P < 0.001) compared to untreated controls (100%) (Figure 3D), and Scx mRNA levels were downregulated to 28.0±2.6% (P = 0.00045, Figure 3E). Although TGF-β is co-dependent on cytoskeletal tension in some cell types such as myofibroblasts [38–39], the addition of the cytoskeleton disrupting reagents C3 transferase (an inhibitor of the Rho signaling pathway) [40] or blebbistatin (an inhibitor of non-muscle myosin II production) [41–42] did not affect TGF-β-mediated ScxGFP expression in tenocytes (Figure S2E). These findings suggest that in tenocytes TGF-β1 regulates Scx expression independently of cytoskeletal tension, though this may need further confirmation.
Since the TGF-βs bind to TGF-β type I receptor and activate the Smad2/3 pathway [35–36], we examined the functional relationship between mechanical force- and TGF-β-mediated Scx induction. The addition of SD208 to primary tenocytes under mechanical force resulted in significantly (88.6%) decreased nuclear levels of phosphorylated Smad2 (pSmad2) in tenocytes. Phosphorylation of Smad2 was very weak and pSmad2 was distributed diffusely, whereas untreated tenocytes showed clear nuclear localization (Figure 3F). Furthermore, the Smad 3 inhibitor SIS3 [43] reduced the expression of ScxGFP and Scx mRNA levels in both TGF-β- and mechanical force-mediated systems by ~35% to ~50% (Figure S2F). Therefore both TGF-β and mechanical force have an important function in the maintenance of Scx expression, and Smad2/3 plays an essential role in both mechanical and biochemical signaling pathways that regulate Scx expression.
Transection interrupts tensile loading, destabilizes ECM structural organization, and releases an excess active TGF-β
In intact adult tissues and organs TGF-β is in a latent form associated in a noncovalent complex with the ECM. In response to injury, local latent TGF-β complexes are converted into active TGF-β [44–45]. To address how mechanical force regulates TGF-β activation and release at a molecular level, adult tenocyte cell lines were generated from Achilles tendons of mice on p53- and p21-null genetic background [46] as the yield of primary tenocytes was insufficient for analysis. These null tenocytes were morphologically indistinguishable from normal wild-type tenocytes (data not shown). When either p53- or p21-null tenocytes were exposed to mechanical force for 3 d in vitro, expression levels of TGF-β1 and Scx mRNAs were remarkably upregulated (12.4±4.3 and 26.9±6.1 fold [n = 3] respectively at 200 μl h−1 flow rate) compared to untreated controls. Released active TGF-β levels in the culture media corresponded to mechanical force strength (Figure 4A). On the other hand, while adding 80 pM TGF-β1 into the medium retained ScxGFP expression for up to 10 days, exposure of primary tenocytes to 160 pM TGF-β1 led to cell death in vitro (Figure 4B). We therefore hypothesized that the sudden interruption of continuous tensile loading by complete tendon transection might have resulted in the destabilization of the ECM’s structural organization and consequent release of excessive amounts of active TGF-β, which caused the massive tenocyte death found in vivo (Figure 1F).
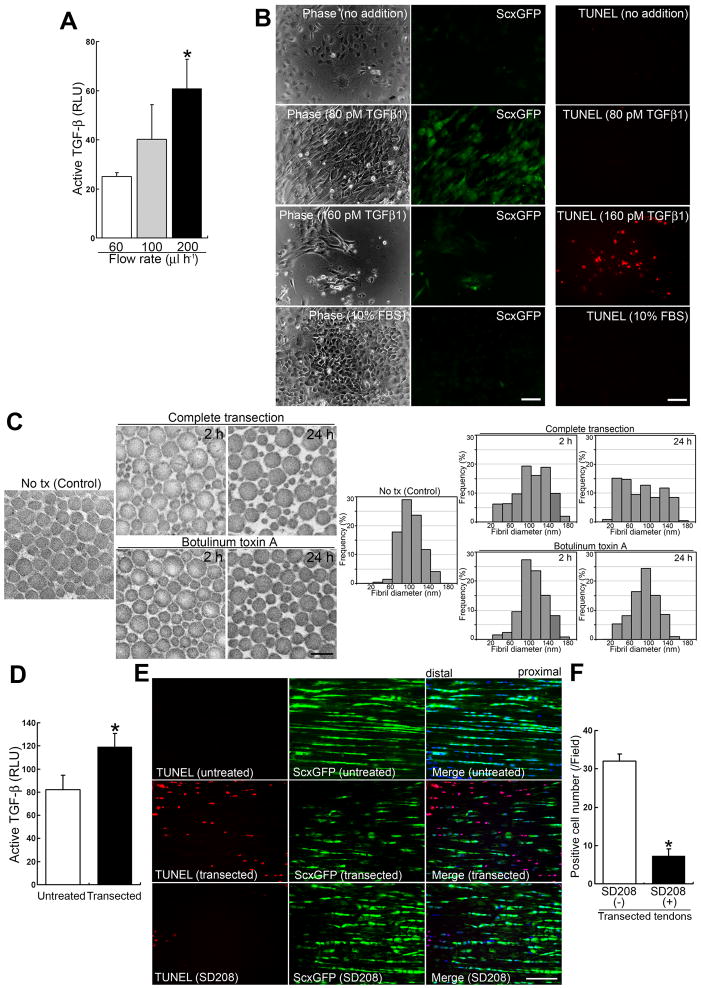
Figure 4. Sudden interruption of continuous tensile loading destabilizes the ECM’s structural organization and allows consequent release of a significant amount of active TGF-β.
(A) Active TGF-β bioassay in culture media from adult tenocyte cell lines cultured in a single network chamber for 5 d under different mechanical forces. Flow rate conditions for 60, 100 and 200 μl h−1 corresponded to 0.01, 0.015 and 0.03 dyne cm−2 mechanical force, respectively. Culture medium (100 μl) from each condition was used for the assay. The data show the amounts released in conditioned media for 1 h under different flow rates. Luciferase activity is presented as relative light units (RLU). Error bars represent standard deviation (n = 3). The values for active TGF-β released in the conditioned media over 1 h were 0.30 ± 0.018 pM under 60 μl h−1 shear stress; 0.52 ± 0.18 pM under 100 μl h−1 shear stress; and 1.01 ± 0.19 pM under 200 μl h−1 shear stress. *, significantly different (P < 0.01) compared to the amount under strain at 60 μl h−1 flow rate.
(B) Effects of the cytokine TGF-β1 on primary tenocytes. Left and middle panels: Phase contrast and fluorescence micrographs of the same area with a GFP filter for each condition. Right panels: The assessment of cell death by TUNEL staining (red). Primary tenocytes were cultured for 48 h, then cytokines were added for a further 7 d. Note that the addition of TGF-β1 at a low concentration (2 ng ml−1; 80 pM) retains the expression of ScxGFP. In contrast to other culture conditions, only a higher concentration (160 pM) leads to tenocyte cell death (13.0 ± 2.7 cells/field [n = 4; field = 0.15 mm2]). Retention of ScxGFP expression was seen at TGF-β1 concentrations as low as 20 pM (data not shown). Neither no addition (DMEM containing 1% FBS) nor 10% FBS maintained ScxGFP expression. Bars = 100 μm.
(C) Ultrastructural analysis of collagen fibrils. Transmission electron micrographs of transverse sections (left panels) and morphometric analysis of fibrils (right panels) in intact (Control), completely transected, and toxin-injected adult Achilles tendon tissues at 2 and 24 h post treatment. Note that collagen fibril ultrastructure in tension-collapsing tendons by complete transection reveals irregular patterns by as early as 2 h post injury. Bar in left panel = 200 nm.
(D) Active TGF-β bioassay in intact (untreated) and transected (at 1.5 h after complete transection) adult Achilles tendons. Luciferase activity is presented as relative light units (RLU). Error bars represent standard deviation (n = 3). Note that the transacted tendon tissues release significant amounts of active TGF-β. *, P < 0.05.
(E) Effects of SD208 on cell death in vivo after complete transection of adult Achilles tendon. TUNEL staining (red; left panels), expression of ScxGFP (green; middle panels) and merged images (right panels) with DAPI (blue) in intact tendons and at 1.5 h after complete transection without (untreated) or with 1.0 μM SD208 treatment. Bar = 50 μm.
(F) Analysis of TUNEL-positive cells at 1.5 h after complete transection without or with SD208 treatment. Error bars represent standard deviation (n = 4; field = 0.07 mm2). Note that SD208 significantly attenuates tenocyte cell death. *, P < 0.001.
Indeed, subsequent analyses of collagen fibril ultrastructure in transected tendons revealed irregular patterns by as early as 2 h post-injury (Figure 4C). In intact tendon, the vast majority of collagen fibrils were 60–120 nm in diameter (70.4%) and only ~2% of fibrils had a diameter <60 nm. By 2 h post injury, the number of larger fibrils with diameter 60–120 nm had decreased from 70% to 45%, whereas the number of thinner fibrils (<60 nm diameter) increased 6.3-fold (12.5%). By 24 h post injury, the collagen fibrils had deteriorated even further: only ~30% of fibrils with diameter 60–120 nm remained, and the number of thinner fibrils (<60 nm diameter) had increased to 30.6%. The number of fibrils with diameter >120 nm also changed over time (16.1% in intact tendon, 30.8% at 2 h, and 20.6% at 24 h post injury). In contrast, unlike completely transected tendons, the relative distribution of fibrils of different diameter in toxin-treated tendons did not change dramatically over time (Figure 4C). Since fibronectin and fibrillin-1 associate with latent TGF-β binding proteins [44–45], their expression patterns were examined but no obvious differences between transected and intact tendon tissues were found (Figure S3). Quantitative analysis of active TGF-β demonstrated that a significantly higher (~1.5-fold) concentration was released into tissues at 1.5 h post injury relative to uninjured tendon (P = 0.018; Figure 4D), whereas the total amount of TGF-β indicated similar levels pre- vs. post injury (631±61.1 relative light units (RLU) vs. 651±69.0 RLU [n = 3]). Such an excessive amount of active TGF-β was indeed related to tenocyte cell death in vivo after injury (Figure 4E). The expression level of Scx mRNA at 1.5 h post injury was 36.4±5.0% (n = 3) of non-treated control. The pre-treatment of tendons with 1.0 μM SD208 for 10 min before injury significantly reduced TUNEL-positive cells, whereas massive tenocyte cell death was observed in the SD208-untreated control transection (P < 0.001; Figure 4E and 4F). Thus the quantity of released active TGF-β from ECM depends on changes in mechanical force and demonstrates a mechanism underlying massive tenocyte death in response to complete transection of Achilles tendons.
Discussion
The present study (1) provides compelling evidence that mechanical forces actually regulate expression of Scx through activation of the TGF-β-/Smad2/3-mediated pathway, which, in turn, is required for maintenance of tendon-specific ECM; and (2) directly links an excess of active TGF-β released from the ECM to adult tendon pathology.
An earlier study has demonstrated that myofibroblast contraction activates and releases latent TGF-β from the ECM in cultured fibroblasts [47]. This supports a functional link between mechanical force and active TGF-β. In normal physiological conditions, every cell senses mechanical signals within a distinct range of magnitude [48]. After tissue injury tissue boundaries disintegrate and ECM architecture is considerably disrupted, causing dramatic mechanical imbalance to cells [39]. Indeed, the elevated TGF-β activity in response to injury causes delayed healing after bone fracture and skeletal muscle injury [49–50]. The suppression of TGF-β1 activity can promote functional recovery after corneal and spinal cord injury [49, 51]. Furthermore, after cancer radiotherapy, TGF-β1 is overexpressed at the targeting sites which develop injury-type response with cell death, excessive fibrosis and tissue atrophy in the surrounding normal tissues [52]. The alteration of mechanical integrity and the modifications that interfere with normal mechanotransduction to mechanical strain could also be implicated in a wide disease spectrum, including muscular dystrophies, arteriosclerosis, osteoporosis and cancer [53]. Thus, the mechanism demonstrated here opens new avenues for understanding how mechanical force is converted into biochemical signals and thereby determines cell fate in a variety of adult tissues or systems in the body. Since multiple mechanisms exist in mechanotransduction pathways, they will interplay as complementary pathways to maintain tissue specific phenotypes.
Tendon injuries are a serious clinical problem since damaged tendon tissues heal slowly and rarely regain normal mechanical strength [14, 30, 54]. These data imply that more extensive use of physical modalities in therapy could benefit patients and improve functional outcome. In tenocytes Scx regulates transcription of Col1a1 through binding to tendon-specific element 2 [20] and the induction of Scx expression in injured tendon may support healing. Furthermore, the application of TGF-β receptor inhibitors to injured tendons as a part of the first-aid treatment immediately after injury could significantly improve the prognosis.
Supplementary Material
Highlights.
Physical forces regulate the release of active TGF-β from the extracellular matrix
Mechanical forces maintain Scleraxis expression through TGF-β-mediated signaling
An excess release of active TGF-β directly links to adult tendon pathology
Acknowledgments
We thank Robb Colbrunn and Dr. Antonie van den Bogert, Musculoskeletal Robotics Testing Core, part of the Cleveland Clinic Musculoskeletal Core Center (a center supported in part by National Institutes of Health grant P30AR-050953), and Andrew Baker and Dr. Kathleen Derwin for biomechanical analysis, Dr. Dick Heinegård for antibodies and Dr. Daniel Rifkin for TMLC cells. We also thank Dr. Véronique Lefebvre for valuable discussions, and Christine Kassuba and Emma Stephenson for editorial assistance. We gratefully acknowledge support from The Cleveland Clinic (to T. Sakai). This work was supported in part by National Institutes of Health research grants DK074538 (to T. Sakai) and EB006203 (to H. Baskaran) and by Sumitomo Foundation, Japan (to T. Sakai).
Abbreviations
- ALK
activin receptor-like kinase
- BMP
bone morphogenic protein
- COMP
cartilage olgomeric matrix protein
- DAPI
4′6-diamidino-2-phenylinodole
- DMEM
Dulbecco’s modified Eagle’s medium
- EGF
epidermal growth factor, ECM, extracellular matrix
- FGF
fibroblast growth factor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GDF
growth differentiation factor
- GFP
green fluorescent protein
- IGF
insulin-like growth factor
- PAI-1
plasminogen activator inhibitor-1
- PCR
polymerase chain reaction
- PDGF
platelet derived growth factor
- Scx
scleraxis
- RT
reverse transcription
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling
Footnotes
Author contributions
Takao Sakai initiated and supervised the project. Dusko Ilic and Takao Sakai conceived ideas and designed experiments. Toru Maeda, Tomoya Sakabe, Ataru Sunaga, Keiko Sakai and Alexander Rivera performed experiments. Douglas Keene carried out electron microscopy studies. Ronen Schweitzer generated ScleraxisGFP transgenic mice. Harihara Baskaran generated microfluidic chambers for the fluid shear stress system. Takako Sasaki generated anti-type III and anti-type I collagen antibodies. Joseph Iannotti initiated the botulinum toxin A experiments. Edward Stavnezer initiated the TGF-β receptor inhibitor SD208 experiments and provided inhibitors. Toru Maeda, Tomoya Sakabe and Takao Sakai analyzed the data. Dusko Ilic, Harihara Baskaran and Takao Sakai wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 3.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 4.Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today. 2008;84:228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- 7.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 8.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 9.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007;236:1677–1682. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 10.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 12.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 13.Iwuagwu FC, McGrouther DA. Early cellular response in tendon injury: the effect of loading. Plast Reconstr Surg. 1998;102:2064–2071. doi: 10.1097/00006534-199811000-00038. [DOI] [PubMed] [Google Scholar]
- 14.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 15.Turton K, Chaddock JA, Acharya KR. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci. 2002;27:552–558. doi: 10.1016/s0968-0004(02)02177-1. [DOI] [PubMed] [Google Scholar]
- 16.Dolly JO, Black J, Williams RS, Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984;307:457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- 17.Sathyamoorthy V, DasGupta BR. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985;260:10461–10466. [PubMed] [Google Scholar]
- 18.Ma J, Shen J, Smith BP, Ritting A, Smith TL, Koman LA. Bioprotection of tendon repair: adjunctive use of botulinum toxin A in Achilles tendon repair in the rat. J Bone Joint Surg Am. 2007;89:2241–2249. doi: 10.2106/JBJS.D.03054. [DOI] [PubMed] [Google Scholar]
- 19.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res. 2007;25:1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 20.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 21.Janakiraman V, Sastry S, Kadambi JR, Baskaran H. Experimental investigation and computational modeling of hydrodynamics in bifurcating microchannels. Biomed Microdevices. 2008;10:355–365. doi: 10.1007/s10544-007-9143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech. 1997;30:79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 23.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002;35:303–309. doi: 10.1016/s0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 24.Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC. A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol. 2008;7:405–416. doi: 10.1007/s10237-007-0104-z. [DOI] [PubMed] [Google Scholar]
- 25.Bird RB, Stewart WE, Lightfoot EN. Transport phenomena. 2. New York: J. Wiley; 2002. [Google Scholar]
- 26.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 2008;105:388–393. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 29.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29:551–563. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 31.Jann HW, Stein LE, Slater DA. In vitro effects of epidermal growth factor or insulin-like growth factor on tenoblast migration on absorbable suture material. Vet Surg. 1999;28:268–278. doi: 10.1053/jvet.1999.0268. [DOI] [PubMed] [Google Scholar]
- 32.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarlane C, Sharma M, Kambadur R. Myostatin is a procachectic growth factor during postnatal myogenesis. Curr Opin Clin Nutr Metab Care. 2008;11:422–427. doi: 10.1097/MCO.0b013e32830007e2. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 35.Moustakas A, Heldin CH. The regulation of TGF{beta} signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 36.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 37.Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 38.Moustakas A, Heldin CH. Dynamic control of TGF-beta signaling and its links to the cytoskeleton. FEBS Lett. 2008;582:2051–2065. doi: 10.1016/j.febslet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Panetti TS, Magnusson MK, Peyruchaud O, Zhang Q, Cooke ME, Sakai T, Mosher DF. Modulation of cell interactions with extracellular matrix by lysophosphatidic acid and sphingosine 1-phosphate. Prostaglandins Other Lipid Mediat. 2001;64:93–106. doi: 10.1016/s0090-6980(01)00102-2. [DOI] [PubMed] [Google Scholar]
- 41.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 42.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 43.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 44.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zajac AL, Discher DE. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr Opin Cell Biol. 2008;20:609–615. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohta M, Kohmura E, Yamashita T. Inhibition of TGF-beta1 promotes functional recovery after spinal cord injury. Neurosci Res. 2009;65:393–401. doi: 10.1016/j.neures.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann G, Henle P, Kusswetter M, Moghaddam A, Wentzensen A, Richter W, Weiss S. TGF-beta1 as a marker of delayed fracture healing. Bone. 2005;36:779–785. doi: 10.1016/j.bone.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47:1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- 52.Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15:350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFranco MJ, Derwin K, Iannotti JP. New therapies in tendon reconstruction. J Am Acad Orthop Surg. 2004;12:298–304. doi: 10.5435/00124635-200409000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.