Abstract
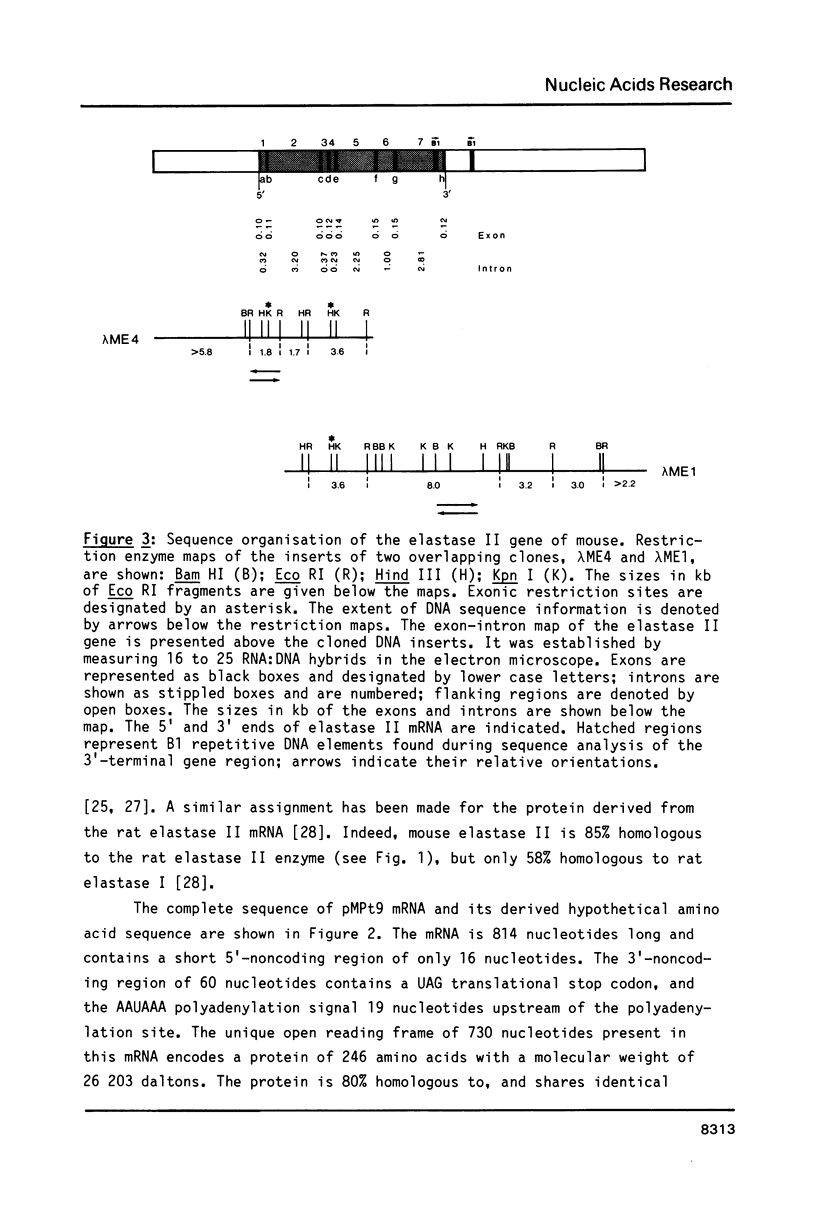
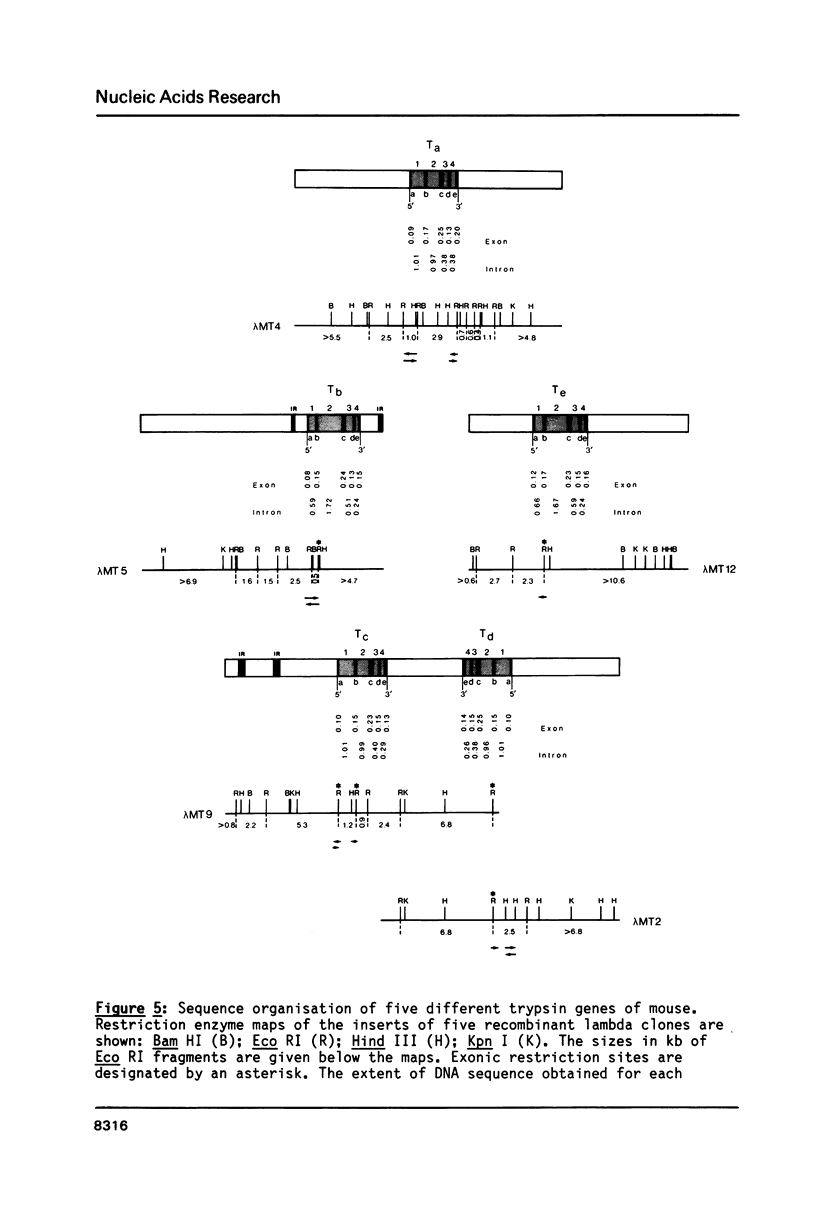
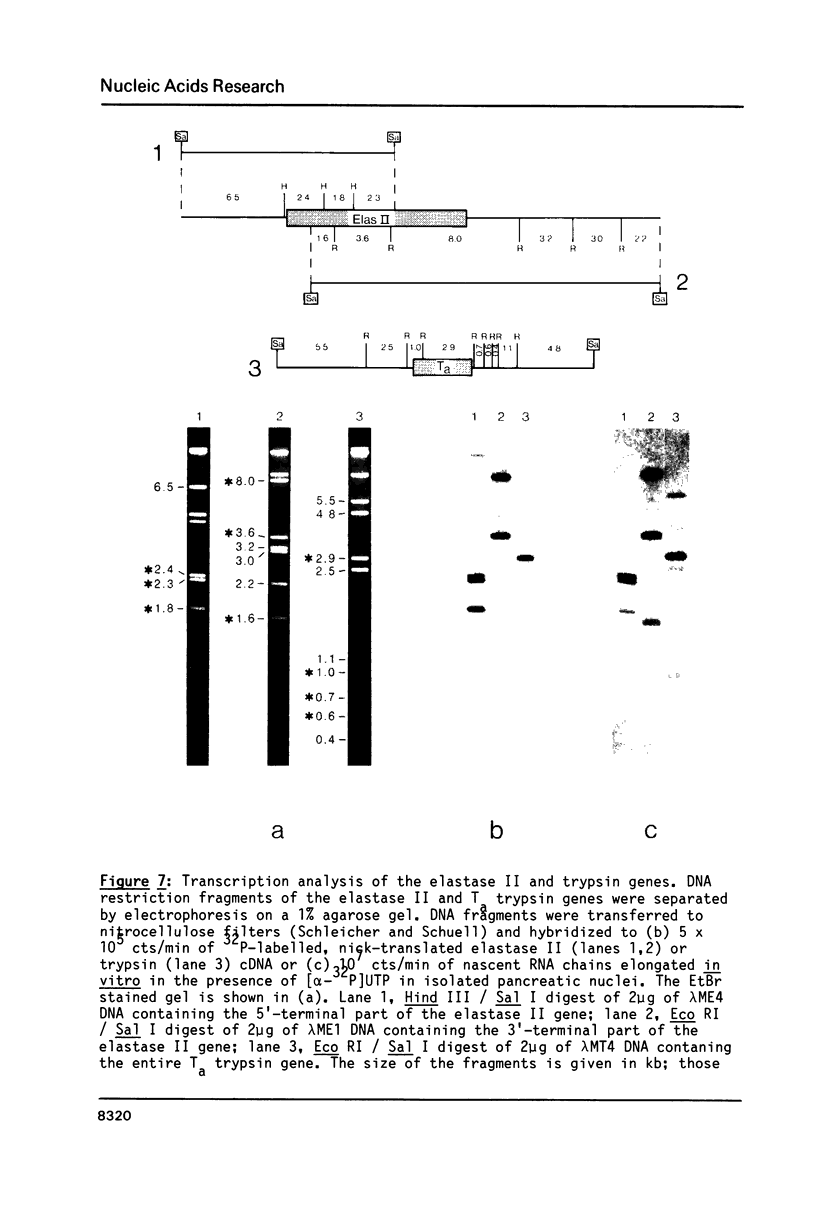
Elastase II and trypsin mRNAs were cloned in form of their cDNAs from pancreas of strain A/J mice, and their complete nucleotide sequences were determined. The elastase II mRNA is 912 nucleotides long and encodes a protein of 271 amino acids. The cloned trypsin mRNA species is 814 nucleotides long and encodes a protein of 246 amino acids. The elastase II gene, which exists as a single copy in the haploid mouse genome, measures 11.2 kb from cap to poly(A) site and is interrupted by at least seven introns. Between 5 and 10 trypsin genes exist in the mouse genome. Five different trypsin genes, two of which are closely linked in a tail-to-tail manner, were studied in detail. They vary in size between 3.4 and 4.0kb, and all are interrupted by four introns. DNA sequence comparison of the elastase II, trypsin and Amy-2a alpha-amylase genes reveals a conserved 13 nucleotide motif in their 5'-flanking regions. The differential accumulation of the elastase II and trypsin mRNAs in the cytoplasm of the acinar pancreatic cell is regulated predominantly at the transcriptional level.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Quinto C., Quiroga M., Valenzuela P., Craik C. S., Rutter W. J. Isolation and sequence of a rat chymotrypsin B gene. J Biol Chem. 1984 Nov 25;259(22):14265–14270. [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S., Grossi G., Hagenbüchle O., Wellauer P. K. Members of the Amy-2 alpha-amylase gene family of mouse strain CE/J contain duplicated 5' termini. J Mol Biol. 1985 Mar 5;182(1):1–10. doi: 10.1016/0022-2836(85)90022-1. [DOI] [PubMed] [Google Scholar]
- Boulet A. M., Erwin C. R., Rutter W. J. Cell-specific enhancers in the rat exocrine pancreas. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3599–3603. doi: 10.1073/pnas.83.11.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Carneiro M., Schibler U. Accumulation of rare and moderately abundant mRNAs in mouse L-cells is mainly post-transcriptionally regulated. J Mol Biol. 1984 Oct 5;178(4):869–880. doi: 10.1016/0022-2836(84)90316-4. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Choo Q. L., Swift G. H., Quinto C., MacDonald R. J., Rutter W. J. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984 Nov 25;259(22):14255–14264. [PubMed] [Google Scholar]
- Del Mar E. G., Largman C., Brodrick J. W., Fassett M., Geokas M. C. Substrate specificity of human pancreatic elastase 2. Biochemistry. 1980 Feb 5;19(3):468–472. doi: 10.1021/bi00544a011. [DOI] [PubMed] [Google Scholar]
- Fehlhammer H., Bode W., Huber R. Crystal structure of bovine trypsinogen at 1-8 A resolution. II. Crystallographic refinement, refined crystal structure and comparison with bovine trypsin. J Mol Biol. 1977 Apr 25;111(4):415–438. doi: 10.1016/s0022-2836(77)80062-4. [DOI] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U., Petrucco S., Van Tuyle G. C., Wellauer P. K. Expression of mouse Amy-2a alpha-amylase genes is regulated by strong pancreas-specific promoters. J Mol Biol. 1985 Sep 20;185(2):285–293. doi: 10.1016/0022-2836(85)90404-8. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Wellauer P. K., Cribbs D. L., Schibler U. Termination of transcription in the mouse alpha-amylase gene Amy-2a occurs at multiple sites downstream of the polyadenylation site. Cell. 1984 Oct;38(3):737–744. doi: 10.1016/0092-8674(84)90269-1. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Rat pancreatic ribonuclease messenger RNA. The nucleotide sequence of the entire mRNA and the derived amino acid sequence of the pre-enzyme. J Biol Chem. 1982 Dec 25;257(24):14582–14585. [PubMed] [Google Scholar]
- MacDonald R. J., Stary S. J., Swift G. H. Two similar but nonallelic rat pancreatic trypsinogens. Nucleotide sequences of the cloned cDNAs. J Biol Chem. 1982 Aug 25;257(16):9724–9732. [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Quinto C., Swain W., Pictet R. L., Nikovits W., Rutter W. J. Primary structure of two distinct rat pancreatic preproelastases determined by sequence analysis of the complete cloned messenger ribonucleic acid sequences. Biochemistry. 1982 Mar 16;21(6):1453–1463. doi: 10.1021/bi00535a053. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroux S., Baratti J., Desnuelle P. Purification and specificity of porcine enterokinase. J Biol Chem. 1971 Aug 25;246(16):5031–5039. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O., Perry R. P. Construction and identification of cDNA clones for mouse ribosomal proteins: application for the study of r-protein gene expression. Gene. 1980 Jul;10(2):113–129. doi: 10.1016/0378-1119(80)90129-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Quinto C., Quiroga M., Swain W. F., Nikovits W. C., Jr, Standring D. N., Pictet R. L., Valenzuela P., Rutter W. J. Rat preprocarboxypeptidase A: cDNA sequence and preliminary characterization of the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(1):31–35. doi: 10.1073/pnas.79.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinovitch M. R., Sreebny L. M., Smuckler E. A. Ribonuclease and ribonuclease inhibitor of the rat parotid gland and its secretion. J Biol Chem. 1968 Jun 25;243(12):3441–3446. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Watson H. C. Three-dimensional structure of tosyl-elastase. Nature. 1970 Feb 28;225(5235):811–816. doi: 10.1038/225811a0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Craik C. S., Stary S. J., Quinto C., Lahaie R. G., Rutter W. J., MacDonald R. J. Structure of the two related elastase genes expressed in the rat pancreas. J Biol Chem. 1984 Nov 25;259(22):14271–14278. [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Van Nest G. A., MacDonald R. J., Raman R. K., Rutter W. J. Proteins synthesized and secreted during rat pancreatic development. J Cell Biol. 1980 Sep;86(3):784–794. doi: 10.1083/jcb.86.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vered M., Gertler A., Burstein Y. Partial amino acid sequence of porcine elastase II. Active site and the activation peptide regions. Int J Pept Protein Res. 1986 Feb;27(2):183–190. doi: 10.1111/j.1399-3011.1986.tb01809.x. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]