Abstract
Bacteriophage lytic enzymes quickly destroy the cell wall of the host bacterium to release progeny phage. Because such lytic enzymes specifically kill the species in which they were produced, they may represent an effective way to control pathogenic bacteria without disturbing normal microflora. In this report, we studied a murein hydrolase from the streptococcal bacteriophage C1 termed lysin. This enzyme is specific for groups A, C, and E streptococci, with little or no activity toward several oral streptococci or other commensal organisms tested. Using purified lysin in vitro, we show that 1,000 units (10 ng) of enzyme is sufficient to sterilize a culture of ≈107 group A streptococci within 5 seconds. When a single dose of lysin (250 units) is first added to the oral cavity of mice, followed by 107 live group A streptococci, it provides protection from colonization (28.5% infected, n = 21) compared with controls without lysin (70.5% infected, n = 17) (P < 0.03). Furthermore, when lysin (500 units) was given orally to 9 heavily colonized mice, no detectable streptococci were observed 2 h after lysin treatment. In all, these studies show that lysin represents a unique murein hydrolase that has a rapid lethal effect both in vitro and in vivo on group A streptococci, without affecting other indigenous microorganisms analyzed. This general approach may be used to either eliminate or reduce streptococci from the upper respiratory mucosal epithelium of either carriers or infected individuals, thus reducing associated disease.
Streptococcus pyogenes (group A β-hemolytic streptococci), the primary etiologic agent of bacterial pharyngitis, is one of few human pathogens that remain uniformly sensitive to penicillin (1). Additionally, the advent of rapid group A streptococcal diagnostic test kits over the last decade has allowed early initiation of antibiotic treatment. Despite these factors, streptococcal-mediated pharyngitis is reported in over 2.5 million people annually in the United States, >80% of these cases occurring in children under 15 years of age (2). However, streptococcal pharyngitis classically is not a reportable disease, and it has been speculated that the documented number of these pharyngitis cases may be considerably underestimated. Additionally, penicillin fails to completely eradicate streptococci in up to 35% of patients treated for pharyngitis (3), and carriage rates as high as 50% have been reported in close contact areas such as day care centers (4). This high carriage rate contributes to the spread of streptococcal pharyngitis (5) and correlates with outbreaks of rheumatic fever (6). Although eradication of the carrier state would reduce the pool of streptococci in the population and thus streptococcal-related diseases, to date the only treatment is an extensive regimen of antibiotics (7) that may increase streptococcal resistance to macrolides, which are often prescribed for patients with penicillin allergies (8).
At the end of a bacteriophage lytic cycle in a sensitive bacterial host, all double-stranded DNA bacteriophages produce a lytic system that consists of a holin and at least one peptidoglycan hydrolase, or “lysin”, capable of degrading the bacterial cell wall. Lysins can be endo-β-N-acetylglucosaminidases or N-acetylmuramidases (lysozymes), which act on the sugar moiety, endopeptidases, which cleave the peptide cross bridge, or more commonly, an N-acetylmuramoyl-l-alanine amidase, which hydrolyzes the amide bond connecting the sugar and peptide constituents. Typically, the holin is expressed in the late stages of phage infection forming a pore in the cell membrane allowing the lysin(s) to gain access to the cell wall peptidoglycan resulting in release of progeny phage (for review, see ref. 9). Lysin, added to sensitive organisms in the absence of bacteriophage, lyses the cell wall producing a phenomenon known as “lysis from without.”
The virulent C1 bacteriophage specifically infect group C streptococci and produce a lysin that has been partially purified and characterized (10, 11). C1 phage lysin can cause “lysis from without” in groups A and E as well as group C streptococci (12, 13). This unique activity has been exploited as a tool in group A streptococcal studies to isolate surface molecules including M proteins (14), to lyse cells for DNA extraction, and to make protoplasts when used in a hypertonic environment (15).
Because there exists a potential use of the C1 phage lysin for the prevention and control of group A streptococcal pharyngitis, we examined its killing ability on these organisms, its actions on other streptococci and oral microflora, and its effectiveness in a mouse model of pharyngitis. This is, to our knowledge, the first report investigating the prophylactic use of a phage encoded lysin in an in vivo model system.
Materials and Methods
Unless otherwise indicated, all materials used were purchased from Sigma and were of the highest purity available.
Bacterial Strains.
Bacterial strains (Table 1) were stored at −80°C and routinely grown in THY [Todd–Hewitt broth, 1% wt/vol yeast extract (both from Difco)] at 37°C. All species grew well in THY, with minor modifications. Adjustments for Porphyromonas gingivalis included anaerobic growth and supplementing the media with 5 mg hemin/100 mg DTT/1 mg menadione. Growth of streptomycin-resistant group A streptococcus T14/46 was supplemented with 200 μg/ml streptomycin sulfate. For preparation of the C1 bacteriophage and production of lysin, group C streptococci were grown in chemically defined media for streptococci (JRH Biosciences, Lenexa, KS).
Table 1.
Bacterial strains tested for lysin sensitivity
| Bacteria | Strain |
Comment |
Source |
|---|---|---|---|
| Set I. Group A Streptococci | |||
| Group A Streptococcus | J17A4 | Grouping strain | 1 |
| Group A Streptococcus | JRS75 | No M protein | 1 |
| Group A Streptococcus | D710 | Class I (M1) | 1 |
| Group A Streptococcus | D471 | Class I (M6) | 1 |
| Group A Streptococcus | A374 | Class I (M12) | 1 |
| Group A Streptococcus | 1RP43 | Class I (M19) | 1 |
| Group A Streptococcus | 1RP256 | Class II (M2) | 1 |
| Group A Streptococcus | D691 | Class II (M11) | 1 |
| Group A Streptococcus | D734 | Class II (M22) | 1 |
| Group A Streptococcus | A945 | Class II (M49) | 1 |
| Group A Streptococcus | A486 variant | No A carbohydrate | 1 |
| Set II. Other Lancefield Groups | |||
| Group B Streptococcus | 090R | 1 | |
| Group C Streptococcus | 26RP66 | 1 | |
| Group D Streptococcus | D76 | 1 | |
| Group E Streptococcus | K131 | 1 | |
| Group F Streptococcus | F68D | 1 | |
| Group G Streptococcus | D166B | 1 | |
| Group L Streptococcus | D167A | 1 | |
| Group N Streptococcus | C559 | 1 | |
| Set III. Oral Streptococci | |||
| Streptococcus crista | PK1408 | AKA CC5A strep. | 2 |
| Streptococcus intermedius | PK2821 | 2 | |
| Streptococcus gordonii | FSS2 | 3 | |
| Streptococcus gordonii | DL1 | 2 | |
| Streptococcus gordonii | PK488 | 2 | |
| Streptococcus gordonii | PK2565 | Blackburn strain | 2 |
| Streptococcus mitis | J22 | 2 | |
| Streptococcus mutans | NG5 | 4 | |
| Streptococcus mutans | Ingbritt 175 | 4 | |
| Streptococcus oralis | H1 | 2 | |
| Streptococcus oralis | PK34 | 2 | |
| Streptococcus parasanguis | PK2564 | 2 | |
| Streptococcus salivarius | ATCC 9222 | 2 | |
| Streptococcus salivarius | ATCC 7945 | 2 | |
| Set IV. Non-strep. Bacteria | |||
| Bacillus pumulis | BJ0050 | 3 | |
| Staphylococcus aureus | RN 6390 | 1 | |
| Staphylococcus epidermidis | BJ0018 | 3 | |
| Escherichia coli | JM83 | Gram-negative | 1 |
| Neisseria lactamicus | Gram-negative | 1 | |
| Porphyromonas gingivalis | W83 | Gram-negative | 5 |
| Pseudomonas aeruginosa | PA01 | Gram-negative | 1 |
1, The Rockefeller University Collection; 2, Paul Kolenbrander, National Institute of Dental and Craniofacial Research, Bethesda, MD; 3, John Mayo, Department of Biochemistry and Molecular Biology, University of Georgia, Athens, GA; 4, Arnold Blelweis, Department of Dental Science, University of Florida, Gainesville, FL; and 5, James Travis, Department of Biochemistry and Molecular Biology, University of Georgia, Athens, GA.
Preparation of C1 Bacteriophage.
To increase titers of the C1 phage, group C streptococcus strain 26RP66 was grown at 37°C to early log phase (preferably OD650 ≈ 0.25) and 1/4 (vol/vol) of prewarmed C1 phage was added and allowed to incubate until complete lysis occurred (≈40 min). The lysate was passed through a 0.45-μm filter (Amicon), and the preparation was stored at 4°C for future use.
Production of Lysin.
Crude phage lysin was prepared as described (10).
Lysin Assay.
Measurement of lysin activity was based on turbidimetric determination of cell lysis. Simple detection of activity during purification used 96-well plates (Costar) and an automated plate reader (Dynatech) measuring a decrease in OD650 of group A streptococcus D471 cells. For quantification of lysin activity used during the remainder of the experiments, an overnight culture of D471 cells was centrifuged (3,000 × g, 10 min), washed twice in enzyme buffer (EB) (5 mM phosphate buffer/1 mM DTT/1 mM EDTA, pH 6.1), and the final OD650 was adjusted to 0.6 in the same buffer. Serial dilutions of lysin containing samples were made in EB in a final volume of 1.0 ml, to which 1.0 ml of the freshly prepared D471 cells were added and mixed. A control contained 1.0 ml of EB with 1.0 ml of D471 cells. After incubation in a water bath at 37°C for 15 min, the OD650 was measured for each dilution, and the reciprocal of the highest dilution that was nearest to half of the control value was defined as the activity of lysin in units/ml. For example, if the mixture from a 1:6,400 dilution produced a drop in OD650 from 0.30 to 0.15 in the 15-min assay, the enzymatic activity was defined as 6,400 units/ml.
To visualize lysin activity during purification, samples from chromatography fractions were applied to disks of sterile filter paper that were applied to a bacterial lawn of D471 cells on brain heart infusion agar. A clearing zone after overnight incubation indicated antibacterial activity.
Lysin Purification.
Fifty milliliters of concentrated crude extract was centrifuged (10,000 × g, 20 min) to remove remaining bacterial debris, and the clear supernatant was dialyzed against EB overnight with a 10-kDa cut-off membrane (Spectra-Por, Spectrum Medical Industries, Los Angeles). The sample was then applied to a 15-ml HiTrap Q column (Amersham Pharmacia–Pharmacia Biotech) that had been equilibrated in EB and eluted in a linear gradient containing EB supplemented with 1 M NaCl. The fraction containing the activity was pooled, dialyzed against EB, and further purified by application to a hydroxylapatite column (Calbiochem), with elution in a linear gradient containing 1 M phosphate buffer, pH 6.1. Fractions containing activity were pooled, dialyzed, and concentrated (Amicon PM-10 membrane) to 10 ml and applied to a Sephacryl-200 (16/60) column (Amersham Pharmacia–Pharmacia Biotech) equilibrated with EB supplemented with 200 mM NaCl for final purification.
In Vitro Lysin Activity on Group A Streptococci.
An overnight culture of group A streptococcus D471 was washed twice, suspended in EB, and an aliquot of serial dilutions was spread on blood agar plates (proteose–peptone agar with 5% defibrinated sheep blood) to determine starting colony count. Lysin was diluted in EB and added to ≈107 D471 cells, and aliquots were removed, diluted, and plated at 5, 30, and 60 sec and 5 and 10 min to assess the remaining viability of the treated cells. All experiments were performed in triplicate with EB serving as the control in place of lysin.
Lysin Activity on Various Bacterial Strains.
All bacterial strains (Table 1) were grown overnight as described above, washed twice in EB, and the OD650 was adjusted to ≈1.0 with EB. Each cell suspension (225 μl) was added in triplicate to a 96-well microtiter plate just before addition of 250 units of purified lysin (25 μl of a 10,000 units/ml stock). The OD650 was monitored for each well over the course of the experiment, and the plates were shaken before each reading to maintain cell suspension. Controls with EB were used for each strain to observe spontaneous lysis of cells and/or sedimentation effects that were not resolved by the shaking. Although no spontaneous lysis was observed, several strains did have ≈10% decrease in OD650 after several hours because of sedimentation. In these cases, the OD650 decrease seen in the controls was added back to the lysin experimental values to compensate for natural settling. The activity of lysin for each strain was reported as the initial velocity of lysis, in −OD/min, based on the time it took to decrease the starting OD by half (i.e., from an OD650 of 1.0 to 0.5). For group A streptococci, this was achieved within the first minute of the assay, whereas it took over an hour to reach this value for some of the Streptococcus gordonii strains. All strains were monitored for 8 h to be sure we were able to detect any observable lysis. Finally, for all strains, an aliquot was taken after 30 min incubation, plated on either brain heart infusion or proteose–peptone blood agar plates, and incubated overnight to assess cell viability.
In Vivo Lysin Activity on Group A Streptococci.
Four- to eight-week-old CD1 Swiss mice were purchased from Charles River Laboratories and housed at the Laboratory Animal Research Center at The Rockefeller University. The group A streptococcus challenge strain was T14/46, which colonizes the oral cavity of mice and allows selection because of its streptomycin resistance (16). One day before experiments, mice were given streptomycin water (5 g/liter) to reduce normal oral bacteria flora. T14/46 streptococci were grown overnight in streptomycin (200 μg/ml) Todd–Hewitt broth (THY), pelleted, and suspended in 1/10 vol of THY. Both crude and purified lysin were adjusted to a concentration of 10,000 units/ml.
The first mouse experiment consisted of mixing 100 μl of EB (control) or crude lysin (1,000 units) with 50 μl (≈107) T14/46 cells in vitro for 5 min and then administering 50 μl of this mixture to the mice (30 μl orally and 10 μl to each nostril) without sedation by using a micropipette. Mice were orally swabbed with calcium alginate fiber tipped ultrafine swabs (Fisher) at 24-h intervals for 7 days and plated on streptomycin blood agar plates to determine colonization status of T14/46. The presence of a single β-hemolytic colony on the streptomycin blood agar was taken to indicate a positive culture. In the second mouse experiment, 25 μl of either EB (control), crude lysin (250 units), or purified lysin (250 units) was first given to the mice orally, followed 5–10 min later by oral and nasal administration of 50 μl (≈107) T14/46 organisms. Again mice were swabbed at daily intervals for 2 days to monitor colonization status of the streptococcal challenge strain. In the third mouse experiment, 16 mice were given 107 streptococci and swabbed at 24-h intervals for 4 days. Of these mice, only 9 that approximated the carrier state by being heavily colonized [colony-forming units (cfus) >300 per swab] for all 4 days were selected for further experimentation. On the 4th day, mice were administered 500 units of purified lysin orally and swabbed 2 h later. Additionally, swabs were taken at 24 and 48 h after lysin treatment (experimental days 5 and 6, respectively).
Electron Microscopy.
Group A streptococci were prepared for electron microscopy as previously described (17). Briefly, streptococci were exposed to lysin and removed at timed intervals, fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate (both from Fisher), pH 6.8, and treated with 1% osmium tetroxide in veronal acetate, pH 6.0, for 1 h. Fixed cells were embedded in 3–4% agar, soaked in 1% uranyl acetate, pH 4.5, for 1 h, and embedded in Epon. Thin sections were stained with either lead citrate alone or 2% aqueous uranyl acetate followed by lead staining. Electron micrographs were performed in collaboration with John Swanson.
Statistical Analysis.
Fisher's exact test was used to compare colonization status for the in vivo experiments. A P value <0.05 was considered significant.
Results
Lysin Preparation.
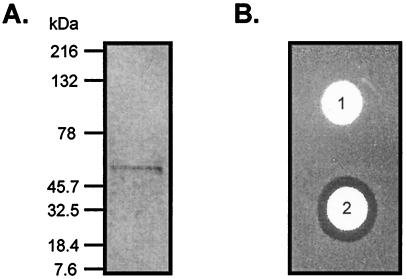
Phage lysin was purified from a crude lysate by column chromatography resulting in a homogeneous preparation with a molecular mass of 50 kDa by SDS/PAGE (Fig. 1A) and lytic activity against group A streptococci (Fig. 1B). However, the apparent molecular mass by gel filtration was ≈100 kDa, which is consistent with previously published reports (10), suggesting that the native enzyme may exist as a dimer. The specific activity of the lysin on group A streptococci is extremely high and appears to function by forming holes in the cell wall, which allows the membrane to become externalized, resulting in its rupture (see below). It appears, therefore, that only a few molecules of enzyme will result in a lytic event, making it difficult to obtain an accurate measure of specific activity. However, on the basis of silver stained gels, we estimate that 1,000 units of enzyme represents ≈10 ng of protein. We obtain yields of about 130,000 units of purified enzyme per liter of crude lysate.
Figure 1.
Purification of lysin. (A) Silver-stained SDS/PAGE of purified lysin. Molecular mass of Kaleidoscope (Bio-Rad) standards is given in kilodaltons. (B) A bacterial lawn of group A streptococci D471 treated with a buffer control disk (1) or a disk containing 250 units of purified lysin (2), as described in Materials and Methods. A clearing zone indicates antibacterial activity.
In Vitro Lysin Activity on Group A Streptococci.
To prove that observed cell lysis, as measured by spectrophotometric loss of turbidity, equates to cell death, streptococcal viability was determined in the presence of various concentrations of lysin. Our data show that complete cell death of ≈107 group A streptococcal cells occurred after exposure to 1,000 units of lysin for 5 sec, and that exposure to 100 units was able to decrease streptococcal viability by an order of 3 logarithms (logs) in 5 sec, 4 logs in 1 min, and 5 logs in 10 min (Table 2).
Table 2.
In vitro killing of group A streptococci by lysin
| Lysin units | Starting count | 5 sec | 30 sec | 60 sec | 5 min | 10 min |
|---|---|---|---|---|---|---|
| 1,000 | 5 × 106 | 0 | 0 | 0 | 0 | 0 |
| 100 | 8.6 × 106 | 1,530 | 1,196 | 771 | 64 | 6 |
| 10 | 9.8 × 106 | >3,000 | >3,000 | >3,000 | >3,000 | >3,000 |
Indicated numbers are cfus.
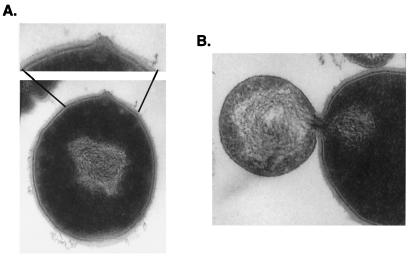
To visualize the action of the lysin on whole streptococci resulting in cell lysis and death, we used thin-section electron microscopy of group A streptococci placed in fixative at various time intervals after exposure to lysin. Within 15 seconds after exposure, a distinct localized degradation of the cell wall can be observed (Fig. 2A), resulting ultimately in the extrusion of the cytoplasmic membrane to the external, hypotonic, environment (Fig. 2B).
Figure 2.
Thin-section electron micrograph of Group A streptococci treated for 15 seconds with lysin. The cell wall is weakened (A), allowing the membrane to extrude through the hole (B) created by lysin. ×50,000.
Bacterial Specificity of Lysin.
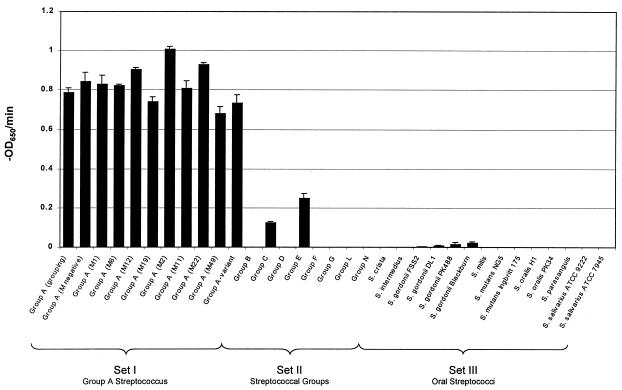
Purified C1 phage lysin was tested for muralytic activity against >40 bacterial strains in a variety of species that were divided into sets (Table 1 and Fig. 3). Set I contained 10 different group A streptococcal strains, including the serological grouping strain, an M-negative strain, 8 distinct M types [representing class I and class II streptococci (18)], and an A variant strain. Lysin was able to equally and completely lyse every strain in this set within 5 min, and no viable colonies were detected after plating cells exposed to lysin for this amount of time. Set II contained eight different Lancefield groups of streptococci (Fig. 3). We found that lysin exhibited activity against groups C and E, although considerably less than that seen with group A strains; however, it was unable to lyse groups B, D, F, G, L, and N streptococci. In agreement with the spectrophotometric observations, no viable colonies were detected for groups C and E streptococci when plated up to 30 min after exposure to lysin. Set III contained representative oral streptococci including Streptococcus crista, Streptococcus intermedius, S. gordonii, Streptococcus mitis, Streptococcus mutans, Streptococcus oralis, Streptococcus parasanguis, and Streptococcus salivarius. Very low but reproducible activity was noted only against all of the S. gordonii strains tested. However, in these bacteria, cell viability remained near starting counts even after 30-min exposure to lysin (4.8 × 107 starting cfus and 4.6 × 107 cfus after 30 min for S. gordonii Blackburn). Set IV contained a mix of Gram-positive bacteria (Bacillus pumulis, Staphylococcus aureus, or Staphylococcus epidermidis) often found in oral flora and Gram-negative (Escherichia coli, Neisseria lactamicus, P. gingivalis, or Pseudomonas aeruginosa) bacteria. Lysin had no effect on these organisms (data not shown in Fig. 3), despite similarities in the peptidoglycan of all Gram-positive organisms.
Figure 3.
Lysin activity on various streptococci. Representative streptococcal strains were exposed to 250 units of purified lysin, and the OD650 was monitored. The activity of lysin for each strain was reported as the initial velocity of lysis, in −OD650/min, on the basis of the time it took to decrease the starting OD by half (i.e., from an OD650 of 1.0 to 0.5). All assays were performed in triplicate, and the data are expressed as means ± standard deviations.
In Vivo Lysin Activity on Group A Streptococci.
In a mouse model, several experimental designs were used to test the in vivo ability of lysin to prevent or eliminate upper respiratory colonization by group A streptococci. In the first experiment, lysin (1,000 units) or buffer was premixed with group A streptococci in vitro and then given orally and nasally to 5 mice per group within 5 min. None of the lysin-treated mice were colonized after 24 h, compared with 5 of 5 buffer-treated control mice (P < 0.01) (Table 3). Following the lysin-treated animals for up to 1 week resulted in the detection of only a single colony of 20 swabs in the lysin-treated mice.
Table 3.
Mouse colonization by lysin- (1,000 units) treated group A streptococci
| Mouse | Day 1 | Day 2 | Day 3 | Day 7 |
|---|---|---|---|---|
| Lysin treated | ||||
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 1 | 0 |
| Total | 0/5 | 0/5 | 1/5 | 0/5 |
| Buffer treated | ||||
| 1 | 26 | 14 | 7 | 0 |
| 2 | >300 | 17 | 100 | 83 |
| 3 | 9 | 0 | 15 | 0 |
| 4 | >300 | >300 | >300 | 220 |
| 5 | 2 | 2 | 30 | 0 |
| Total | 5/5 | 4/5 | 5/5 | 2/5 |
Indicated numbers are cfus.
In the second mouse experiment, animals were pretreated orally with a single dose (250 units) of crude (n = 10) or purified (n = 11) lysin before challenge with ≈107 streptococci. Lysin again showed a protective effect by reducing the incidence of colonization from 70.5% (n = 17) in lysin-treated to 28.5% (n = 21) in control animals after 24 h (P < 0.03) (Table 4). There was no statistical difference in the protection provided by crude lysin (3/10 infected) versus purified lysin (3/11 infected) (P = 1.0). Notably, of the lysin-treated mice that were colonized, cfu counts were consistently low (1–11 colonies), mouse 4 being the exception, and either these cfus remained low or the colonization was cleared by 48 h. In contrast, in addition to 1 death, cfus of colonized buffer-treated mice all increased at 48 h.
Table 4.
Pretreatment of mice with lysin (250 units) prevents streptococcal infections
| Lysin
treated
|
Buffer control
|
||||
|---|---|---|---|---|---|
| Mouse | Day 1 | Day 2 | Mouse | Day 1 | Day 2 |
| Crude
lysin | |||||
| 1 | 0 | 0 | 1 | 1 | 14 |
| 2 | 0 | 0 | 2 | 33 | 250 |
| 3 | 9 | 0 | 3 | 0 | 0 |
| 4 | >300 | >300 | 4 | 0 | 0 |
| 5 | 1 | 0 | 5 | 4 | 12 |
| 6 | 0 | 0 | 6 | 1 | 2 |
| 7 | 0 | 0 | 7 | 6 | >300 |
| 8 | 0 | 0 | 8 | 6 | 0 |
| 9 | 0 | 0 | 9 | >300 | Dead |
| 10 | 0 | 0 | 10 | 83 | >300 |
| Purified
lysin |
11 | 10 | >300 | ||
| 11 | 0 | 0 | 12 | 0 | 0 |
| 12 | 1 | 0 | 13 | 0 | n.d. |
| 13 | 0 | 0 | 14 | 150 | n.d. |
| 14 | 1 | 1* | 15 | 0 | n.d. |
| 15 | 11 | 10* | 16 | >300 | n.d. |
| 16 | 0 | 0 | 17 | 200 | n.d. |
| 17 | 0 | n.d. | |||
| 18 | 0 | n.d. | |||
| 19 | 0 | n.d. | |||
| 20 | 0 | n.d. | |||
| 21 | 0 | n.d. | |||
| Total | 6/21 | 3/16 | Total | 12/17 | 8/12 |
n.d., no data collected; indicated numbers are cfus.
Isolated streptococci remained sensitive to lysin treatment in vitro.
In the third set of mouse experiments, 9 mice that had been heavily colonized (>300 cfus per swab) for 4 days were given a single dose of 500 units of lysin orally and monitored for subsequent colonization changes (Table 5). Negative 2-h postlysin oral swabs from all mice (0/9) suggested complete eradication of streptococci from the mucosa (day 4 prelysin vs. 2 h postlysin swab, P < 0.001). However, when these animals were followed for an additional 24 and 48 h, 2 animals revealed positive cultures, and a third animal died. Culturing of the rebound or surviving streptococci from 24-h and 48-h swabs of lysin-treated mice in this and the previous mouse experiments failed to identify any lysin-resistant colonies.
Table 5.
Elimination of group A streptococci from the mucosal surface of colonized mice (500 units lysin/mouse)
| Mouse | Strep (107) ↓ | Day 1 | Day 2 | Day 3 | Day 4 | Lysin ↓ | Postlysin treatment
|
||
|---|---|---|---|---|---|---|---|---|---|
| Day 4, 2 hr | Day 5, 24 hr | Day 6, 48 hr | |||||||
| 1 | >300 | >300 | >300 | >300 | 0 | 0 | 200* | ||
| 2 | >300 | >300 | >300 | >300 | 0 | 50* | 0 | ||
| 3 | >300 | >300 | >300 | >300 | 0 | 0 | 0 | ||
| 4 | >300 | >300 | >300 | >300 | 0 | 0 | 0 | ||
| 5 | >300 | >300 | >300 | >300 | 0 | Dead | Dead | ||
| 6 | >300 | >300 | >300 | >300 | 0 | n.d. | 0 | ||
| 7 | >300 | >300 | >300 | >300 | 0 | n.d. | 0 | ||
| 8 | >300 | >300 | >300 | >300 | 0 | n.d. | 0 | ||
| 9 | >300 | >300 | >300 | >300 | 0 | n.d. | 0 | ||
| Total colonized | 9/9 | 9/9 | 9/9 | 9/9 | 0/9 | 2/5 | 2/9 | ||
n.d., no data collected; indicated numbers are cfus..
Isolated streptococci remained sensitive to lysin treatment in vitro.
Discussion
Cell wall hydrolases digest the rigid bacterial cell wall, rendering the organism susceptible to osmotic lysis. In humans, one such cell wall hydrolase, lysozyme (N-acetylmuramidase), is an important part of the immune system through its presence in neutrophils as well as being secreted in tears and saliva (19). However, lysozyme is a general hydrolase, and surface proteins or a polysaccharide capsule present on many bacteria often hinder the ability of this enzyme to properly access the peptidoglycan. Therefore, prophylactic use of muralytic enzymes that have been shown to cleave the peptidoglycan of desired organisms represents an additional antimicrobial approach. To date, the only such enzyme tested extensively is lysostaphin, an endopeptidase produced by Staphylococcus simulans with the ability to cleave the pentaglycine cross bridge of the S. aureus peptidoglycan (20). Lysostaphin has been shown to be an effective alternative for the treatment of experimental aortic valve endocarditis caused by a S. aureus isolate with reduced susceptibility to vancomycin (21). In addition to lysozyme and lysostaphin, bacteriophage cell wall hydrolases (lysins) may prove to be a novel approach to control bacterial infections in general. Our work presented here suggests that the oral administration of the C1 phage lysin may be a useful reagent in controlling the group A streptococcal carrier state and for killing infectious streptococci present in the saliva and mucus of infected individuals.
The C1 phage lysin has previously been shown to be an N-acetylmuramoyl-l-alanine amidase, cleaving the amide bond between the N-acetylmuramic acid and l-alanine connecting the sugar and peptide moieties of the peptidoglycan (22). Although this bond is common to all Gram-positive cell walls, it has been suggested that phage lysins in general require a specific recognition or binding domain in addition to their catalytic domain. Garcia et al. show that amidase lysins from pneumococcal phages, as well as pneumococcal endolysins, contain a binding domain that recognizes the choline present in the pneumococcal cell wall (23). Additionally, when chimeras were made consisting of amidase catalytic domains without activity on pneumococcal cell walls and known choline-binding domains, the new proteins were fully active against these cell walls (reviewed in ref. 24). Yet, these tests were performed only on pneumococcal cell walls, so it is not known whether this activity was restricted to only these bacteria. In a more extensive investigation of species specificity by an amidase lysin, Loessner et al. showed that a cloned Listeria monocytogenes phage amidase lysin was active against all 6 species and 16 serovars of Listeria, but lacked activity against the other 23 tested Gram-positive species (25).
Our results confirm previous findings that the C1 phage lysin is active against groups A, C, and E streptococci (12, 13). We also show that the C1 phage lysin has a higher rate of activity on group A cells than group C cells, the natural host of the C1 phage (Fig. 3). The reason for this is unclear; however, in nature, phage lytic enzymes come in contact with the peptidoglycan from the cell membrane side rather than the extracellular environment. Therefore, under these conditions, the C1 lysin may be more active against group C cell wall. The majority of the oral streptococci were completely unaffected, and we observed only minor lysis in the S. gordonii species. It is questionable whether this low activity on the S. gordonii would have any physiological relevance in vivo because we needed to assay the lysin for several hours rather than minutes to detect any activity on these cells. This finding is corroborated by Maxted, who showed lysin to be capable of slowly lysing suspensions of group H streptococci (now classified as S. sanguis or S. gordonii) but never saw any effect of this enzyme on a bacterial lawn of these same streptococci (12), suggesting the observed lysis is slower than the growth rate of these organisms. Given that the C1 phage lysin shows intraspecies specificity, this lysin represents the most highly evolved peptidoglycan hydrolase described to date. Although group A streptococci cause the majority of streptococcal-mediated pharyngitis, group C streptococci have also been implicated in clinical cases (26), whereas group E streptococci are not considered human pathogens. Taken together, the data suggest that C1 lysin may be uniquely suited to target the pharyngitis-causing streptococci.
Mice have been used experimentally to study the ability of group A streptococci to colonize the mucosal epithelium (16), although, except for M-type 50, they are not normally susceptible to or carriers of streptococci in nature. We used this mouse model system to show that orally administered lysin can either prevent the acquisition of streptococci or significantly decrease the number of adherent streptococci. We showed, on the basis of oral swab data, complete elimination of the organisms from the oral mucosa 2 h after a single treatment of mice heavily colonized with group A streptococci. However, the mouse model does have limitations that should be considered when interpreting these observations. Whereas the inoculum dose received by animals in protection experiments may have been higher (107 cfu) than any likely human exposure, this dose was given within 10 min of lysin administration. The clearance rate of lysin from the oral mucosa has not been determined, so it is impossible to extrapolate the long-term protective effects that a single lysin dose may provide until these experiments can be conducted. If used on a regular schedule (daily, weekly) or formulated to increase the residence time on the mucosa, the observed effects may be extended. In experiments with previously colonized mice, it is possible that streptococci had sufficient time to become internalized within epithelial cells and establish an infection. Although, based on oral swabs, we were able to remove all of the organisms present in the oral pharynx with a single dose of lysin, internalized streptococci may be able to repopulate the oral mucosa several hours or days after treatment. This may explain the slight rebound of cfus noted in some of the mice at the 24- and 48-h swab in Table 5. Recolonization, however, was not the result of lysin-resistant bacteria, because organisms isolated from lysin-treated animals were found to be sensitive to lysin. Thus, periodic treatment with lysin may reduce the spread of group A streptococci in the community.
At any given time, up to 50% of the population carry group A streptococci asymptomatically in their oropharynx (3). Because these streptococci are uniquely human pathogens, the carrier state is the reservoir for group A streptococci in the environment. A carrier state may be promoted by a variety of conditions, including poor patient compliance to antistreptococcal therapy, poor intestinal absorption of penicillin, and streptococcal invasion into mucosal epithelial cells (for review, see ref. 7). Reduction of this group A streptococcal reservoir from the carrier would have a marked impact on the dissemination of streptococci in the population and streptococcal diseases in general; however, presently this may be accomplished only by treatment with antibiotics. Although this may work in limited situations, it is not a practical approach for entire populations because of its effects on the normal flora and the resulting increase in antibiotic resistant bacteria.
Our results show that oral administration of the C1 phage lysin is nondisruptive toward the indigenous oral microflora tested and is rapidly lethal for group A streptococci residing on the mucosal epithelium. Additionally, preliminary irritation tests show lysin to be a nonirritant to the mucosal epithelium (data not shown). One question that needs to be addressed regarding the use of lysin in humans is in vivo production of bioactive cell wall fragments generated by rapid streptococcal lysis. Although increased inflammatory response as a result of these fragments is a concern with any lytic antibacterial, the topical application of lysin would considerably limit the potential of such a response. In addition, because only nanogram quantities of lysin applied orally are needed to lyse streptococci, it is unlikely that a mucosal immune response to the lysin molecule itself would occur, although further experimentation is needed to confirm this hypothesis. Nonetheless, our data indicate that lysin represents a possible alternative to antibiotic therapy for the streptococcal carrier state and warrants continued investigation.
Although our results show promise that the C1 phage lysin may help reduce bacterial carriage and/or augment current antibiotic treatment of pharyngitis, this is only one example of the potential use of such phage enzymes. Several pathogenic bacteria, such as S. aureus and Streptococcus pneumoniae, have been associated with high human carriage rates that may be directly attributable to disease (27). In addition to these organisms, bacteriophages are found in nearly all pathogenic bacteria, including Clostridium botulinum, Yersinia pestis, Bacillus anthracis, Mycobacterium tuberculosis, Salmonella typhi, and Borrelia burgdorferi, to name a few (28, 29). In all these systems, with the exception of certain filamentous phage, the phages are released as the result of lytic enzymes. In light of our results with the C1 phage lysin, similar strategies may be used for the control of these pathogens in the development of what may be termed “enzybiotics.”
Acknowledgments
This work was supported in part by U.S. Public Service Grant AI11822 and by a grant from New Horizons Diagnostics (to V.A.F.). We particularly thank John Swanson for his electron micrographs. For donations of various bacterial strains, as indicated in Table 1, we thank Paul Kolenbrander, John Mayo, Arnold Bleiweis, and James Travis. Finally, we thank Mary Windels for her assistance with the animal experiments and production of crude lysin.
Abbreviations
- cfu
colony-forming unit
- EB
enzyme buffer
References
- 1.Macris M H, Hartman N, Murray B, Klein R F, Roberts R B, Kaplan E L, Horn D, Zabriskie J B. Pediatr Infect Dis J. 1998;17:377–381. doi: 10.1097/00006454-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Schappert S M, Nelson C. Vital Health Stat. 1999;13:1–122. [PubMed] [Google Scholar]
- 3.Pichichero M E. Pediatr Rev. 1998;19:291–302. doi: 10.1542/pir.19-9-291. [DOI] [PubMed] [Google Scholar]
- 4.Feldman S, Bisno A L, Lott L, Dodge R, Jackson R E. J Pediatr. 1987;110:783–787. doi: 10.1016/s0022-3476(87)80024-0. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen L, Levy D, Ferroni A, Gehanno P, Berche P. J Clin Microbiol. 1997;35:2111–2114. doi: 10.1128/jcm.35.8.2111-2114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver C. J Antimicrob Chemother. 2000;1:13–21. doi: 10.1093/jac/45.suppl_1.13. [DOI] [PubMed] [Google Scholar]
- 7.Tanz R R, Shulman S T. Pediatr Annals. 1998;27:281–285. doi: 10.3928/0090-4481-19980501-07. [DOI] [PubMed] [Google Scholar]
- 8.York M K, Gibbs L, Perdreau-Remington F, Brooks G F. J Clin Microbiol. 1999;37:1727–1731. doi: 10.1128/jcm.37.6.1727-1731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young R. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti V A, Gotschlich E C, Bernheimer A W. J Exp Med. 1971;133:1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raina J L. J Bacteriol. 1981;145:661–663. doi: 10.1128/jb.145.1.661-663.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxted W R. J Gen Microbiol. 1957;16:584–594. doi: 10.1099/00221287-16-3-584. [DOI] [PubMed] [Google Scholar]
- 13.Krause R M. J Exp Med. 1957;106:365–384. doi: 10.1084/jem.106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischetti V A, Jones K F, Scott J R. J Exp Med. 1985;161:1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler J, Holland J, Terry J M, Blainey J D. J Gen Microbiol. 1980;120:27–33. doi: 10.1099/00221287-120-1-27. [DOI] [PubMed] [Google Scholar]
- 16.Bessen D, Fischetti V A. J Immunol. 1990;145:1251–1256. [PubMed] [Google Scholar]
- 17.Swanson J, Hsu K, Gotschlich E C. J Exp Med. 1969;130:1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessen D, Jones K F, Fischetti V A. J Exp Med. 1989;169:269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klockars M, Reitamo S. J Histochem Cytochem. 1975;23:932–940. doi: 10.1177/23.12.1104708. [DOI] [PubMed] [Google Scholar]
- 20.Schaffner W, Melly M A, Hash J H, Koenig M G. Yale J Biol Med. 1967;39:215–229. [PMC free article] [PubMed] [Google Scholar]
- 21.Patron R L, Climo M W, Goldstein B P, Archer G L. Antimicrob Agents Chemother. 1999;43:1754–1755. doi: 10.1128/aac.43.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischetti V A, Zabriskie J B, Gotschlich E C. In: Fifth International Symposium on Streptococcus pyogenes. Haverkorn M J, editor. Amsterdam: Excerpta Medica; 1972. pp. 26–36. [Google Scholar]
- 23.Garcia E, Garcia J L, Garcia P, Arraras A, Sanchez-Puelles J M, Lopez R. Proc Natl Acad Sci USA. 1988;85:914–918. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez R, Garcia E, Garcia P, Garcia J L. Microb Drug Resist. 1997;3:199–211. doi: 10.1089/mdr.1997.3.199. [DOI] [PubMed] [Google Scholar]
- 25.Loessner M J, Wendlinger G, Scherer S. Mol Microbiol. 1995;16:1231–1241. doi: 10.1111/j.1365-2958.1995.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 26.Turner J C, Fox A, Fox K, Addy C, Garrison C Z, Herron B, Brunson C, Betcher G. J Clin Microbiol. 1993;31:808–811. doi: 10.1128/jcm.31.4.808-811.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Eiff C, Becker K, Machka K, Stammer H, Peters G. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann H-W, DuBow M S. Viruses of Prokaryotes: General Properties of Bacteriophages. Boca Raton, FL: CRC; 1987. [Google Scholar]
- 29.van Regenmortel M H V, Fauquet C M, Bishop D H L, Carstens E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeoch D J, Pringle C R, Wickner R B. Virus Toxonomy. San Diego: Academic; 2000. [Google Scholar]