Abstract
Alcoholic and nonalcoholic fatty liver diseases are potentially pathological conditions that can progress to steatohepatitis, fibrosis, and cirrhosis. These conditions affect millions of people throughout the world in part through poor lifestyle choices of excess alcohol consumption, overnutrition, and lack of regular physical activity. Abnormal mitochondrial and cellular redox homeostasis has been documented in steatohepatitis and results in alterations of multiple redox-sensitive signaling cascades. Ultimately, these changes in signaling lead to altered enzyme function and transcriptional activities of proteins critical to mitochondrial and cellular function. In this article, we review the current hypotheses linking mitochondrial redox state to the overall pathophysiology of alcoholic and nonalcoholic steatohepatitis and briefly discuss the current therapeutic options under investigation. Antioxid. Redox Signal. 15, 485–504.
Introduction
The mitochondrion has historically been described as the powerhouse of the cell through the aerobic metabolism of acetyl-CoA by the tricarboxylic acid (TCA) cycle and oxidative phosphorylation to produce adenosine triphosphate (ATP). With the dramatic increase in metabolic disorders such as obesity, the metabolic syndrome, and fatty liver disease (FLD) in Westernized societies, recent emphasis has been placed on examining the role of mitochondrial dysfunction in these disorders. To date, numerous reports have implicated hepatic mitochondrial dysfunction in the pathogenesis of FLD, which has a histological spectrum ranging from simple steatosis to steatohepatitis, advanced fibrosis, and cirrhosis.
A major role of mitochondria is to maintain cellular homeostasis through maintenance of oxidation–reduction (redox) balance. Redox reactions lie at the heart of cellular physiology, as a integral part of the production of ATP and signaling the overall energy state of the cell. Small changes in the mitochondrial redox state alters energy homeostasis and the function of many enzymes whose activities are under redox control. However, our knowledge of the cellular and tissue-specific mitochondrial redox state and its role in the initiation and progression of metabolic disorders is still incomplete. This limited understanding of mitochondrial energetics has led to lack of progress in development of appropriate therapeutics for these disorders (213), making a more complete understanding of mitochondrial energetics within FLD critical.
FLD is currently the most common liver disorder affecting the United States and world population with an estimated prevalence of 35% among Americans (111, 149) and can be classified into alcoholic liver disease and nonalcoholic fatty liver disease (NAFLD). Steatohepatitis represents the progression of simple hepatic steatosis to a histopathology displaying increased inflammation, fibrosis, and cell death. Alcoholic steatohepatitis (ASH) has a prevalence of 2% in the general population, whereas nonalcoholic steatohepatitis (NASH) has a prevalence of 3%, increasing with other comorbidities, such as obesity, insulin resistance, type 2 diabetes, dyslipidemia, and cardiovascular disease (CVD) (5, 123, 232). These estimates are complicated by the lack of an accepted noninvasive diagnostic technique for steatohepatitis (51, 149), raising the possibility for an even greater prevalence in the general population. NAFLD is considered an independent risk factor for CVD (7, 181, 205), and is associated with increased mortality (49, 150). Perhaps more importantly, the presence of steatohepatitis drastically increases the likelihood of disease progression to more pathological, and perhaps irreversible, liver diseases such as fibrosis, cirrhosis, liver failure, and hepatocarcinoma (5, 123).
In this review, we examine the current hypotheses implicating mitochondrial redox state in the pathophysiology of ASH and NASH, and the current therapeutic options under investigation.
Hepatic Mitochondrial Biochemistry
Hepatic mitochondrial physiology
Mitochondrial function is critical to cellular physiology as mitochondria play an important role in energy supply, fat and glucose metabolism, antioxidant defense, Ca2+ homeostasis, and apoptosis. A brief description of cellular and mitochondrial physiology in the liver will follow, with special emphasis on the redox machinery and reactive oxygen species (ROS) production capabilities within the hepatocyte.
Carbohydrate metabolism
The liver represents a major site of glucose uptake and storage and the major site for insulin clearance. Under normal insulin-sensitive conditions, insulin inhibits glycogenolysis and gluconeogenesis by the liver, resulting in suppressed glucose production. However, FLD is often associated with hepatic insulin resistance in which the most common feature is the inability of insulin to cease hepatic glucose production (52).
During episodes of elevated plasma insulin and glucose, the liver metabolizes glucose in four primary pathways: (i) ATP production through glycolysis/TCA cycle/oxidative phosphorylation, (ii) ketogenesis, (iii) glycogen storage, and (iv) de novo lipogenesis of free fatty acids (FFAs), which appears to play a role in both ASH and NASH (207). Under normal physiological conditions, insulin initiates a transient prolipogenic state in hepatocytes to store the excess glucose as FFAs and triacylglycerols (TAGs), primarily through activation of the sterol regulatory element binding protein-1c (SREBP-1c) pathway. SREBP-1c activation results in increased transcription of several enzymes directly related to fatty acid synthesis/storage (acetyl-CoA carboxylase [ACC], fatty acid synthase, glyceraldehyde-3-phosphate acyltransferase, diacylglycerol acyltransferase), as well as upregulating SREBP-1c expression and additional transcription factors (peroxisome proliferator-activated receptor-γ [PPARγ], liver X receptor, and cyclic adenosine monophosphate response element binding protein) that can modulate the prolipogenic environment. Considerable evidence implicates these transcription factors in the presence of excess hepatic lipid in rodent models of both disease states (72, 96, 131, 167, 191, 210, 227). Recently, our group has reported elevated levels of several of these enzymes and transcription factors in a rodent model that spontaneously develops NASH (173, 175). Importantly, human stable isotope studies have documented the involvement of increased de novo lipogenesis in the development of FLD (46, 47).
Lipid metabolism
Hepatic β-oxidation fuels gluconeogenesis and the synthesis of ketone bodies, 3-hydroxybutyrate and acetoacetate, which are utilized as alternative sources of energy by extrahepatic organs, like the brain, when blood glucose levels are low. Liver cells are exposed to FFAs from four potential sources: (i) circulating plasma FFAs derived from the adipose TAG stores, (ii) hydrolysis of TAG within chylomicrons in the portal blood supply, (iii) de novo lipogenesis, or (iv) hydrolysis of intracellular TAGs. FFAs lipolyzed from visceral adipocytes are dumped directly into the portal vein where they circulate through the liver and, with the suppressed oxidative capacity seen in insulin resistance, will overload hepatocytes with lipid and promote hepatic TAG storage. Once taken up and converted to acyl-CoAs, the metabolism of these FFAs in the liver occurs through oxidation, ketogenesis, or esterification into TAG, which can be stored in the cytoplasm or excreted as very low-density lipoproteins (VLDL). The oxidation of FFAs occurs primarily in mitochondria and is controlled by carnitine palmitoyltransferase-1 (CPT-1)-mediated conversion of acyl-CoA into acyl-carnitine, which is transported into the mitochondrial matrix. Subsequently, FFAs undergo β-oxidation with production of acetyl-CoA, which can enter the TCA cycle, resulting in production of NADH to drive oxidative phosphorylation or are converted to ketone bodies for nonoxidative disposal (Fig. 1).
FIG. 1.
Cellular FAO. Long chain nonesterified fatty acids are actively transported through the plasma membrane, converted to acyl-CoAs, conveyed through the cytoplasm by fatty acid binding proteins, and transported into the mitochondrial matrix through the action of CPTs. The acyl-CoAs enter the beta-oxidation cycle that produces reducing equivalents as NADH, acetyl-CoAs, and chained shortened acyl-CoAs. The beta-oxidation of acyl-CoAs requires four enzymatic reactions: dehydrogenation by acyl-CoA dehydrogenase (AD), hydration by enoyl-CoA hydratase (2), oxidation by L-β-hydroxyacyl-CoA dehydrogenase (3), and thiolysis by β-ketothiolase(4). The last three enzymatic steps of beta-oxidation are carried out by MTP (2,3,4). The acetyl-CoA produced can then be shuttled to ketogenesis, enter the TCA cycle, or be used in steroidogenesis. CPT, carnitine palmitoyltransferase; TCA, tricarboxylic acid; MTP, mitochondrial trifunctional protein; FAO, fatty acid oxidation.
Changes in fatty acid oxidation (FAO) in each of these pathophysiologies will be discussed in greater detail below. The dynamics of TAG synthesis and secretion in steatohepatitis lie outside the focus of this review; however, elevated TAG is a prerequisite for the diagnosis of steatosis and VLDL secretion has been observed to be diminished in individuals with steatohepatitis (26, 62, 100, 137).
Energy homeostasis
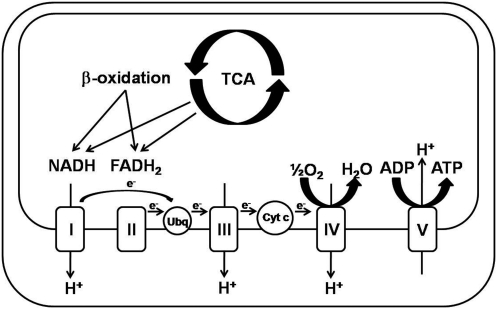
Maintaining sufficient cellular ATP is the primary function of mitochondria. Reducing equivalents (NADH/FADH2) produced primarily through the β-oxidation of fatty acids and the TCA cycle are utilized to pump hydrogen ions out of the mitochondrial matrix into the intermembrane space (213). This creates the mitochondrial proton electrochemical gradient (ΔΨ) that is necessary not only to power the synthesis of ATP by ATP synthase but also to generate heat and transport proteins into the matrix. The electron transport chain (ETC) is responsible for the movement of the hydrogen ions from the matrix into the mitochondrial intermembrane space as electrons from NADH/FADH2 are transported and ultimately catalyze the reduction of O2 to water (Fig. 2). The production of excess ROS through changes in reducing potential of NADH and alterations in the ETC are observed in steatohepatitis.
FIG. 2.
Oxidative phosphorylation. Utilization of reducing equivalents generated through β-oxidation and the TCA cycle to fuel the pumping of hydrogen from the mitochondrial matrix into the intermembrane space by the flow of electrons through the complexes of the ETC and, ultimately, reducing molecular oxygen to water. The movement of hydrogen back into the mitochondrial matrix, down this electrochemical gradient, through Complex V (ATP synthase) provides the necessary energy for the production of ATP. Cyt c, cytochrome c; FADH2, reduced flavin adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; Ubq, ubiquinone; ETC, electron transport chain; ATP, adenosine triphosphate.
Mitochondrial genome
The mitochondria are unique among cellular organelles in that they contain DNA that is required for appropriate development and function. This nucleic material is a circular, double-stranded DNA of ∼16,500 nucleotides located in the mitochondrial matrix (213). The mitochondrial DNA (mtDNA) contains genes that encode 13 ETC proteins, 2 mitochondrial ribosomal proteins, and all the mitochondrial transfer RNA. Each mitochondria contains 5–10 mtDNA molecules and the total copy number per cell approaches several thousand (60, 86, 189). Within the matrix, mitochondria possess simple DNA repair capabilities (44); however, mtDNA is extremely sensitive to oxidative damage due to its location near the inner mitochondrial membrane where most of the ROS is produced, and also due to the lack of histones and more sophisticated repair mechanisms (16, 60).
Mitochondrial Alterations in Steatohepatitis
As mentioned briefly above, the presence of steatohepatitis has dynamic effects on mitochondrial physiology as observed in alterations in β-oxidation and CPT-1 activity, dysfunctional ETC phenotypes, reduced ATP homeostasis (13, 86), as well as other mitochondrial physiological processes. These alterations in mitochondrial function can be attributed in large part to interconnected disturbances in either redox nodes (NAD/NADH, NADP/NADPH, reduced glutathione [GSH]/oxidized glutathione [GSSG], and Thioredoxins) or ROS and reactive intermediate production (Fig. 3), which propagate further alterations in mitochondrial function through additional changes in mitochondria physiological processes influenced by the redox state.
FIG. 3.
Factors affecting mitochondrial dysfunction in steatohepatitis. Observed clustering of interrelated factors linked to mitochondrial antioxidant status and redox nodes and steatohepatitis.
Redox nodes
The intricate and expansive role of the mitochondrial redox state in cellular physiology recently has been reviewed in detail (213). The importance of mitochondrial redox control encompasses not only oxidative phosphorylation leading to ATP production but also redox balance throughout the entire cell and signaling pathways that affect gene transcription. The redox state of the cell is determined by the ratios of reduced-to-oxidized state of soluble electron transport molecules and redox-sensitive amino acids (e.g., NADPH, NADH, thioredoxins 1 and 2, GSH, and cysteine) (Fig. 4). Each of these nodes is in some fashion coupled to the redox state of the other nodes based on cellular localization. For example, in mitochondria, NADPH/NADP+ node is dependent on the redox state of thioredoxin 2 and GSH/GSSG (82). Together, these nodes function to regulate ROS levels in mitochondria and the redox state of thiol/disulfide in multiple cellular compartments. Through changes in compartmental redox states, dynamic changes in a wide variety of signaling pathways can be synchronized throughout the cell. This can be observed when GSH levels are reduced in the liver of mice; alterations in mitochondrial function and activation of various stress signaling pathways result in increased steatosis, inflammation, and mitochondrial injury (30). Also, the importance of these redox nodes for cellular function can be observed in the studies demonstrating embryonic lethality upon gene knockout of key redox enzymes (39, 94).
FIG. 4.
Redox nodes in mitochondrial ROS management. Involvement of four redox nodes (GSH/GSSG, Trx(SH)2/TrxSS, NADP+/NADPH, and NAD+/NADH) in the reduction of ROS in the mitochondria. In this example, the reduction of H2O2, produced through the dismutation of mitochondrial superoxide (O2−) by Mn-SOD, is mediated by several enzymes that require the efficient maintenance of the mitochondrial redox nodes. GPx, glutathione peroxidase; GSH, reduced glutathione; GSHR, glutathione reductase; GSSG, oxidized glutathione; Mn-SOD, manganese-superoxide dismutase; NAD+, nicotinamide adenine dinucleotide; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NNT, nicotinamide nucleotide transhydrogenase; Prx, peroxiredoxin; TrxR, thioredoxin reductase; Trx(SH)2, reduced thioredoxin; TrxSS, oxidized thioredoxin; ROS, reactive oxygen species.
Of interest to this review is the maintenance of mitochondrial NADH/NAD+, GSH/GSSG, thioredoxin 2, and NADPH/NADP+ nodes. As mentioned previously, reducing equivalents are transferred from dietary calories through the action of various catabolic pathways to the ETC utilizing NADH as a soluble carrier. The movement of electrons down the ETC produces ROS of varying degrees based on mitochondrial conditions. The regulation of mitochondrial ROS levels is primarily accomplished by the action of manganese-superoxide dismutase (Mn-SOD) and glutathione peroxidase. The action of Mn-SOD produces H2O2 from superoxide produced by inefficient transfer of electrons through the ETC, which is further reduced to H2O by glutathione peroxidase and peroxiredoxin. This reduction of H2O2 is powered by the GSH/GSSG and thioredoxin nodes, respectively, which is coupled to the NADPH/NADP+ node by the action of glutathione reductase and thioredoxin reductase, which catalyze the reduction of the soluble carriers using NADPH as the reducing equivalent (213). The mitochondrial NADPH/NADP+ node is then directly coupled back to NADH/NAD+ by the action of nicotinamide nucleotide transhydrogenase to produce NADPH. Thus, any perturbation in the components of this mitochondrial redox cycle would result in an increase in mitochondrial redox state and the occurrence of oxidative damage to mitochondrial structures (Fig. 4).
Sources of ROS and reactive intermediates
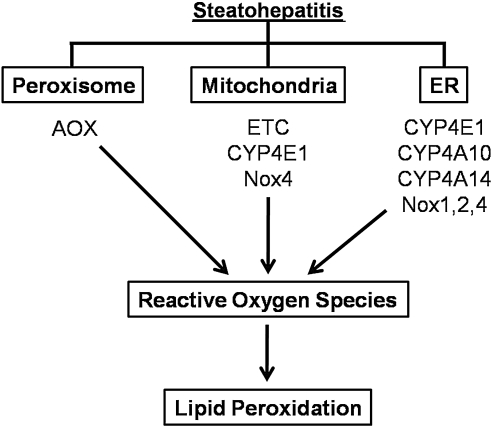
Both ASH and NASH produce deleterious changes in mitochondrial and cytoplasmic redox nodes with subsequent reduction in cellular antioxidant systems, resulting in increased potential for the development of oxidative stress within the cell. This oxidative stress is commonly manifested as increased production of ROS and reactive intermediates beyond the cellular capacity to mediate these species levels. This elevation of ROS and reactive intermediates can result in modified cellular constituents or initiation of stress-sensitive signaling cascades. Under normal physiological conditions, low-level, localized ROS production functions as a modulator of metabolic pathways such as TCA cycle and mitochondrial biogenesis (4, 84), and is a vital component to signal transduction in cascades such as insulin signaling (73, 74, 119, 120). However, elevated endogenous ROS production in the cell is capable of exerting pathophysiological effects on mitochondria through direct modification of biomolecules or changes in cellular signaling pathways. These potential sources for reactive intermediates are outlined below and in Figure 5.
FIG. 5.
Sites of ROS production in steatohepatitis. Cellular localization and enzymes responsible for ROS production observed in steatohepatitis. AOX, acyl-CoA oxidase; CYP, cytochrome P450 enzyme; ER, endoplasmic reticulum; NOX, NADPH oxidase.
Mitochondria
Mitochondria are considered the major site of cellular ROS production, with up to 1%–2% of mitochondrial oxygen consumption, resulting in ROS production under normal conditions (18). This ROS production occurs through reduction of molecular oxygen to superoxide (O2−) by Complex I and III of the ETC (9, 109, 147, 166, 230) (Fig. 6). The O2− produced is capable of rapidly generating additional ROS and reactive intermediates through interaction with various cellular components. However, under normal conditions, mitochondrial Mn-SOD, localized in the matrix, catalyzes the dismutation of the superoxide to H2O2 (160), which is subsequently reduced to H2O by the action of glutathione peroxidase and peroxiredoxin in a GSH-dependent reaction (160). This leakage of electrons from the ETC to produce ROS has been shown to be directly related to the magnitude of the ΔΨ (18). Mitochondrial ROS production is elevated under conditions where an increase in NADH production and subsequent increase in ΔΨ is not coupled to an increase in ATP production. Importantly, it has been observed that incrementally small changes in ΔΨ result in significant changes in mitochondrial ROS production (133). This can be observed under conditions of increased β-oxidation of FFAs in NASH (13) and elevated mitochondrial NADH due to alcohol metabolism in ASH (165). Additionally, extra-mitochondrial signaling events can alter mitochondrial ROS production. For example, tumor necrosis factor α (TNFα) induced release of cytochrome c from mitochondria decreases ETC efficiency and results in increased mitochondrial ROS production (109, 161). Further, ROS-sensitive signaling pathways can lead to activation of nuclear factor-κB-mediated increases in TNFα expression (225), perpetuating the increase in oxidative stress.
FIG. 6.
Mitochondrial ROS production. Under normal conditions, 1%–2% of electrons entering the ETC are transferred to molecular oxygen by Complexes I and III to produce superoxide (O2−). Increases in the mitochondrial electrochemical gradient through uncoupling of the production of reducing equivalents from ATP synthesis results in increased production of O2−. Additionally, any decrease in expression or post-translational modification of ETC components that further decreases the efficiency of electron transport results in increased mitochondrial ROS production.
NAD(P)H oxidase
The NAD(P)H oxidase (NOX) enzymes represent a family of membrane-bound enzymes capable of ROS production (superoxide and H2O2). The role of NOX enzymes in the pathogenesis of steatohepatitis has been reviewed previously (43, 78, 103). The NOX enzymes contain a flavin-cytochrome subunit that is capable of oxidizing molecular oxygen to superoxide utilizing NAD(P)H as the reducing equivalent (110). At least one NOX enzyme isoform is expressed in all cell types that make up the liver and its vasculature [hepatocytes (80, 134), Kupffer cells (45), hepatic stellate cells (176), and endothelial cells (98)]. Increases in TNFα levels in the liver and systemic circulation in steatohepatitis have been observed to be directly responsible for increased NOX activity and subsequently increased ROS production (103, 104) resulting in increased fibrosis development and apoptosis (12, 41, 42, 136, 176, 217).
Cytochrome P450
The cytochrome P450 enzymes, primarily CYP2E1, are a family of mixed function oxidases. In steatohepatitis, CYP2E1 and 4A10 are capable of producing lipid peroxidation products and have been observed to have increased liver expression and activation in steatohepatitis (25, 180, 216) and in comorbidities associated with steatohepatitis (151). These observed increases in cytochrome P450 activity make it an important source of ROS in hepatocytes under pathophysiological conditions (31, 75, 188). Additionally, CYP enzymes are upregulated in models of NAFLD (91). Of particular interest to this review is that CYP2E1 can be localized to mitochondria and that this localization is dependent on specific signaling cascades (178, 179). Initially, the presence of an inducible cytochrome P450 localized to the mitochondrial matrix was described that shared the exact same n-terminus and catalytic activity as CYP2E1 (178). Further, it was shown that CYP2E1 can be localized to the mitochondrial matrix due, in part, to cyclic adenosine monophosphate-dependent serine phosphorylation within the n-terminus of the protein (179). The presences of ROS-producing CYP2E1 within mitochondria could be responsible for many of the observed mitochondrial pathophysiologies observed due to upregluated cytochrome P450 in models of NAFLD.
Peroxisomes
Since peroxisomal fatty acid entry is not carnitine dependent or sensitive to inhibitors of CPT-1, it is thought that mitochondria and peroxisomes work in concert to prevent lipid accumulation in a high-fat environment. In the liver, peroxisomes function as a sight of β-oxidation of FFAs, in particular, very long chain (>C18) FFAs, branched chain FFAs, and bile acid intermediates (190). However, ROS production, primarily H2O2, is increased with the rate limiting step in peroxisomal β-oxidation, acyl-CoA oxidase (190) and may overwhelm cellular defense systems. Like mitochondria, the peroxisome has an array of enzymes to neutralize the ROS produced by the β-oxidation of FFA, including catalase. However, under conditions that result in a fivefold transcriptional increase in peroxisomal β-oxidative capacity, the elevation in antioxidant enzyme transcription is <10% (53, 168). This disparity in ROS production to ROS removal capacity represents a potential source of increased cellular ROS in the elevated lipid environment of steatohepatitis. It is likely that antioxidative enzyme systems, such as glutathione peroxidase and superoxide dismutase, would need to be upregulated in concert with peroxisomal FAO in order for beneficial effects to be realized.
Lipid peroxidation products
Lipid peroxides (LPO), most commonly observed as the degradation products malondialdehyde and 4-hydroxynonenal, refer to any lipid that has been oxidized by ROS to a peroxide form. This reaction occurs most frequently with polyunsaturated FFAs as they contain a number of the required methylene carbons between unsaturated carbon–carbon double bonds. These products can be produced anywhere in the cell that are exposed to ROS, and the reaction results in the propagation of additional LPO through creation of lipid peroxyl radicals. Within mitochondria, LPO can be produced from ROS though the leakage of electrons from the ETC or mitochondria-localized 2E1 (172). If not properly neutralized by the antioxidant system, the LPO produced can result in damage to mtDNA (40, 87, 88), inactivation of COX IV of the ETC (28, 29), and initiation of apoptosis through induction of the mitochondrial permeability transition (MPT) (108).
Alcoholic Steatohepatitis
FLD attributable to alcohol consumption is a major cause of morbidity and mortality and is one of the largest liver-related disease states in the United States and the world (113, 123). Human knowledge of excessive alcohol consumption producing negative health outcomes has been documented for thousands of years (113). However, in the past 50 years, considerable work has begun to dissect the mechanistic impact of alcohol exposure on liver function and pathology. In this section, we review the current dogma and ongoing research areas as they relate to alterations in hepatic mitochondrial function in conditions of acute and chronic alcohol exposure.
Antioxidant status/redox nodes
Acute metabolism of alcohol by cytosolic alcohol dehydrogenase to acetaldehyde, and acetaldehyde to acetate by mitochondrial aldehyde dehydrogenase, utilize NAD+ as electron acceptor, resulting in increased NADH production (165). This increase in NADH can inhibit dehydrogenase reactions in the cytoplasm and mitochondria, such as in the TCA cycle and β-oxidation, disrupting the energy supply and reducing FAO (61, 114). The increase in NADH/NAD+ ratio does not appear to persist with chronic ethanol consumption and may be insufficient to explain alcohol-induced fatty liver (183). However, the change in redox status may be an initiating factor in the future development of fatty liver during chronic alcohol consumption due to the creation of a pro-oxidant state through depletion of antioxidant capabilities as described below.
After both acute and chronic alcohol consumption, increases in Mn-SOD transcription and activity have been observed in rodent models (102). Also, adenoviral overexpression of Mn-SOD has been observed to protect hepatocytes against CYP2E1 alcohol-mediated cytotoxicity (158). However, recent in vitro experiments describe ethanol-induced cytotoxicity through inhibition of Mn-SOD through oxidative modification of the enzyme via ethanol-induced ROS-specific mechanism (221). Additionally, a reduction in mitochondrial glutathione peroxidase activity (10) and GSH levels (33, 35, 55, 56, 66, 85), resulting in lower antioxidant capability, was observed in in vitro and in vivo models of alcohol exposure. These decreases are at least partially mediated through decreased GSH transport into mitochondria (55, 56). Ethanol also sensitizes hepatocyte mitochondria to the pro-oxidant effects of TNFα (34), which in turn perpetuates greater mitochondrial ROS production (76, 153).
Fatty acid oxidation
Alcohol consumption has classically been accompanied by decreased mitochondrial β-oxidation (165), and the control of lipid metabolism to either oxidation or lipogenesis is a critical axis in the pathophysiology of ASH. The regulation and activity of adenine monophosphate-activated protein kinase (AMPK) lies at the center of this axis and has been described as the master switch in cellular metabolism (117). By phosphorylating ACC, AMPK reduces production of malonyl-CoA, which is a powerful allosteric inhibitor of CPT-1-mediated mitochondrial FAO and an intermediate necessary for de novo lipogenesis. Additionally, AMPK phosphorylates and activates malonyl-CoA decarboxylase, which catalyzes the elimination of malonyl-CoA, further reducing de novo lipogenesis and increasing FAO. Alcohol has been shown to suppress AMPK activity and thus to increase ACC activity, resulting in decreased palmitate oxidation (229) in cell culture, presumably through increased ACC production of malonyl-CoA. Addition of chemical activators of AMPK (metformin and aminoimidazole carboxamide ribonucleotide) and constitutively active AMPK abrogated the alcohol-induced increases in ACC activity and increased oxidation of palmitate. These in vitro data are corroborated by an observed reduction in AMPK activity and lower FAO in livers from rodents after ethanol feeding (71).
Additionally, transcriptional control of the enzymes involved in FAO has been observed to be dysfunctional in ASH. PPARα, a member of the nuclear hormone receptor family, controls transcription of many of the enzymes involved in cellular FAO (112, 231). In cell culture, alcohol exposure inhibits PPARα reporter gene activation in the presence or absence of a PPARα agonist (64). This effect was blocked by inhibition of alcohol dehydrogenase, but augmented by aldehyde dehydrogenase inhibition, implicating acetaldehyde involvement in the experiments. In vivo studies also demonstrate that PPARα activity and PPARα target gene transcription was downregulated in rodent models of alcohol exposure (58, 139). Whereas, in vivo administration of a PPARα agonist during ethanol treatment increased PPARα target gene expression and FAO (58).
Increases in ROS observed in ASH also can lead to the oxidative modification of amino acid residues that are critical to the catalytic function of enzymes involved in FAO. The β-oxidation enzymes (thiolase, acyl-CoA dehydrogenase, enoyl-CoA hydratase, and hydroxy-acyl-CoA dehydrogenase) have been observed to be oxidatively modified in chronic ethanol consumption (135), with 3-ketoacyl-CoA thiolase activity being diminished (the other enzyme activities were not measured) (135, 196) (Fig. 7). These changes in β-oxidation in ASH are important as we have previously demonstrated that a primary disruption in mitochondrial β-oxidation induces hepatic steatosis in a rodent model of NAFLD (91). These decreases in β-oxidation capacity could lead to significant lipid accumulation during alcohol consumption through reductions in lipid catabolism (228). Thus, an alcohol-induced oxidative stress mechanism could explain the observed decreases in β-oxidation observed in ethanol-treated animals (23).
FIG. 7.
Mitochondrial FAO. The mitochondria represents the site of the majority of oxidation of cellular fatty acids, as acyl-CoAs, and the entry and initiation of fatty acid metabolism is controlled by the activity of CPT. Reduced expression and activity observed in steatohepatitis limits mitochondrial FAO and perpetuates excess cellular lipid levels. Fatty acids within the mitochondria undergo β-oxidation through a 4-enzyme pathway that produces acetyl-CoA and a 2-carbon shortened acyl-CoA that can undergo further oxidation. The produced reducing equivalents are utilized by the ETC, whereas the acetyl-CoA enters the TCA cycle resulting in the production of CO2 and additional reducing equivalents or reduced to produce ketone bodies. Decreased functioning of the enzymes of β-oxidation by oxidative modification or genetic manipulation has been shown to result in increased steatosis and is present in progressive steatohepatitis. KO, knockout.
Oxidative phosphorylation
The ETC is composed of five polypeptide complexes (complex I–V) and cytochrome c, which function to transfer electrons from reduced NADH and FADH2 to the ultimate electron acceptor of aerobic metabolism, oxygen, to produce H2O (Fig. 2). Complexes I and III utilize energy from the transfer of these electrons to pump protons out of the mitochondrial matrix, producing the ΔΨ. This gradient is used primarily to provide the necessary energy for ATP synthase to catalyze the creation of ATP from ADP. Complex I and II transfer two electrons from NADH and FADH2, respectively, to ubiquinone to produce ubiquinol. Complex III transfers the electrons from ubiquinol to cytochrome c and then to Complex IV, which catalyzes the reduction of molecular oxygen to H2O. As mentioned previously, at normal efficiency and oxidative phosphorylation coupling, Complex I and III leak 1%–2% of the electrons in the ETC to oxygen to produce superoxide (9, 18, 109, 147, 166, 230). Increases in the ΔΨ, either through increases in reducing equivalents or decreased ETC efficiency, lead to substantial increases in ROS production by Complexes I and III (109, 161, 165) (Fig. 6). Together, all the ETC complexes utilize multiple flavin, iron–sulfur centers, copper centers, and cytochromes to transfer the electrons from the reduced electron carriers to oxygen rendering their individual function sensitive to oxidative modification under conditions of oxidative stress (213).
Acute exposure of hepatocytes to ethanol increases ROS production through complexes I and III (9, 11). Chronic exposure of rodents to alcohol has been observed to lead to decreased activities of Complex I, III, and IV in isolated mitochondria (209). Additionally, ethanol exposure leads to decreased expression of protein subunits of ETC complexes (203, 209). As previously mentioned, ethanol exposure sensitizes mitochondria to cellular TNFα signaling pathways. One such pathway is the observed increase in TNFα-mediated acidic sphingomyelinase production of ceramide in models of chronic alcohol consumption (57). This increased ceramide has been shown to directly inhibit Complex III with increases in mitochondrial ROS production and apoptotic cell death (67, 77). Also, increased mitochondrial lipid peroxidation products inhibit Complex IV through adduct formation with histidine residues of subunit IV (28), and ethanol-induced production of mitochondrial malondialdehyde resulted in Complex IV inhibition via formation of lysine adducts in both subunit IV and V (29). Thus, increased mitochondrial ROS production during alcohol consumption can perpetuate a pathophysiological condition that favors greater ROS production through decreases in ETC efficiency and capacity.
Uncoupling proteins
The production of ATP by oxidative phosphorylation couples the creation of the ΔΨ during transport of electrons through the ETC to ATP synthesis utilizing the potential energy of the ΔΨ. The uncoupling proteins (UCPs) represent a family of mitochondrial proteins that function to dissipate the ΔΨ, uncoupling mitochondrial respiration from ATP synthesis (99). The contribution to initiation and progression of steatohepatitis based on changes in expression and function of UCPs has been proposed (194). In addition, a recent report found a significant increase in liver UCP-2 expression in mice after chronic exposure to ethanol (170). However, to date very little research in the study of ASH specifically has been focused in this area, and will be discussed in greater detail in the section related to NASH.
mtDNA damage and replication
Considerable literature exists documenting the decrease in mtDNA after acute and chronic exposure to alcohol (20, 40, 60, 124, 189), occurring mainly through ROS-mediated oxidative damage upon acute ethanol exposure (20, 81, 124, 125, 198). One possible consequence of changes in mtDNA structure would be decreased replication through blockage of the replication enzymatic machinery (86). The apparent unsophisticated nature of mtDNA repair mechanisms enhances the build-up of oxidative mtDNA lesions (86), which results in preferential degradation of damaged mtDNA (124). These decreases in mtDNA during chronic ethanol consumption are not only the result of increased ROS-mediated degradation but also the apparent lack of an adaptive increase in resynthesis (40). These changes in mtDNA have been shown to be directly associated with decreased expression of mitochondrial proteins encoded on mtDNA (38).
Mitochondrial permeability transition
Apoptosis has been observed as a frequent cellular outcome after acute and chronic ethanol exposure (68, 69, 86). One important component of the mitochondrial involvement in the control of apoptosis is the precise, multi-point regulation of the mitochondrial permeability transition (MPT). The activation of the MPT due to cellular stress results in opening of the permeability transition pore across the inner and outer mitochondrial membrane (86). Pore opening results in mitochondrial swelling and dissipation of the proton electrochemical gradient, with subsequent membrane rupture and release of additional proapoptotic stimuli (e.g., cytochrome c, pro-caspase-9, and apoptosis-inducing factor) (3). The status of the MPT pore is regulated by several thiol groups (15), and is susceptible to the oxidizing conditions observed under states of elevated ROS production such as ethanol consumption.
As such, chronic ethanol consumption in rodents has been observed to sensitize mitochondria from isolated hepatocytes to numerous proapoptotic stimuli (154). ROS produced from ethanol exposure due to CYP2E1 activation and the ETC has been implicated in the initiation of apoptosis through MPT activation with resultant increases in cell death (24, 220). Also, a dynamic augmentation of ethanol-mediated MPT activation has been observed in numerous studies with the co-administration of TNFα utilizing immortalized and primary hepatocytes (153–155, 220). One widely observed mechanism for this observed potentiation is the ethanol-mediated hepatocyte sensitization to TNFα, resulting in greater ceramide production with subsequent increases in apoptotic cell death via inhibition of ETC complex III (6, 67, 77).
Also, this interaction between ethanol and TNFα has a substantially negative impact on the cytosolic and mitochondrial GSH pool (68, 69). The lowering of both of these pools of antioxidant reducing power potentiates a proapoptotic cellular physiology under conditions of oxidative stress. Additionally, ROS-mediated elevations in lipid peroxidation products, particularly 4-hydroxynonenal, have been shown to be powerful inducers of the MPT (108). This is mediated, in part, through an increase in the observed number of protein adducts of proteins involved in the control of the MPT and apoptosis (145, 203). Finally, an additive effect of ethanol-induced reductions in mtDNA and increased susceptibility to ethanol-induced MPT activation and apoptosis may exist in chronic alcohol consumption (86).
Nonalcoholic Steatohepatitis
The increase in high-fat/high-carbohydrate caloric intake coupled with decreased levels of physical activity is the primary initiator of the clustering of disease states that have become known as the metabolic syndrome. The development of FLD has been shown to be associated with many of the comorbidities within the metabolic syndrome and thus has been deemed the liver manifestation of the metabolic syndrome (5). Considerable research in NAFLD has focused on areas and mechanisms already studied for alcoholic FLD. Nevertheless, there is a paucity of data on the natural history and progression of NAFLD. Recently, our group has begun to describe the natural history of NAFLD in a hyperphagic Otsuka Long-Evans Tokushima Fatty (OLETF) rat that spontaneously develops steatohepatitis (173–175). However, the natural history and progression of steatosis to steatohepatitis in human NASH is still poorly understood. Below is a review of the current theories behind the development of NASH and the involvement of the liver mitochondria in these processes.
Antioxidant status/redox nodes
Oxidative stress is a widely observed phenotype in NASH (122, 148, 177, 222), which may partially be due to decreased antioxidant capacity. As such, microarray analysis of liver biopsies from patients with NASH demonstrate decreased transcriptional capacity for the removal of mitochondrial ROS (199). This decreased transcriptional capacity observed in the microarray data is supported by the observed reduction in antioxidant capacity through decreases in hepatic GSH content and SOD activity in patients with NASH (211). In addition, the observed decreases in liver antioxidant capacity coincide with increased systemic oxidative stress from subjects with NASH (106). Further, we have recently demonstrated that the hepatic activity of SOD is significantly reduced in young OLETF animals, with a subsequent increase in GSSG levels in older OLETF animals, a rodent model of obesity and NASH (175). As mentioned during the discussion of ASH, these changes in antioxidant capacity, particularly low mitochondrial glutathione and SOD activity, predispose mitochondria to various oxidative injuries with perpetuation of mitochondrial dysfunction and elevated ROS production.
Interestingly in NASH, hepatic mitochondria are able to maintain a relatively normal NADH/NAD+ ratio in the face of dysfunctional ETC (13), due in large part to the observed elevation in ketogenesis in NASH (132, 185) and the utilization of NADH to produce ketone bodies from acetoacetate. The NADH/NAD+ being maintained may also be related to the lowering of the ΔΨ by increased expression of UCPs, which could improve the efficiency of the ETC. This possibility will be discussed below.
Fatty acid oxidation
Many studies have recognized the potential importance of impaired hepatic FAO and speculated that mitochondrial abnormalities may be involved in NAFLD (13, 21, 54, 91, 144, 162, 185). Previous studies have shown that mitochondrial oxidative capacity is decreased in liver tissue of patients and animal models with hepatic steatosis (70, 157), and hepatic CPT-1 expression has recently been shown to be decreased in NASH patients (138) and in rodent models of NASH (194). However, observations in NASH patients and rodent models of NASH regarding hepatic FAO are mixed (25, 132, 175, 185).
To better understand the importance of mitochondrial function and FAO in the etiology of NAFLD and progression to NASH, our laboratory has employed the strategy of utilization of three different animal models: (i) with a primary genetic mutation in mitochondrial β-oxidation, (ii) a model of overeating and obesity, and (iii) a model of low aerobic fitness and the metabolic syndrome (91, 173–175, 206). We have generated a mouse model for a null mutation causing complete mitochondrial trifunctional protein (MTP) deficiency. The MTP is a heterotrimeric protein that consists of four α-subunits and four β-subunits and catalyzes three of the four chain-shortening reactions in the mitochondrial β-oxidation of long-chain fatty acids (Fig. 7). The homozygous (MTPa−/−) mice lack both MTP α- and β-subunits, have biochemical changes identical to those of human deficiency, and suffer neonatal hypoglycemia and sudden death 6–36 h after birth. Analysis of the histopathologic changes in the MTPa−/− pups revealed rapid development of hepatic steatosis after birth, followed by acute degeneration and necrosis of the cardiac and diaphragmatic myocytes, which may be the reason for the underlying etiology for sudden death (90). We have more recently documented that aging in mice heterozygous for the MTP defect (MTPa+/−) causes development of insulin resistance and hepatic steatosis when rodents are fed normal chow, in association with an age-dependent decline in hepatic MTP expression in our mice. This provided direct evidence that genetic impairment of mitochondrial β-oxidation of fatty acids causes both hepatic steatosis and insulin resistance in mice. MTP heterozygous animals also had higher hepatic antioxidative activity, increased expression of cytochrome P-450 2E1, and mitochondrial ultrastructural abnormalities, consistent with increased hepatic oxidative stress. We also have preliminary data to suggest that a high-fat diet, in combination with the genetic defect, induces hepatic steatosis at a much earlier age in the MTPa+/− mice and further increases ROS formation in these animals (J.A. Ibdah, unpublished results).
In our second series of studies, we have characterized the natural progression of NAFLD and NASH in the OLETF rat, a hyperphagic, obese, rodent model (175) that we liken to overweight, overeating, sedentary humans. Young OLETF rats display reduced hepatic cytochrome c protein content, mitochondrial CPT-1 activity, and complete (to CO2) and total FAO before the development of hepatic steatosis or insulin resistance (175). In addition, OLETF animals exhibit a progressive loss of hepatic mitochondrial content and function that contributes to the progression of NAFLD and occurs in conjunction with the worsening in glycemic control and a loss of hepatic antioxidative capacity, increased hepatic oxidative stress, reduced hepatic mitochondrial content and function, and disruption in hepatic mitochondria health. Shown in Figure 8 are representative electron microscopy images of livers from OLETF and Long-Evans Tokushima Otsuka (lean controls) animals. Figure 8 demonstrates huge lipid droplets and the apparent disruption in nuclear health in the OLETF liver, as well as hypodense and more rounded mitochondria in the OLETF animals. This occurs in conjunction with disruption in outer and inner mitochondrial membrane integrity and an apparent disruption in mitochondrial cristea (175).
FIG. 8.
Ultrastructural changes in steatohepatitis. Representative transmission electron microscopic images of from Otsuka Long-Evans Tokushima Fatty rats (A) with steatohepatitis and healthy controls (B). In image A, the Otsuka Long-Evans Tokushima Fatty liver demonstrates large, perinuclear lipid droplets and a disruption in nuclear integrity.
Our third rodent model for studying NAFLD is the high- and low-capacity rats (HCR/LCR) that were created by Drs. Steve Britton and Lauren Koch (101). These animals were selectively bred over several generations for high and low endurance running, resulting in 2 divergent strains with grossly different intrinsic endurance exercise capacities (∼7-fold different) and aerobic fitness (∼30% different) (219). The LCR rats display a higher incidence of both CVD and metabolic syndrome risk factors than HCR rats. Using these animals, we sought to investigate if low aerobic capacity was linked to impaired hepatic metabolism and steatosis development (206). Indeed, hepatic mitochondrial content (citrate synthase activity and cytochrome c protein), mitochondrial FAO, and peroxisomal activity (acyl-CoA oxidase and catalase activity) were significantly reduced in the LCR compared with the HCR rats. LCR livers also displayed a lipogenic phenotype with higher protein content of both SREBP-1c and acetyl CoA carboxylase. These differences in the LCR rats were associated with hepatic steatosis and lipid peroxidation at an early age and elevated markers of liver injury (fibrosis and apoptosis) at the time of natural death, findings not observed in the HCR rat. The LCR rats also developed characteristics of the metabolic syndrome, including increased abdominal fat, hyperinsulinemia, hyperlipidemia, and hypertension (206).
Collectively, these findings provide strong evidence and possible mechanistic links between low or impaired mitochondrial FAO and the development and progression of FLD. However, these observations in rodent models need to be more closely examined in human conditions.
Oxidative phosphorylation
The activities in all five complexes of oxidative phosphorylation have been observed to be lower in subjects with NASH (83, 157). In addition, we have recently shown that hepatic cytochrome c protein content is reduced in a rodent model of NASH (175). Also, lower cytochrome c oxidase and mitochondrial membrane potentials were observed in mice that developed NAFLD after high-fat diet feeding (126). Lower liver ATP levels have been observed in rodent models of NASH (193), and human subjects with NASH demonstrate impaired ability to produce ATP after ATP-depleting challenge, which is due in part to reduced ATP synthase activity (157). This reduction in ATP synthase activity could be due in part to observed oxidative modification of the βF1 subunit as described in rodents (208). However, recent rodent experiments have demonstrated that maximal ATP synthase activity was not decreased through NASH development, and the observed decreases in ATP levels were hypothesized to be due to the observed decreases in Complex I and II activity (193).
Uncoupling proteins
As indicated previously, expression and activity of UCPs are described to uncouple mitochondrial respiration from oxidative phosphorylation by diminishing the proton electrochemical gradient. As such, under conditions of hyperpolarization of the inner mitochondrial membrane, UCPs may function to protect the cell through decreasing the production of mitochondrial ROS (141) and regulating mitochondrial apoptosis signals (170). In the liver, expression of UCP-2 has been observed to be increased in steatosis (27, 175, 201) and hepatocytes exposed to lipid (37), which may be due in part to elevations in lipid peroxidation products (50). However, it also has been found that obesity-related FLD is unaltered in UCP-2 deficient mice (8). Increased expression of UCP-2 during NASH has been associated with compromised ATP synthesis capability (27, 36), possibly through the lowering of the proton electrochemical gradient (95). This UCP-2-mediated decrease in hepatocyte ATP levels is associated with the observed aggravation of Fas-mediated hepatocyte cell death in a rodent model of steatosis (63). The acute increase in UCP-2 expression under increased lipid load and increased proton electrochemical gradient may occur as a cytoprotective mechanism to limit elevated ROS production. However, chronically elevated hepatocyte UCP-2 expression in NASH, with resultant decreases in ATP levels, may be associated with a pathophysiological phenotype by increasing the susceptibility to proapoptotic signals.
mtDNA damage and replication
Although not completely characterized, mtDNA has been observed to be severely depleted in patients with NASH (83), and the levels of oxidative damage in mtDNA is inversely associated with hepatic ROS levels in both mice models of FLD (65). As mentioned previously, mtDNA is particularly susceptible to oxidative damage, and the depletion of mtDNA may be due in part to the pathological increases in ROS observed in NASH (13). Additionally, the decrease in mtDNA attributable to increased ROS production in NASH may form an additive association with the commonly observed age-related decreases in mtDNA (163). These observed decreases in mtDNA lead to decreased expression of mtDNA encoded proteins, which could contribute to the documented alterations in mitochondrial function observed in NASH (83, 184).
Mitochondrial permeability transition
The activation of the MPT pores in NASH follows many of the same paradigms as in ASH, as the underlying increase in ROS and decreases in antioxidant status in both pathophysiologies lead to MPT activation (68, 69). The lipotoxicity induced by elevated hepatic lipids in steatosis and increased ROS is a hallmark of apoptosis in NASH (128). Recently our group has observed increased apoptosis in a novel rodent model of steatosis that demonstrate reduced hepatic oxidative capacity (206). TNFα signaling in steatohepatitis also is prevalent in the apoptosis observed in NASH (153). Additionally, elevated lipids initiate apoptosis through activation of endoplasmic stress signaling pathways in hepatoytes and rodent models of steatosis (214, 215).
Clinical Management of ASH and NASH
Currently, the treatment of steatohepatitis is complicated by the lack of acceptable noninvasive diagnostic techniques, presence of comorbidities, incomplete knowledge of natural history, and poor patient compliance with all modes of long-term care regimens. However, considerable work has been published outlining strategies that can be employed to manage and treat steatohepatitis (143, 212). Below is a brief description of current therapeutic options for the management and treatment of steatohepatitis.
Lifestyle modification
Lifestyle modifications targeted at increasing physical activity and reducing energy intake are recommended by healthcare providers for optimal health and are the most common prescribed therapy for individuals found to have NASH. In addition, the most important modification for an individual with ASH is the cessation of alcohol consumption and improved dietary habits. Considerable recent work has reviewed the previous studies, strategies, and recommendations of lifestyle modification as treatment for steatohepatitis (59, 97, 130, 143, 156), and new human trials continue to be reported describing successful treatment of liver pathology utilizing lifestyle modification paradigms (89, 152, 164). Managing body weight is likely the best overall method in the treatment of NASH. Since insulin resistance and obesity play a central role in NASH, weight loss is the mainstay of treatment. The weight loss program should be centered on the caloric restriction rather than alteration of dietary contents, with a prescription of both aerobic and resistance training exercise. Initial goals from the patient should be a 10% reduction in body weight by the combination of exercise training and energy restriction. This should be targeted over a 6-month period, and it is important that individuals continue exercising to maintain the lost weight and for overall cardiometabolic health. Recent investigations support these recommendations and suggest that being habitually more physically active (159), doing higher amounts of physical activity during leisure-time (233), and routinely performing or increasing one's physical activity levels to ≥150 min/week is inversely associated with NAFLD (200). In addition, having higher cardiorespiratory fitness also is inversely associated with NAFLD and NASH (32, 107). Further, a recent randomized controlled trial found that body weight reduction of 7%–10% through a lifestyle modification of caloric restriction and exercise training improved liver histology in biopsy-proven NASH patients (164). Moreover, our recent findings in the OLETF and the HCR/LCR rodent models further support daily exercise and increased aerobic fitness in the protection from development of NAFLD and the importance of hepatic mitochondrial content and function in this process (173, 174, 206). However, it should be noted that not all individuals are able and/or willing to successfully adopt a regularly active lifestyle or successfully lost weight and keep it off. Therefore, additional therapeutics are desperately needed for the management of NASH.
Specific nutrient supplementation as a part of the improved dietary habits of lifestyle modification also has been investigated. Activation of the PPAR family may produce beneficial outcomes in steatohepatitis. The lipid dietary component, omega-3 polyunsaturated fatty acids (n-3 PUFAs) are known PPAR agonists (129), and dietary supplementation with n-3 PUFAs has been observed to ameliorate steatosis and decrease the degree of liver injury in a rodent model of NASH (204). In addition, it recently was demonstrated that dietary supplementation with L-carnitine significantly reduced serum TNFα and improved liver function and histology in a cohort of patients with steatohepatitis (121). Carnitine supplementation has the potential benefit of detoxifying accumulated acyl-CoA intermediates and replenishing the intramitochondrial carnitine pool; however, carnitine supplementation has been questioned because of the accumulation of long-chain fatty acylcarnitines that may cause cardiac arrthythmias (17).
Pharmaceutical and antioxidant therapies
The most widely utilized pharmacological interventions for the treatment of type 2 diabetes represent attractive tools for the treatment of steatohepatitis; these include the PPAR agonists, fibrates and thiazolidinediones (TZDs), and the AMPK activator, metformin. TZDs and metformin are oral glucose-lowering medications used to treat type 2 diabetes that enhance insulin sensitivity and signaling. TZDs bind to the PPARs and improve insulin sensitivity, in part, by facilitating enhanced TAG storage by adipocytes, suppressing the ectopic storage of lipids into liver and skeletal muscle. It has been shown that PPAR agonists (fibrates, PPARα; TZDs, PPARγ) not only improve insulin sensitivity by suppressing the ectopic storage of lipids into liver, but also increase hepatic FAO and improve hepatic insulin sensitivity (226). Several human trials have observed improvement in steatosis and liver histology in steatohepatitis after TZD therapy alone or in conjunction with other treatment modalities (2, 14, 142, 171, 186). However, the observed beneficial effects of TZD on steatohepatitis disappear after cessation of therapy, suggesting that long-term TZD administration is necessary for continued therapeutic benefit in NASH (118). With the current safety issues regarding long-term TZD therapy (146), the usefulness of this agent in the treatment of steatohepatitis is questionable.
The activation of PPARα with n-3 PUFA supplementation has been shown to decrease the severity of NASH in rodents (204). Additional studies utilizing pharmaceutical PPARα agonists have also described improvement in steatosis potentially through the increased expression of enzymes involved in lipid oxidation, and decreased fibrosis in a rodent model of NASH (92, 93, 169). In addition, the biguanide, metformin, also has been used extensively as an insulin-sensitizer in the treatment of diabetes and has been shown to have efficacy in the treatment of steatohepatitis (115, 127, 202). Metformin also has been shown to reduce hepatic lipogenesis and increase FAO in the ob/ob mouse model of hepatic steatosis (115). Metformin can activate AMPK in liver (234) and this activation can result in increased CPT-1 activity and increased expression of proteins involved in mitochondrial respiration (218). Metformin also reduces apoptosis by inhibiting the MPT (79).
Inhibition of the inflammatory and proapoptotic action of TNFα also has been observed to be beneficial in the treatment of ASH and NASH. In NASH, administration of the TNFα inhibitor, pentoxifylline, has been observed to improve liver function and histology in a rodent model of steatohepatitis and in human clinical trials (1, 105, 187). While in a rodent model of ASH, administration of the TNFα antibody formulation, infliximab, resulted in attenuation of ethanol-induced liver injury (224). However, human clinical trials utilizing infliximab, in conjunction with steroid administration as a therapy of ASH, have produced equivocal results (140, 197).
Due to the observed elevations in oxidative stress in steatohepatitis, antioxidant therapies could play a role in disease treatment, and considerable literature is dedicated to the study of various antioxidant molecules. Corticosteroids, pentoxifylline, propylthiouracil, and anti-TNFα have all been used to treat patients with alcoholic hepatitis with marginal success. In particular, studies have observed the potential beneficial effects of vitamin E and vitamin E derivatives in treating NASH (48, 116), but findings are mixed in the treatment of ASH with antioxidant therapies (19). Additionally, ursodeoxycholic acid has been shown to increase mitochondrial GSH levels and protect the mitochondrial proton electrochemical gradient in rodents (192). Recently, a randomized human clinical trial documented a significant improvement in NASH in nondiabetic subjects after long-term vitamin E administration compared to pioglitazone (186). Additionally, the observation of reduced systemic coenzyme Q10 levels in patients with NAFLD (223) has lead to limited investigation of the effects of dietary supplementation of coenzyme Q. Coenzyme Q10 supplementation has been observed to lower oxidative stress and markers of inflammation in a mouse, without modulating lipid peroxidation or improving systemic insulin resistance (195). Additionally, coenzyme Q9 monomethyl ether administration increased VLDL assembly in rats with high-fat-diet-induced steatosis (22, 182), with no observed improvement in NAFLD or systemic insulin resistance (182).
However, as with other pathophysiologies, the use of antioxidant therapies for the treatment of patients with steatohepatitis needs greater investigation due to the equivocal nature of the observations. Most importantly, these investigation need to encompass additional randomized control studies to evaluate the use of vitamin E or ursodeoxycholic therapies in the treatment of human NASH.
Conclusions
In this review we have outlined mechanisms by which mitochondrial function is observed to be negatively impacted in the presence of steatohepatitis. The observed changes in mitochondrial function and alterations in redox status in steatohepatitis are strikingly similar in overall phenotype within ASH and NASH. With such similarities, a detailed understanding of all research that spans steatohepatitis is critical for researchers and healthcare professionals. Continued research into the resolution of the inflammatory state observed in steatohepatitis and its effects on mitochondrial function are vital. In addition, critical evaluation of potential therapies for the prevention and treatment of FLD, including lifestyle modifications, is strongly encouraged.
Abbreviations Used
- ACC
acetyl-CoA carboxylase
- AMPK
adenine monophosphate activated protein kinase
- AOX
acyl-CoA oxidase
- ASH
alcoholic steatohepatitis
- ATP
adenosine triphosphate
- CPT
carnitine palmitoyltransferase
- CVD
cardiovascular disease
- CYP
cytochrome P450
- Cyt c
cytochrome c
- ETC
electron transport chain
- FADH2
flavin adenine dinucleotide
- FAO
fatty acid oxidation
- FFA
free fatty acid
- FLD
fatty liver disease
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GSHR
glutathione reductase
- GSSG
oxidized glutathione
- HCR
high capacity rat
- KO
knockout
- LCR
low capacity rat
- LPO
lipid peroxides
- Mn-SOD
manganese-superoxide dismutase
- MPT
mitochondrial permeability transition
- mtDNA
mitochondrial DNA
- MTP
mitochondrial trifunctional protein
- NAD+
nicotinamide adenine dinucleotide
- NADH
reduced nicotinamide adenine dinucleotide
- NADP+
nicotinamide adenine dinucleotide phosphate
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NNT
nicotinamide nucleotide transhydrogenase
- NOX
NADPH oxidase
- OLETF
Otsuka Long-Evans Tokushima fatty
- PPAR
peroxisome proliferator-activated receptor
- Prx
peroxiredoxin
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SREBP
sterol regulatory element binding protein
- TAG
triacylglycerol
- TCA
tricarboxylic acid
- TNFα
tumor necrosis factor α
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- TrxSS
oxidized thioredoxin
- TZD
thiazolidinediones
- Ubq
ubiquinone
- UCP
uncoupling protein
- VLDL
very low density lipoprotein
Acknowledgments
This work was partially supported by NIH grants DK 068210 (J.A.I.) and F32 DK-83182 (R.S.R.).
References
- 1.Adams LA. Zein CO. Angulo P. Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 2.Aithal GP. Thomas JA. Kaye PV. Lawson A. Ryder SD. Spendlove I. Austin AS. Freeman JG. Morgan L. Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 3.Alnemri ES. Hidden powers of the mitochondria. Nat Cell Biol. 1999;1:E40–E42. doi: 10.1038/10034. [DOI] [PubMed] [Google Scholar]
- 4.Applegate MA. Humphries KM. Szweda LI. Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 5.Argo CK. Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Arora AS. Jones BJ. Patel TC. Bronk SF. Gores GJ. Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 7.Aygun C. Kocaman O. Sahin T. Uraz S. Eminler AT. Celebi A. Senturk O. Hulagu S. Evaluation of metabolic syndrome frequency and carotid artery intima-media thickness as risk factors for atherosclerosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2008;53:1352–1357. doi: 10.1007/s10620-007-9998-7. [DOI] [PubMed] [Google Scholar]
- 8.Baffy G. Zhang CY. Glickman JN. Lowell BB. Obesity-related fatty liver is unchanged in mice deficient for mitochondrial uncoupling protein 2. Hepatology. 2002;35:753–761. doi: 10.1053/jhep.2002.32028. [DOI] [PubMed] [Google Scholar]
- 9.Bailey SM. Pietsch EC. Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999;27:891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SM. Patel VB. Young TA. Asayama K. Cunningham CC. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res. 2001;25:726–733. [PubMed] [Google Scholar]
- 11.Bailey SM. Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 12.Bataller R. Schwabe RF. Choi YH. Yang L. Paik YH. Lindquist J. Qian T. Schoonhoven R. Hagedorn CH. Lemasters JJ. Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begriche K. Igoudjil A. Pessayre D. Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Belfort R. Harrison SA. Brown K. Darland C. Finch J. Hardies J. Balas B. Gastaldelli A. Tio F. Pulcini J. Berria R. Ma JZ. Dwivedi S. Havranek R. Fincke C. DeFronzo R. Bannayan GA. Schenker S. Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi P. Petronilli V. Di Lisa F. Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci. 2001;26:112–117. doi: 10.1016/s0968-0004(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 16.Bogenhagen DF. Repair of mtDNA in vertebrates. Am J Hum Genet. 1999;64:1276–1281. doi: 10.1086/302392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet D. Martin D. Pascale De L. Villain E. Jouvet P. Rabier D. Brivet M. Saudubray JM. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 18.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabre E. Nutrition in alcoholic steatohepatitis: more of the same or something new? Curr Opin Clin Nutr Metab Care. 2008;11:626–631. doi: 10.1097/MCO.0b013e32830b5d1e. [DOI] [PubMed] [Google Scholar]
- 20.Cahill A. Wang X. Hoek JB. Increased oxidative damage to mitochondrial DNA following chronic ethanol consumption. Biochem Biophys Res Commun. 1997;235:286–290. doi: 10.1006/bbrc.1997.6774. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell SH. Swerdlow RH. Khan EM. Iezzoni JC. Hespenheide EE. Parks JK. Parker WD., Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 22.Cano A. Ciaffoni F. Safwat GM. Aspichueta P. Ochoa B. Bravo E. Botham KM. Hepatic VLDL assembly is disturbed in a rat model of nonalcoholic fatty liver disease: is there a role for dietary coenzyme Q? J Appl Physiol. 2009;107:707–717. doi: 10.1152/japplphysiol.00297.2009. [DOI] [PubMed] [Google Scholar]
- 23.Cederbaum AI. Lieber CS. Beattie DS. Rubin E. Effect of chronic ethanol ingestion on fatty acid oxidation by hepatic mitochondria. J Biol Chem. 1975;250:5122–5129. [PubMed] [Google Scholar]
- 24.Cederbaum AI. Wu D. Mari M. Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic Biol Med. 2001;31:1539–1543. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani N. Gorski JC. Asghar MS. Asghar A. Foresman B. Hall SD. Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 26.Charlton M. Sreekumar R. Rasmussen D. Lindor K. Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 27.Chavin KD. Yang S. Lin HZ. Chatham J. Chacko VP. Hoek JB. Walajtys-Rode E. Rashid A. Chen CH. Huang CC. Wu TC. Lane MD. Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 28.Chen J. Schenker S. Frosto TA. Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE). Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta. 1998;1380:336–344. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 29.Chen J. Petersen DR. Schenker S. Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin Exp Res. 2000;24:544–552. [PubMed] [Google Scholar]
- 30.Chen Y. Yang Y. Miller ML. Shen D. Shertzer HG. Stringer KF. Wang B. Schneider SN. Nebert DW. Dalton TP. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- 31.Chitturi S. Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 32.Church TS. Kuk JL. Ross R. Priest EL. Biltoft E. Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Colell A. Garcia-Ruiz C. Morales A. Ballesta A. Ookhtens M. Rodes J. Kaplowitz N. Fernandez-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-L-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 34.Colell A. Garcia-Ruiz C. Miranda M. Ardite E. Mari M. Morales A. Corrales F. Kaplowitz N. Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 35.Colell A. Coll O. Garcia-Ruiz C. Paris R. Tiribelli C. Kaplowitz N. Fernandez-Checa JC. Tauroursodeoxycholic acid protects hepatocytes from ethanol-fed rats against tumor necrosis factor-induced cell death by replenishing mitochondrial glutathione. Hepatology. 2001;34:964–971. doi: 10.1053/jhep.2001.28510. [DOI] [PubMed] [Google Scholar]
- 36.Cortez-Pinto H. Chatham J. Chacko VP. Arnold C. Rashid A. Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 37.Cortez-Pinto H. Zhi Lin H. Qi Yang S. Odwin Da Costa S. Diehl AM. Lipids up-regulate uncoupling protein 2 expression in rat hepatocytes. Gastroenterology. 1999;116:1184–1193. doi: 10.1016/s0016-5085(99)70022-3. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham CC. Coleman WB. Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990;25:127–136. doi: 10.1093/oxfordjournals.alcalc.a044987. [DOI] [PubMed] [Google Scholar]
- 39.Dalton TP. Dieter MZ. Yang Y. Shertzer HG. Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 40.Demeilliers C. Maisonneuve C. Grodet A. Mansouri A. Nguyen R. Tinel M. Letteron P. Degott C. Feldmann G. Pessayre D. Fromenty B. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology. 2002;123:1278–1290. doi: 10.1053/gast.2002.35952. [DOI] [PubMed] [Google Scholar]
- 41.De Minicis S. Bataller R. Brenner DA. NADPH oxidase in the liver: defensive, offensive, or fibrogenic? Gastroenterology. 2006;131:272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 42.De Minicis S. Seki E. Uchinami H. Kluwe J. Zhang Y. Brenner DA. Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 43.De Minicis S. Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol. 2008;23(Suppl 1):S98–S103. doi: 10.1111/j.1440-1746.2007.05277.x. [DOI] [PubMed] [Google Scholar]
- 44.DiMauro S. Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001;106:18–26. doi: 10.1002/ajmg.1392. [DOI] [PubMed] [Google Scholar]
- 45.Ding A. Nathan C. Analysis of the nonfunctional respiratory burst in murine Kupffer cells. J Exp Med. 1988;167:1154–1170. doi: 10.1084/jem.167.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diraison F. Moulin P. Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly KL. Smith CI. Schwarzenberg SJ. Jessurun J. Boldt MD. Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dufour JF. NASH and thiazolidinediones: not to be taken lightly. J Hepatol. 2007;47:451–453. doi: 10.1016/j.jhep.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Dunn W. Xu R. Wingard DL. Rogers C. Angulo P. Younossi ZM. Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Echtay KS. Esteves TC. Pakay JL. Jekabsons MB. Lambert AJ. Portero-Otin M. Pamplona R. Vidal-Puig AJ. Wang S. Roebuck SJ. Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50(Suppl):S412–S416. doi: 10.1194/jlr.R800089-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabbrini E. Sullivan S. Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahimi HD. Reinicke A. Sujatta M. Yokota S. Ozel M. Hartig F. Stegmeier K. The short- and long-term effects of bezafibrate in the rat. Ann NY Acad Sci. 1982;386:111–135. doi: 10.1111/j.1749-6632.1982.tb21410.x. [DOI] [PubMed] [Google Scholar]
- 54.Farrell GC. Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Checa JC. Kaplowitz N. Garcia-Ruiz C. Colell A. Miranda M. Mari M. Ardite E. Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Checa JC. Kaplowitz N. Garcia-Ruiz C. Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Checa JC. Colell A. Mari M. Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29:151S–157S. [PubMed] [Google Scholar]
- 58.Fischer M. You M. Matsumoto M. Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 59.Frith J. Day CP. Robinson L. Elliott C. Jones DE. Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol. 2010;52:112–116. doi: 10.1016/j.jhep.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Fromenty B. Grimbert S. Mansouri A. Beaugrand M. Erlinger S. Rotig A. Pessayre D. Hepatic mitochondrial DNA deletion in alcoholics: association with microvesicular steatosis. Gastroenterology. 1995;108:193–200. doi: 10.1016/0016-5085(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 61.Fromenty B. Berson A. Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. J Hepatol. 1997;26(Suppl 1):13–22. doi: 10.1016/s0168-8278(97)82328-8. [DOI] [PubMed] [Google Scholar]
- 62.Fujita K. Nozaki Y. Wada K. Yoneda M. Fujimoto Y. Fujitake M. Endo H. Takahashi H. Inamori M. Kobayashi N. Kirikoshi H. Kubota K. Saito S. Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 63.Fulop P. Derdak Z. Sheets A. Sabo E. Berthiaume EP. Resnick MB. Wands JR. Paragh G. Baffy G. Lack of UCP2 reduces Fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression. Hepatology. 2006;44:592–601. doi: 10.1002/hep.21310. [DOI] [PubMed] [Google Scholar]
- 64.Galli A. Pinaire J. Fischer M. Dorris R. Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 65.Gao D. Wei C. Chen L. Huang J. Yang S. Diehl AM. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1070–G1077. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Ruiz C. Morales A. Colell A. Ballesta A. Rodes J. Kaplowitz N. Fernandez-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Ruiz C. Colell A. Mari M. Morales A. Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Ruiz C. Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21(Suppl 3):S3–S6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Ruiz C. Fernandez-Checa JC. Redox regulation of hepatocyte apoptosis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S38–S42. doi: 10.1111/j.1440-1746.2006.04644.x. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Ruiz I. Rodriguez-Juan C. Diaz-Sanjuan T. del Hoyo P. Colina F. Munoz-Yague T. Solis-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44:581–591. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Villafranca J. Guillen A. Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie. 2008;90:460–466. doi: 10.1016/j.biochi.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 72.Gavrilova O. Haluzik M. Matsusue K. Cutson JJ. Johnson L. Dietz KR. Nicol CJ. Vinson C. Gonzalez FJ. Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein BJ. Mahadev K. Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldstein BJ. Mahadev K. Wu X. Zhu L. Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal. 2005;7:1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 76.Goossens V. Grooten J. De Vos K. Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gudz TI. Tserng KY. Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 78.Guichard C. Moreau R. Pessayre D. Epperson TK. Krause KH. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem Soc Trans. 2008;36:920–929. doi: 10.1042/BST0360920. [DOI] [PubMed] [Google Scholar]
- 79.Guigas B. Detaille D. Chauvin C. Batandier C. De Oliveira F. Fontaine E. Leverve X. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004;382:877–884. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzelian PS. Bissell DM. Meyer UA. Drug metabolism in adult rat hepatocytes in primary monolayer culture. Gastroenterology. 1977;72:1232–1239. [PubMed] [Google Scholar]
- 81.Halliwell B. Aruoma OI. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281:9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- 82.Hansen JM. Go YM. Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 83.Haque M. Sanyal AJ. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best Pract Res Clin Gastroenterol. 2002;16:709–731. doi: 10.1053/bega.2002.0325. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto T. Hussien R. Oommen S. Gohil K. Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 85.Hirano T. Kaplowitz N. Tsukamoto H. Kamimura S. Fernandez-Checa JC. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology. 1992;16:1423–1427. doi: 10.1002/hep.1840160619. [DOI] [PubMed] [Google Scholar]
- 86.Hoek JB. Cahill A. Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hruszkewycz AM. Evidence for mitochondrial DNA damage by lipid peroxidation. Biochem Biophys Res Commun. 1988;153:191–197. doi: 10.1016/s0006-291x(88)81207-5. [DOI] [PubMed] [Google Scholar]
- 88.Hruszkewycz AM. Bergtold DS. The 8-hydroxyguanine content of isolated mitochondria increases with lipid peroxidation. Mutat Res. 1990;244:123–128. doi: 10.1016/0165-7992(90)90060-w. [DOI] [PubMed] [Google Scholar]
- 89.Huang MA. Greenson JK. Chao C. Anderson L. Peterman D. Jacobson J. Emick D. Lok AS. Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 90.Ibdah JA. Paul H. Zhao Y. Binford S. Salleng K. Cline M. Matern D. Bennett MJ. Rinaldo P. Strauss AW. Lack of mitochondrial trifunctional protein in mice causes neonatal hypoglycemia and sudden death. J Clin Invest. 2001;107:1403–1409. doi: 10.1172/JCI12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ibdah JA. Perlegas P. Zhao Y. Angdisen J. Borgerink H. Shadoan MK. Wagner JD. Matern D. Rinaldo P. Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Ip E. Farrell GC. Robertson G. Hall P. Kirsch R. Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 93.Ip E. Farrell G. Hall P. Robertson G. Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 94.Jakupoglu C. Przemeck GK. Schneider M. Moreno SG. Mayr N. Hatzopoulos AK. de Angelis MH. Wurst W. Bornkamm GW. Brielmeier M. Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jezek P. Possible physiological roles of mitochondrial uncoupling proteins—UCPn. Int J Biochem Cell Biol. 2002;34:1190–1206. doi: 10.1016/s1357-2725(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 96.Ji C. Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 97.Johnson NA. George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology. 2010;52:370–381. doi: 10.1002/hep.23711. [DOI] [PubMed] [Google Scholar]
- 98.Jones SA. O'Donnell VB. Wood JD. Broughton JP. Hughes EJ. Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 99.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 100.Kharbanda KK. Todero SL. Ward BW. Cannella JJ., 3rd Tuma DJ. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem. 2009;327:75–78. doi: 10.1007/s11010-009-0044-2. [DOI] [PubMed] [Google Scholar]
- 101.Koch LG. Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 102.Koch OR. Pani G. Borrello S. Colavitti R. Cravero A. Farre S. Galeotti T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med. 2004;25:191–198. doi: 10.1016/j.mam.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 103.Kono H. Rusyn I. Yin M. Gabele E. Yamashina S. Dikalova A. Kadiiska MB. Connor HD. Mason RP. Segal BH. Bradford BU. Holland SM. Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kono H. Rusyn I. Uesugi T. Yamashina S. Connor HD. Dikalova A. Mason RP. Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 105.Koppe SW. Sahai A. Malladi P. Whitington PF. Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 106.Koruk M. Taysi S. Savas MC. Yilmaz O. Akcay F. Karakok M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci. 2004;34:57–62. [PubMed] [Google Scholar]
- 107.Krasnoff JB. Painter PL. Wallace JP. Bass NM. Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–1166. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kristal BS. Park BK. Yu BP. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J Biol Chem. 1996;271:6033–6038. doi: 10.1074/jbc.271.11.6033. [DOI] [PubMed] [Google Scholar]
- 109.Kushnareva Y. Murphy AN. Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lambeth JD. Kawahara T. Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lazo M. Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 112.Lee GY. Kim NH. Zhao ZS. Cha BS. Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378:983–990. doi: 10.1042/BJ20031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol. 2008;23(Suppl 1):S2–S8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 114.Lieber CS. Hepatic, metabolic and toxic effects of ethanol: 1991 update. Alcohol Clin Exp Res. 1991;15:573–592. doi: 10.1111/j.1530-0277.1991.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 115.Lin HZ. Yang SQ. Chuckaree C. Kuhajda F. Ronnet G. Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 116.Loguercio C. Federico A. Trappoliere M. Tuccillo C. de Sio I. Di Leva A. Niosi M. D'Auria MV. Capasso R. Del Vecchio Blanco C. The effect of a silybin-vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:2387–2395. doi: 10.1007/s10620-006-9703-2. [DOI] [PubMed] [Google Scholar]
- 117.Long YC. Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lutchman G. Modi A. Kleiner DE. Promrat K. Heller T. Ghany M. Borg B. Loomba R. Liang TJ. Premkumar A. Hoofnagle JH. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424–429. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 119.Mahadev K. Wu X. Zilbering A. Zhu L. Lawrence JT. Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- 120.Mahadev K. Zilbering A. Zhu L. Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 121.Malaguarnera M. Gargante MP. Russo C. Antic T. Vacante M. Avitabile T. Li Volti G. Galvano F. l-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis-a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338–1345. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 122.Malinska H. Oliyarnyk O. Hubova M. Zidek V. Landa V. Simakova M. Mlejnek P. Kazdova L. Kurtz TW. Pravenec M. Increased liver oxidative stress and altered PUFA metabolism precede development of non-alcoholic steatohepatitis in SREBP-1a transgenic spontaneously hypertensive rats with genetic predisposition to hepatic steatosis. Mol Cell Biochem. 2010;335:119–125. doi: 10.1007/s11010-009-0248-5. [DOI] [PubMed] [Google Scholar]
- 123.Mann RE. Smart RG. Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209–219. [PMC free article] [PubMed] [Google Scholar]
- 124.Mansouri A. Gaou I. De Kerguenec C. Amsellem S. Haouzi D. Berson A. Moreau A. Feldmann G. Letteron P. Pessayre D. Fromenty B. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–190. doi: 10.1016/s0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- 125.Mansouri A. Demeilliers C. Amsellem S. Pessayre D. Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–743. [PubMed] [Google Scholar]
- 126.Mantena SK. Vaughn DP. Andringa KK. Eccleston HB. King AL. Abrams GA. Doeller JE. Kraus DW. Darley-Usmar VM. Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marchesini G. Brizi M. Bianchi G. Tomassetti S. Zoli M. Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 128.Marra F. Gastaldelli A. Svegliati Baroni G. Tell G. Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Masterton GS. Plevris JN. Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 130.Mathurin P. Louvet A. Dharancy S. Treatment of severe forms of alcoholic hepatitis: where are we going? J Gastroenterol Hepatol. 2008;23(Suppl 1):S60–S62. doi: 10.1111/j.1440-1746.2007.05286.x. [DOI] [PubMed] [Google Scholar]
- 131.Matsusue K. Haluzik M. Lambert G. Yim SH. Gavrilova O. Ward JM. Brewer B., Jr. Reitman ML. Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miele L. Grieco A. Armuzzi A. Candelli M. Forgione A. Gasbarrini A. Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98:2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 133.Miwa S. Brand MD. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem Soc Trans. 2003;31:1300–1301. doi: 10.1042/bst0311300. [DOI] [PubMed] [Google Scholar]
- 134.Mizukami Y. Matsubara F. Matsukawa S. Izumi R. Cytochemical localization of glutaraldehyde-resistant NAD(P)H-oxidase in rat hepatocytes. Histochemistry. 1983;79:259–267. doi: 10.1007/BF00489788. [DOI] [PubMed] [Google Scholar]
- 135.Moon KH. Hood BL. Kim BJ. Hardwick JP. Conrads TP. Veenstra TD. Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 136.Morgan MJ. Kim YS. Liu ZG. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 137.Musso G. Gambino R. De Michieli F. Cassader M. Rizzetto M. Durazzo M. Faga E. Silli B. Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 138.Nakamuta M. Kohjima M. Morizono S. Kotoh K. Yoshimoto T. Miyagi I. Enjoji M. Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2005;16:631–635. [PubMed] [Google Scholar]
- 139.Nanji AA. Dannenberg AJ. Jokelainen K. Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther. 2004;310:417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 140.Naveau S. Chollet-Martin S. Dharancy S. Mathurin P. Jouet P. Piquet MA. Davion T. Oberti F. Broet P. Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 141.Negre-Salvayre A. Hirtz C. Carrera G. Cazenave R. Troly M. Salvayre R. Penicaud L. Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- 142.Neuschwander-Tetri BA. Brunt EM. Wehmeier KR. Oliver D. Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 143.Neuschwander-Tetri BA. Lifestyle modification as the primary treatment of NASH. Clin Liver Dis. 2009;13:649–665. doi: 10.1016/j.cld.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 144.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 145.Niemela O. Parkkila S. Pasanen M. Iimuro Y. Bradford B. Thurman RG. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res. 1998;22:2118–2124. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 146.Nissen SE. Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 147.Nohl H. Gille L. Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol. 2005;69:719–723. doi: 10.1016/j.bcp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 148.Oliveira CP. da Costa Gayotto LC. Tatai C. Della Bina BI. Janiszewski M. Lima ES. Abdalla DS. Lopasso FP. Laurindo FR. Laudanna AA. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6:399–406. doi: 10.1111/j.1582-4934.2002.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ong JP. Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. doi: 10.1016/j.cld.2007.02.009. vii. [DOI] [PubMed] [Google Scholar]
- 150.Ong JP. Pitts A. Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 151.O'Shea D. Davis SN. Kim RB. Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56:359–367. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- 152.Oza N. Eguchi Y. Mizuta T. Ishibashi E. Kitajima Y. Horie H. Ushirogawa M. Tsuzura T. Nakashita S. Takahashi H. Kawaguchi Y. Oda Y. Iwakiri R. Ozaki I. Eguchi T. Ono N. Fujimoto K. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol. 2009;44:1203–1208. doi: 10.1007/s00535-009-0115-x. [DOI] [PubMed] [Google Scholar]
- 153.Pastorino JG. Simbula G. Yamamoto K. Glascott PA., Jr. Rothman RJ. Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–29798. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 154.Pastorino JG. Marcineviciute A. Cahill A. Hoek JB. Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem Biophys Res Commun. 1999;265:405–409. doi: 10.1006/bbrc.1999.1696. [DOI] [PubMed] [Google Scholar]
- 155.Pastorino JG. Hoek JB. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 156.Patel AA. Torres DM. Harrison SA. Effect of weight loss on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2009;43:970–974. doi: 10.1097/MCG.0b013e3181b57475. [DOI] [PubMed] [Google Scholar]
- 157.Perez-Carreras M. Del Hoyo P. Martin MA. Rubio JC. Martin A. Castellano G. Colina F. Arenas J. Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 158.Perez MJ. Cederbaum AI. Adenovirus-mediated expression of Cu/Zn- or Mn-superoxide dismutase protects against CYP2E1-dependent toxicity. Hepatology. 2003;38:1146–1158. doi: 10.1053/jhep.2003.50479. [DOI] [PubMed] [Google Scholar]
- 159.Perseghin G. Lattuada G. De Cobelli F. Ragogna F. Ntali G. Esposito A. Belloni E. Canu T. Terruzzi I. Scifo P. Del Maschio A. Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683–688. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 160.Pessayre D. Mansouri A. Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 161.Pessayre D. Fromenty B. Mansouri A. Mitochondrial injury in steatohepatitis. Eur J Gastroenterol Hepatol. 2004;16:1095–1105. doi: 10.1097/00042737-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 162.Pessayre D. Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 163.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2007;22(Suppl 1):S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 164.Promrat K. Kleiner DE. Niemeier HM. Jackvony E. Kearns M. Wands JR. Fava JL. Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Purohit V. Gao B. Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raha S. Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 167.Rahman SM. Schroeder-Gloeckler JM. Janssen RC. Jiang H. Qadri I. Maclean KN. Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–1117. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 168.Rao MS. Reddy JK. Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis. 1987;8:631–636. doi: 10.1093/carcin/8.5.631. [DOI] [PubMed] [Google Scholar]
- 169.Rao MS. Papreddy K. Musunuri S. Okonkwo A. Prevention/reversal of choline deficiency-induced steatohepatitis by a peroxisome proliferator-activated receptor alpha ligand in rats. In Vivo. 2002;16:145–152. [PubMed] [Google Scholar]
- 170.Rashid A. Wu TC. Huang CC. Chen CH. Lin HZ. Yang SQ. Lee FY. Diehl AM. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131–1138. doi: 10.1002/hep.510290428. [DOI] [PubMed] [Google Scholar]
- 171.Ratziu V. Giral P. Jacqueminet S. Charlotte F. Hartemann-Heurtier A. Serfaty L. Podevin P. Lacorte JM. Bernhardt C. Bruckert E. Grimaldi A. Poynard T. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 172.Raza H. Prabu SK. Robin MA. Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 173.Rector RS. Thyfault JP. Laye MJ. Morris RT. Borengasser SJ. Uptergrove GM. Chakravarthy MV. Booth FW. Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008;586(Pt 17):4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rector RS. Thyfault JP. Morris RT. Laye MJ. Borengasser SJ. Booth FW. Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 175.Rector RS. Thyfault JP. Uptergrove GM. Morris EM. Naples SP. Borengasser SJ. Mikus CR. Laye MJ. Laughlin MH. Booth FW. Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reinehr R. Becker S. Eberle A. Grether-Beck S. Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem. 2005;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 177.Rensen SS. Slaats Y. Nijhuis J. Jans A. Bieghs V. Driessen A. Malle E. Greve JW. Buurman WA. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Robin MA. Anandatheerthavarada HK. Fang JK. Cudic M. Otvos L. Avadhani NG. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contains an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J Biol Chem. 2001;276:24680–24689. doi: 10.1074/jbc.M100363200. [DOI] [PubMed] [Google Scholar]
- 179.Robin MA. Anandatheerthavarada HK. Biswas G. Sepuri NB. Gordon DM. Pain D. Avadhani NG. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J Biol Chem. 2002;277:40583–40593. doi: 10.1074/jbc.M203292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Robin MA. Sauvage I. Grandperret T. Descatoire V. Pessayre D. Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005;579:6895–6902. doi: 10.1016/j.febslet.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 181.Rubinstein E. Lavine JE. Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:380–385. doi: 10.1055/s-0028-1091982. [DOI] [PubMed] [Google Scholar]
- 182.Safwat GM. Pisano S. D'Amore E. Borioni G. Napolitano M. Kamal AA. Ballanti P. Botham KM. Bravo E. Induction of non-alcoholic fatty liver disease and insulin resistance by feeding a high-fat diet in rats: does coenzyme Q monomethyl ether have a modulatory effect? Nutrition. 2009;25:1157–1168. doi: 10.1016/j.nut.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 183.Salaspuro MP. Shaw S. Jayatilleke E. Ross WA. Lieber CS. Attenuation of the ethanol-induced hepatic redox change after chronic alcohol consumption in baboons: metabolic consequences in vivo and in vitro. Hepatology. 1981;1:33–38. doi: 10.1002/hep.1840010106. [DOI] [PubMed] [Google Scholar]
- 184.Santamaria E. Avila MA. Latasa MU. Rubio A. Martin-Duce A. Lu SC. Mato JM. Corrales FJ. Functional proteomics of nonalcoholic steatohepatitis: mitochondrial proteins as targets of S-adenosylmethionine. Proc Natl Acad Sci USA. 2003;100:3065–3070. doi: 10.1073/pnas.0536625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanyal AJ. Campbell-Sargent C. Mirshahi F. Rizzo WB. Contos MJ. Sterling RK. Luketic VA. Shiffman ML. Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 186.Sanyal AJ. Chalasani N. Kowdley KV. McCullough A. Diehl AM. Bass NM. Neuschwander-Tetri BA. Lavine JE. Tonascia J. Unalp A. Van Natta M. Clark J. Brunt EM. Kleiner DE. Hoofnagle JH. Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Satapathy SK. Garg S. Chauhan R. Sakhuja P. Malhotra V. Sharma BC. Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–1952. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- 188.Schattenberg JM. Wang Y. Singh R. Rigoli RM. Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 189.Schon EA. Bonilla E. DiMauro S. Mitochondrial DNA mutations and pathogenesis. J Bioenerg Biomembr. 1997;29:131–149. doi: 10.1023/a:1022685929755. [DOI] [PubMed] [Google Scholar]
- 190.Schrader M. Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 191.Schroeder-Gloeckler JM. Rahman SM. Janssen RC. Qiao L. Shao J. Roper M. Fischer SJ. Lowe E. Orlicky DJ. McManaman JL. Palmer C. Gitomer WL. Huang W. O'Doherty RM. Becker TC. Klemm DJ. Jensen DR. Pulawa LK. Eckel RH. Friedman JE. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem. 2007;282:15717–15729. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Serviddio G. Pereda J. Pallardo FV. Carretero J. Borras C. Cutrin J. Vendemiale G. Poli G. Vina J. Sastre J. Ursodeoxycholic acid protects against secondary biliary cirrhosis in rats by preventing mitochondrial oxidative stress. Hepatology. 2004;39:711–720. doi: 10.1002/hep.20101. [DOI] [PubMed] [Google Scholar]
- 193.Serviddio G. Bellanti F. Tamborra R. Rollo T. Romano AD. Giudetti AM. Capitanio N. Petrella A. Vendemiale G. Altomare E. Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur J Clin Invest. 2008;38:245–252. doi: 10.1111/j.1365-2362.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 194.Serviddio G. Sastre J. Bellanti F. Vina J. Vendemiale G. Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 2008;29:22–35. doi: 10.1016/j.mam.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 195.Sohet FM. Neyrinck AM. Pachikian BD. de Backer FC. Bindels LB. Niklowitz P. Menke T. Cani PD. Delzenne NM. Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 2009;78:1391–1400. doi: 10.1016/j.bcp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 196.Song BJ. Moon KH. Olsson NU. Salem N., Jr. Prevention of alcoholic fatty liver and mitochondrial dysfunction in the rat by long-chain polyunsaturated fatty acids. J Hepatol. 2008;49:262–273. doi: 10.1016/j.jhep.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Spahr L. Rubbia-Brandt L. Pugin J. Giostra E. Frossard JL. Borisch B. Hadengue A. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. 2001;35:582–589. doi: 10.1016/s0168-8278(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 198.Spencer JP. Jenner A. Chimel K. Aruoma OI. Cross CE. Wu R. Halliwell B. DNA strand breakage and base modification induced by hydrogen peroxide treatment of human respiratory tract epithelial cells. FEBS Lett. 1995;374:233–236. doi: 10.1016/0014-5793(95)01117-w. [DOI] [PubMed] [Google Scholar]
- 199.Sreekumar R. Rosado B. Rasmussen D. Charlton M. Hepatic gene expression in histologically progressive nonalcoholic steatohepatitis. Hepatology. 2003;38:244–251. doi: 10.1053/jhep.2003.50290. [DOI] [PubMed] [Google Scholar]
- 200.St George A. Bauman A. Johnston A. Farrell G. Chey T. George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 201.Starkel P. Sempoux C. Leclercq I. Herin M. Deby C. Desager JP. Horsmans Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J Hepatol. 2003;39:538–546. doi: 10.1016/s0168-8278(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 202.Stein LL. Dong MH. Loomba R. Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: current status. Adv Ther. 2009;26:893–907. doi: 10.1007/s12325-009-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Suh SK. Hood BL. Kim BJ. Conrads TP. Veenstra TD. Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 204.Svegliati-Baroni G. Candelaresi C. Saccomanno S. Ferretti G. Bachetti T. Marzioni M. De Minicis S. Nobili L. Salzano R. Omenetti A. Pacetti D. Sigmund S. Benedetti A. Casini A. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol. 2006;169:846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Targher G. Bertolini L. Padovani R. Rodella S. Zoppini G. Zenari L. Cigolini M. Falezza G. Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 206.Thyfault JP. Rector RS. Uptergrove GM. Borengasser SJ. Morris EM. Wei Y. Laye MJ. Burant CF. Qi NR. Ridenhour SE. Koch LG. Britton SL. Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsukamoto H. She H. Hazra S. Cheng J. Wang J. Fat paradox of steatohepatitis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S104–S107. doi: 10.1111/j.1440-1746.2007.05294.x. [DOI] [PubMed] [Google Scholar]
- 208.Vendemiale G. Grattagliano I. Caraceni P. Caraccio G. Domenicali M. Dall'Agata M. Trevisani F. Guerrieri F. Bernardi M. Altomare E. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 2001;33:808–815. doi: 10.1053/jhep.2001.23060. [DOI] [PubMed] [Google Scholar]
- 209.Venkatraman A. Landar A. Davis AJ. Chamlee L. Sanderson T. Kim H. Page G. Pompilius M. Ballinger S. Darley-Usmar V. Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- 210.Vidal-Puig A. Jimenez-Linan M. Lowell BB. Hamann A. Hu E. Spiegelman B. Flier JS. Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Videla LA. Rodrigo R. Orellana M. Fernandez V. Tapia G. Quinones L. Varela N. Contreras J. Lazarte R. Csendes A. Rojas J. Maluenda F. Burdiles P. Diaz JC. Smok G. Thielemann L. Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 212.Vuppalanchi R. Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wallace DC. Fan W. Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang D. Wei Y. Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 215.Wei Y. Wang D. Topczewski F. Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 216.Weltman MD. Farrell GC. Hall P. Ingelman-Sundberg M. Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 217.Wheeler MD. Kono H. Yin M. Nakagami M. Uesugi T. Arteel GE. Gabele E. Rusyn I. Yamashina S. Froh M. Adachi Y. Iimuro Y. Bradford BU. Smutney OM. Connor HD. Mason RP. Goyert SM. Peters JM. Gonzalez FJ. Samulski RJ. Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 218.Winder WW. Holmes BF. Rubink DS. Jensen EB. Chen M. Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 219.Wisloff U. Najjar SM. Ellingsen O. Haram PM. Swoap S. Al-Share Q. Fernstrom M. Rezaei K. Lee SJ. Koch LG. Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 220.Wu D. Cederbaum AI. Ethanol-induced apoptosis to stable HepG2 cell lines expressing human cytochrome P-4502E1. Alcohol Clin Exp Res. 1999;23:67–76. [PubMed] [Google Scholar]
- 221.Yang ES. Lee JH. Park JW. Ethanol induces peroxynitrite-mediated toxicity through inactivation of NADP+-dependent isocitrate dehydrogenase and superoxide dismutase. Biochimie. 2008;90:1316–1324. doi: 10.1016/j.biochi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 222.Yang S. Zhu H. Li Y. Lin H. Gabrielson K. Trush MA. Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 223.Yesilova Z. Yaman H. Oktenli C. Ozcan A. Uygun A. Cakir E. Sanisoglu SY. Erdil A. Ates Y. Aslan M. Musabak U. Erbil MK. Karaeren N. Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 224.Yin M. Wheeler MD. Kono H. Bradford BU. Gallucci RM. Luster MI. Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 225.Yin M. Gabele E. Wheeler MD. Connor H. Bradford BU. Dikalova A. Rusyn I. Mason R. Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001;34:935–942. doi: 10.1053/jhep.2001.28888. [DOI] [PubMed] [Google Scholar]
- 226.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 227.You M. Fischer M. Deeg MA. Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 228.You M. Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 229.You M. Matsumoto M. Pacold CM. Cho WK. Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 230.Young TA. Cunningham CC. Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: studies using myxothiazol. Arch Biochem Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 231.Yu S. Rao S. Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3:561–572. doi: 10.2174/1566524033479537. [DOI] [PubMed] [Google Scholar]
- 232.Zakhari S. Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- 233.Zelber-Sagi S. Nitzan-Kaluski D. Goldsmith R. Webb M. Zvibel I. Goldiner I. Blendis L. Halpern Z. Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 234.Zhou G. Myers R. Li Y. Chen Y. Shen X. Fenyk-Melody J. Wu M. Ventre J. Doebber T. Fujii N. Musi N. Hirshman MF. Goodyear LJ. Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]