Abstract
The ‘bystander effect’ phenomenon has challenged the traditional framework for assessing radiation damage by showing radiation induced changes in cells which have not been directly targeted, but are neighbors to or receive medium from directly hit cells. Our group performed a range of single and serial low dose irradiations on two genetically distinct strains of mice. Bladder explants established from these mice were incubated in culture medium, which was used to measure death responses in a keratinocyte reporter system. The study revealed that the medium harvested from bladder tissues’ (ITCM) from acutely irradiated C57BL6 but not Balb/c mice, was able to induce clonogenic death. Administration of a priming dose(s) before a challenge dose to both C57BL6 and Balb/c mice stimulated reporter cell survival irrespective of the time interval between dose(s) delivery. When ITCM corresponding to both strains of mice was measured for its calcium mobilization inducing ability, results showed an elevation in intracellular calcium levels that was strain dependent. This indicates that genotype determined the type of bystander signal/response that was produced after exposure to low and acute doses of radiation. However, serial exposure conditions modified bystander signal production to induce similar effects that were characterized by excessive growth.
Keywords: Ratiometric calcium measurement, Priming dose, challenge dose, apoptosis, transfer medium, ITCM
INTRODUCTION
Low doses of radiation may cause cells to produce signals that are capable of altering the survival and response of non-irradiated cells (Nagasawa and Little 1992, Deshpande et al. 1996; Mothersill and Seymour 1997; Watson et al. 2000: Lewis et al. 2001; Lorimore and Wright 2003). These effects have been termed the bystander effect, and once induced, this phenomenon may become a permanent characteristic of the cell population (extensively reviewed in Mothersill and Seymour 2006a; 2006b; 2006c). Although the precise mechanism is unknown, there is substantial evidence that bystander signals may be transmitted by direct gap junction communication (Azzam et al. 1998) and by media soluble factors (Mothersill and Seymour, 1997).
Reports dating back to the 1950’s have revealed that radiation exposure at one site could impose damage in distant, non irradiated sites (Parsons et al. 1954; Souto 1962, Hollowell and Littlefield 1967). Research on bystander responses is largely based on in vitro experiments using high LET radiation (Nagasawa and Little 1992; Deshpande et al. 1996; Prise et al. 1998; Zhou et al. 2000; Prise et al. 2006), mostly as the result of microbeam technology that allowed for targeted exposures to extranuclear sites as well as extra-cellular or neighboring cells (Sedelnikova et al. 2007 and Zhou et al. 2009). Although recently medium transfer techniques and co-culture have gained in popularity. Investigation into the in vivo generation of such factors has also been explored in fish and rodent models (Mothersill et al. 2005 and 2007). Some studies have looked into sex and tissue specific changes in the mouse genome after acute and chronic exposures to ionizing radiation (Kovalchuk et al. 2004a and 2004b; Besplug et al. 2005) and revealed that an up-regulation of various hematopoietic signaling pathways exists after exposure to low dose, chronic levels of radiation. In addition, Lorimore et al. (2008) showed genotype-dependent induction of chromosomal instability in un-irradiated hemaopoietic stem cells after exposure to conditioned medium from bone marrow cells of in vivo gamma irradiated mice.
Bystander effects may manifest themselves in various forms, ranging from delayed genomic instability, apoptosis, cell cycle delay, micronucleus formation, delayed mutations and changes in gene expression (Kadhim et al. 1992; Seymour and Mothersill 1997; Mothersill and Seymour 1998, Lorimore et al. 1998; Wu et al. 1999, Morgan 2003). The exact nature of the transducing mechanism is unclear, however studies such as those by Lyng et al. (2002a; 2002b; 2006) have shown rapid calcium induction, loss of mitochondrial membrane potential, and increase in reactive oxygen species in cells receiving culture medium taken from various generations of cells post exposure.
Seymour and Mothersill (2006) discussed that when patients blood samples were taken after radiation treatment had commenced, the conditioned media harvested from the samples, resulted in greater levels of adaptation in reporters in vitro than if the pre treatment blood sample was assayed. Similarly, Maguire et al (2007) showed an increase in cell sparing of 15% in reporters after they received a priming dose before the challenge dose. It is postulated that a small priming insult high enough to cause damage results in the activation of repair systems. This in turn results in the accumulation of various repair proteins at the site of damage, which aids in the reduction of subsequent damage that may occur as a result of the challenge dose (Crawford and Davies 1994). In fact, Ikushima et al. (1996), showed a higher rate of DNA double strand rejoining induced after exposure to challenge doses in adapted versus nonadapted cells.
Our group aimed to investigate two aspects of the bystander effect using an in vivo mouse model. The first of these explored the role of genetic predisposition in the in vivo generation of bystander signals. The second explored whether bystander signal(s) can be modified in vivo if mice were exposed to a low priming dose delivered before a higher challenge dose. Biological markers of cell death such as clonogenic survival and intracellular calcium measurements which can trigger apoptosis were analyzed as endpoints of biological signal production using our well established reporter system.
METHODS
Mouse Models and In Vivo Irradiations
The mice used in this experiment were C57BL6 and Balb/c mice, which were bred and housed in the AECL bioresearch facility (Chalk River, ON). Specifically, the mice were placed on ventilated racks supplied with HEPA filtered air, autoclaved feed and were given reverse osmosis water to drink so as to ensure a pathogen free environment and optimal health conditions. C57BL6 mice have been well established in previous adaptive response studies (Mitchel et al 2008), therefore, radio-senstive Balb/c mice were used to compare the effects of in vivo radiation on two genetically contrasting genotypes. The mice were approximately four month old, non-pregnant females who received whole body irradiation carried out with either a Co-60 gamma beam 150C (for adaptive doses), or a Co-60 GammaCell 200 (for challenge doses) irradiator. The dose rate for the gamma beam exposures was 0.5 mGy/min. The dose rate for the gamma cell 200 varied according to distance from the beam, between 162 and 168 mGy/min for the mice under study. The mice were sacrificed 24 hours after receiving the final dose. Bladders were surgically extracted from the mice under sterilized conditions, placed in sterilized transport medium, and couriered overnight to McMaster University. These bladders were received in three separate lots, with a total of 22 C57BL6 mice [4 were given 0 Gy, 2 were given 2 Gy, 4 were given 20 mGy, 4 were given 20 mGy followed by 2 Gy after four hours, 4 were given 20 mGy followed by another 2 Gy after 24 hours, and finally 4 were given 20 mGy followed by another 20 mGy followed by another 20 mGy followed by 2 Gy (all doses were separated by 48 hours)]. A total of 16 Balb/c mice bladder samples were shipped [3 were given 0 Gy, 3 were given 20 mGy, 3 were given 2 Gy, 2 were given 20 mGy followed by 2 Gy after 4 hours, 2 were given 20 mGy after 24 hours, and lastly 2 were given 20 mGy followed by 20 mGy followed by another 20 mGy followed by 2 Gy (all doses were separated by 48 hours).
Bladder Explant Assay
Full details of the method are available in Mothersill et al. 2001. Briefly, upon arrival at McMaster University, bladders were placed into sterilized petri dishes (VWR, Burlington, ON) and chopped into three pieces, approximately 2 mm2 in size. Tissue explants were then plated as single explants in sterile T25 cm2 40 ml flasks (Falcon, Franklin Lanes, NJ) containing 2 mL of RPMI 1640 (Gibco, Burlington, ON) culture medium supplemented with 60mL of Fetal Bovine Serum (Invitrogen, Burlington, ON), 5 mL of Penicillian (10,000 units) – Streptomycin (10,000 μg) (Gibco, Burlington, ON), 5 mL of L-Gluthamine at 200 mM (100x) (Gibco, Burlington, ON), 0.5 μg/ml hydrocortisone (Sigma-Aldrich, Oakville, ON), and 12.5 mL of 1M HEPES buffer solution (Gibco, Burlington, ON). The tissue explants were incubated at 37° C in a 5% carbon dioxide and 95% humidity incubator. After 48 hours, the medium from the tissue explants (referred to as ITCM) was harvested and frozen in 2 mL aliquots. The explants were replenished with 2 mL of serum free medium (KGM, Clonetics Cooperation) and incubated for 10–2 days and fixed for analysis for a separate study not discussed in this paper.
Reporter Cell Culture
HPV transfected human foreskin kertinocytes were maintained in 500 mL of RPMI 1640 (Gibco, Burlington,ON) that was supplemented as described above. All cells were maintained in sterilized T75 cm2 flasks (Falcon, Franklin Lanes, NJ) within 37° C. The media was changed when the cells reached 80%–00% confluence. 24 hours later they were sub-cultured. Cells were detached from the flask surface by using a 1:1 solution of 0.25 % trypsin and 1 mM EDTA (Gibco, Burlington, ON) in Dulbecco’s Phosphate Solution (1x) (Gibco, Burlington, ON). 2 mL of the cell solution containing approximately 1 million cells was transferred into a new T75cm2 flask containing 20 ml of medium to maintain stocks. This procedure was carried out under sterile conditions in a Class II biosafety unit.
Clonogenic Assay and Medium Change
1 mL of the detached cell solution was placed in 10 mL isotonic buffer (VWR, Burlington, ON) and the cell concentration of this aliquot was counted using a Coulter Counter model Z2 (Beckman Coulter, Fullerton, CA). A threshold was pre-set to the size suitable for HPV-G cells and the total cell count was corrected for background materials of similar size present in the solution. Once the corrected cell concentration was derived, the stock cell solution underwent a series of dilutions (1:10, 1:100, 1:1000) allowing for plating of appropriate cell numbers for survival (Puck and Marcus, 1956).
To assess the effect of the irradiated tissue conditioned medium or control tissue conditioned medium (ITCM or CTCM) on unirradiated reporter HPV cells, frozen conditioned medium that was harvested previously was thawed, filtered through a 0.22 micron filter (VWR, Mississauga, ON)and added (5 mL of ITCM) to HPV-G reporters (Mothersill and Seymour 1997). The reporters were destined to receive the CM were plated at a density in the range of 500–000 cells/flask. 100 μL of the conditioned medium (CM) was reserved for calcium flux measurements. After exposure to the ITCM, the reporters were incubated for 10–4 days at 37° C. Once macroscopic colonies were observed, the cells were stained with 2 mL carbon fushin for 4 minutes and then rinsed off with water. Colonies were then counted using the 50 cell threshold described in Puck and Marcus (1956) in which cells that reached this limit were classified as true survivors. In order to compare treatments, percentage survival was calculated using the formula: Percent Survival = 100 x [PE of the treatment/PE of controls]. PE stands for plating efficiency determined by number of colonies counted/number of colonies plated. All data was normalized to the 0 Gy controls by first calculating MCF (mean correction factor): Average of (100/colony count of 0 Gy controls). All treatments were then normalized by using the formula: (MCF x percent survival of treatment) = Adjusted Percent Survival.
Ratiometeric Calcium Measurements
HPV-G cells were plated at 300,000 cells per dish (MatTek, Ashland, MA) and incubated for 72 hours. Upon formation of a monolayer, the cells were washed twice with a buffer containing 130 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 1mM CaCl2, 1mM MgCl2(pH=7.4) Cells were then incubated with 10 uL of Fura-2 (420 μM) for 30 minutes at 37 ° C. The dye was discarded and the cells were washed with the same buffer three times and 300 uL of buffer was added to each plate for measurement purposes. Once Fura- 2 was excited, measurements at 380nm and 345 nm were recorded every 2 seconds for 9 minutes. After 45 seconds to 1 minute of recording, 100μL of the ITCM was added to the cells. The pre- event time frame was chosen so as to firmly establish a baseline reading. All measurements were made in the dark at room temperature.
Ratio of fluorescence at the two wavelengths versus time was then graphed using the Sigma Plus software system, thus illustrating the kinetics of free cytosolic calcium induction.
Image Analysis
Randomly selected colonies each treatment flask were photographed daily (for 8 – 10 days) using a CCD camera that is mounted on a microscope. The images were imported into the Image Pro 9.0 software system and colonies were then measured for area values using the manual tag function. Area measurement data for all the colonies were daily imported into an excel worksheet where they were averaged. Area growth was displayed as average area growth ± SEM per day and compared across treatments using ANOVA statistical test with P<0.05 as the limit of significance.
Statistical Analysis
The clonogenic survival data are presented as a mean± SEM, where each treatment had N=3 recipient flasks, unless otherwise stated. Significance of differences was tested using ANOVA, with P<0.05 as the limit of significance. Similarly, the calcium flux data are presented as a mean ± SEM from measurements taken from five different cells.
RESULTS
Percent Survival and Growth Rate of Reporters Given Transfer Medium from irradiated C57BL6 and Balb/c Mice
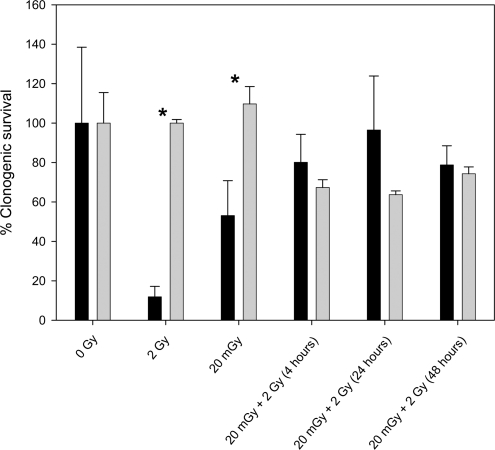
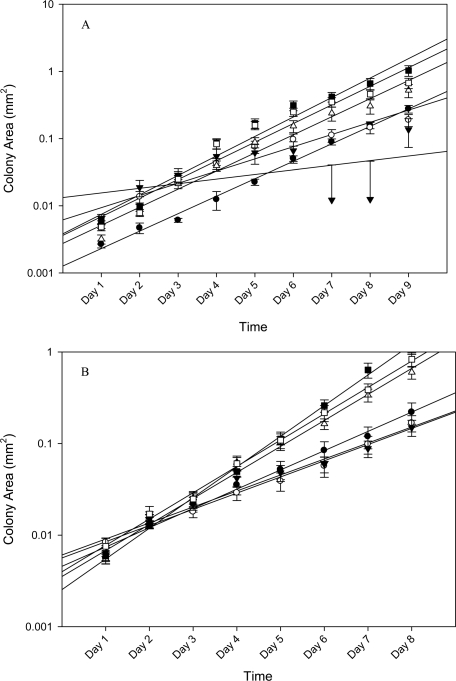
Results showed that ITCM collected from 2 Gy irradiated C57BL6 mice significantly (p<0.05) reduced the percent survival of reporters from 100% in unirradiated mice to 12% ± 2.835 (fig 1). As expected, these reporters also displayed a severe reduction in their growth rates, which was measured as the slope of the area growth curve (fig 2A), from m= 0.0293 in the unirradiated controls to 0.008 (p<0.05). However, ITCM collected from 20 mGy irradiated mice had a percent reporter survival of 53% ± 10.260. Bladder medium harvested from mice given a 20 mGy priming dose, twenty four hours before a 2 Gy challenge dose (24 hour group), had a significant increase in reporter percent survival (80% ± 11.551), and also displayed enhanced growth rates [m =0.0585] that exceeded even the reporters given ITCM from the unirradiated mice. (fig 3A). However, reporter percent survival as well as the corresponding growth rate was seen to decrease when the time interval between the priming and challenge dose was four hours rather than twenty four hours. Similarly, when multiple priming doses (3x) were administered to mice forty-eight hours before a challenge dose, the corresponding ITCM caused a decrease in both the percent of surviving reporter colonies and their rate of division when compared to the ITCM from the 24 hour group.
FIGURE 1.
HPV- G reporter colony survival was measured in reporters that received ITCM from bladder explants extracted from (black) C57BL6 mice that were exposed to 0 gy, 2 Gy,20 mGy, 20 mGy followed by 2 Gy four hours later, 20 mGy followed by 2 Gy twenty four hours later, and 20 mGy, then 20 mGy, then 20 mGy followed by 2 Gy, at fourty eight hour intervals (N=4/treatment)(gray) Balb/c mice that were exposed to 0 Gy, 20 mGy, 2 Gy, 20 mGy + 2 Gy (4 hours), 20 mGy + 2 Gy (24 hours apart), and 20, 20, 20 mGy followed by 2 Gy (48 hour intervals). N= a minimum of 3 flaks per treatment. All deviations were represented as ±S.E.M. Significance was determined using student t test statistical analysis where p<0.05:
FIGURE 2.
Randomly selected colonies (N= 25 colonies/treatment of Balb/c mice and N= 20 to 50 colonies across the treatments of C57BL6 mice) were serially photographed and the corresponding colony area was tracked over the course of the incubation time. Cellular Growth was defined by the equation Y = mx - b, where Y is area in mm2, m is the slope or growth rate and b is the y-intercept. For panel (A): (black circle) 0 Gy, m= 0.0293 (black triangle) 2 Gy, m= 0.008 (gray circle) 20 mGy, m= 0.0226 (white triangle) 20 mGy followed by 2 Gy given 4 hours apart, m = 0.0585 (black square) 20 mGy followed by 2 Gy, given 24 hours apart, m= 0.1176 (white square) 20 mGy followed by another 20 mGy and another 20 mGy followed by 2 Gy, given at 48 hour intervals, m= 0.0806. For panel B: (black circle) 0 Gy, m= 0.0307 (black triangle) 2 Gy, m= 0.0176 (gray circle) 20 mGy, m= 0.0194 (white triangle) 20 mGy followed by 2 Gy given 4 hours apart, m = 0.07425 (black square) 20 mGy followed by 2 Gy, given 24 hours apart, m= 0.3727 (white square) 20 mGy followed by another 20 mGy and another 20 mGy followed by 2 Gy, given at 48 hour intervals, m= 0.0979.
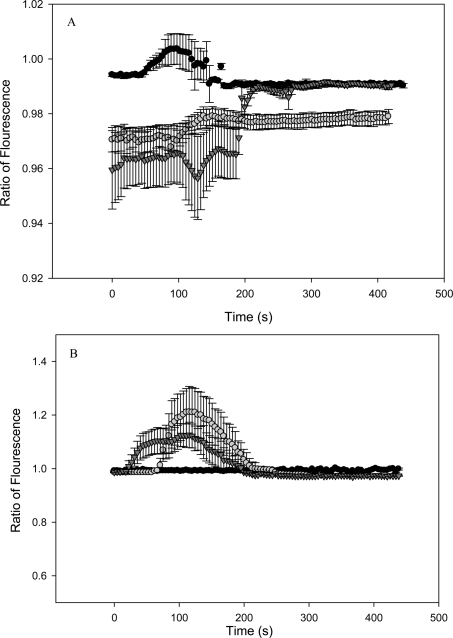
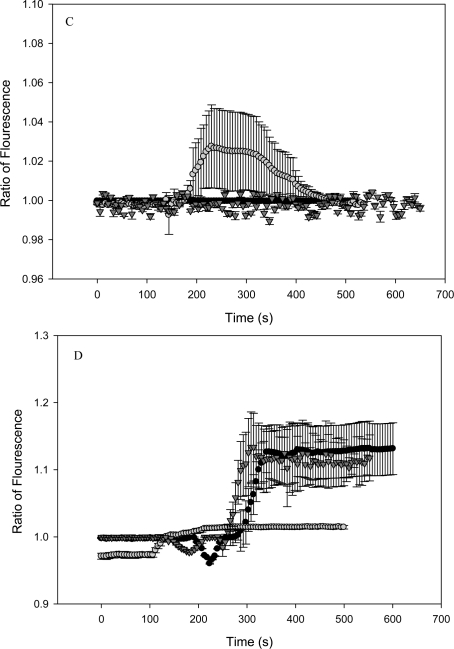
FIGURE 3.
Cytosolic calcium is measured in HPV-G reporters that received transfer medium from bladder explants extracted from C57BL6 (normal apoptotic response) mice that were exposed to (graph A): (black circle) 0 Gy, (gray circle) 2 Gy, (black triangle) 20 mGy. Graph B: (black circle)20 mGy followed by 2 Gy four hours later, (gray circle) 20 mGy followed by 2 Gy twenty four hours later, and (black triangle) 20 mGy, then 20 mGy, then 20 mGy followed by 2 Gy, at fourty eight hour intervals. Graph C depicts calcium measurements from reporters given ITCM from Balb/c mice that were exposed to (black circle) 0 Gy, (black triangle) 20 mGy, (gray circle) 2 Gy. Graph D displays calcium measurements from reporters given ITCM from Balb/c mice given (gray circle) 20 mGy followed by 2 Gy, four hours later, (black circle) 20 mGy + 2 Gy (24 hours apart), and (black triangle) 20, 20, 20 mGy followed by 2 Gy (48 hour intervals). (All measurements were taken as an average from N=5 cells) and all deviations were represented as ±S.E.M.
In contrast, acutely irradiated (2 Gy and 20 mGy) Balb/c mice failed to elicit a death response in the corresponding reporters (fig 1) when compared to reporters given ITCM from unirradiated Balb/c mice. However, their growth [m= 0.0176 and 0.0194 respectively] was considerably slower following the medium transfer when compared to the reporters given ITCM from unirradiated mice [m= 0.0307] (fig 3B). When Balb/c mice were primed with 20 mGy four hours (4 hour group) and twenty four hours (24 hour group) before a challenge dose, the corresponding ITCM caused a reduction in the percentage of surviving reporters when compared to the reporters given ITCM taken from the acutely exposed mice. Similarly, ITCM from mice repeatedly primed with 20 mGy (delivered 48 hours apart) before a 2 Gy challenge dose, also reduced the percent of surviving reporters. Regardless of lower colony counts, reporters that were given ITCM from repeatedly exposed mice possessed growth rates that surpassed that of the reporters given ITCM from unirradiated mice (fig 3B).
Calcium Flux in Reporters Receiving ITCM from Irradiated C57BL6 and Balb/c Mice
Medium that was harvested from bladder explants originating from un-irradiated C57BL6 mice failed to induce a calcium flux in reporters (fig 2A). However, ITCM from 2 Gy and 20 mGy irradiated mice (fig 2A) induced a transient increase in intracellular calcium levels that lasted for approximately 130 – 200 seconds. A transient calcium flux was also induced by ITCM taken from C57BL6 mice that were primed with 20 mGy, four hours before a 2 Gy challenge dose (fig 2B). However, ITCM obtained from mice that were primed twenty four hours prior to the challenge dose (24 hour group), showed an initial decrease in intracellular calcium concentration at the time of addition of the medium. This was followed by slight increase in the calcium levels which remained elevated for the remaining 250 seconds (fig 2B). Similarly, ITCM from mice in the 48 hour group (20 +20 +20 mGy followed by 2 Gy, given 48 hours apart) induced an increase in calcium levels at a faster rate and with a greater magnitude then the reporters exposed to the ITCM from mice in he 24 hour group (20 + 2 Gy, 24 hours apart).
The bladder medium from irradiated Balb/c showed a slightly different pattern of calcium induction. ITCM corresponding to the Balb/c mice exposed to 2 Gy of radiation induced a transient calcium flux, where as the ITCM established from 20 mGy and 0 Gy irradiated mice, failed to do so (Fig 2C). ITCM established from Balb/c mice in the 24 hour group (20 mGy + 2 Gy exposures given 24 hours apart), showed a slight increase in the cytosolic calcium concentration that remained elevated (fig 2D). ITCM from mice in the 48 hour group (20 mGy + 20 mGy + 20 mGy + 2 Gy, forty eight hour apart), showed a calcium induction response that was characterized by a transient decrease in calcium levels at the time of medium addition. This was followed by a rapid and persistent increase of intracellular calcium levels that lasted throughout the measurement period (approximately 300 seconds) (Fig 2D). ITCM associated with Balb/c mice from the 24 and the 48 hour group showed a similar magnitude of calcium induction (by a factor of approximately 0.2). However, the ITCM taken from C57BL6 mice in the 24 hour and 48 hour group showed an increase in calcium levels around the magnitude of 0.02 and 0.3 respectively.
DISCUSSION
The results presented by our group provide further evidence that bystander factors are produced in vivo at the time of irradiation, moreover, they are transferred through medium harvested from murine bladder explants to completely unirradiated HPV-G reporter cells. The fact that these signals are able to induce biological responses in vitro, days after production, confirms that not only are these factors long lived, but they are soluble by nature. These findings have been previously reported by various groups such as Mothersill et al. (2005, 2006, 2007) and O’Dowd et al. (2006) which have shown the production of bystander factor(s) after in vivo and in vitro irradiation of various species of mice and fish.
Results clearly demonstrated tremendous variation in the growth response of reporter cells post- ITCM exposure. Acutely irradiated C57BL6 mice were able to produce pro-apoptotic bystander factors at the time of irradiation where as the acutely irradiated Balb/c mice failed to induce a death response in reporter cells. This suggests that genetic background may influence the type of bystander factors that are produced after acute radiation exposures. These results are supported by similar work by Mothersill et al (1999 and 2005) that have also shown genetically dependant variations in biological responses to radiation. The first response was seen in C57BL6 mice bladder explant cells, which showed a pro-apoptotic response that was associated with ‘chromosomal stability’. The second response was characterized by the survival of damaged CBAH explant cells, and was associated with chromosomal instability. By extending this logic to the present results, it is possible to infer that the failure of ITCM from irradiated Balb/c mice to induce reporter cell death could perhaps represent a deregulated response that results from the inability of tissues to assess or detect radiation induced damage. Interestingly, Chen et al. (2005) revealed that Balb/c mice show a higher proportion of immunosuppressive cells (CD4+CD25+) when compared to C57BL6 mice, which resulted in the suppression of autoimmune responses against pathogens and induced tolerance against antigens. This type of an immuno-compromised environment would have difficulties detecting radiation-induced damage in cells, and as a result, would fail to produce factors that would regulate growth of other cells. For example, Lorimore et al. (2001) discussed how genetically stable C57BL6 mice (haemopietic tissues) showed considerable macrophage activation and neutrophil infiltration post in vivo irradiation when compared to the CBA/Ca mice. These cells also serve as a persistent source for various cytokines as well as oxygen/nitrogen species that are known to act as key players in the propagation of bystander mediated effects (Lorimore et al. 2001). Furthermore, macrophages isolated from CBA/Ca mice produced more citrulline and NO, which are powerful inflammatory mediators (Coates et al. 2008) which can produce a damaging microenvironment that may initiate malignant transformation (Wright and Coates 2006). In contrast, the macrophages associated with C57BL6 mice produced polyamines and proline, which act as antioxidants and stimulate tissue regeneration (Coates et al. 2008). In view of this, one can speculate that the survival phenotype seen in reporters post exposure to Balb/c mice ITCM may be the consequence of a dysfunctional immune response that generates more proxidants than antioxidants, thus creating a state of enhanced oxidative stress that promotes various types of pathological conditions such as cancer. In fact, specific types of tumors have been associated with chronic inflammation and production of ROS/NOS by tissue macrophages or neutrophils (Jackson et al. 1989; Weitzman and Gordon 1990). On the other hand, the toxicity of ITCM from irradiated C57BL6 mice served as a regulatory mechanism that promoted cell death so as to eliminate damaged cells from the population.
Repeatedly exposing C57BL6 mice (prime + challenge dose) to radiation caused them to modify the nature of the bystander signal they produced from pro-death (in acutely irradiated mice) to pro-survival. Whether this growth is adaptive or not is unclear at this point. Despite their genetic differences, ITCM from repeatedly exposed Balb/c mice also significantly stimulated colony growth rates (table 1.2) in the corresponding reporters. This suggests that biological response in reporters may be partly modulated by exposure conditions. This supports previous work reported by Seymour and Mothersill (2006) which showed that blood serum (from patients undergoing radiotherapy) samples that initially produced the greatest bystander effect also showed the greatest adaptive response when blood serum was re-sampled six weeks post the last therapy treatment. These results allow for the conclusion that bystander factor(s) produced by the tissue are being modified as a result of repeated exposures, in that they induce radio resistance that may or may not be adaptive. However, our group’s data show that genetic background of the C57BL6 and Balb/3 mice were such that in response to chronic radiation, they both elicited the production of distinct pro-survival type signals (despite the fact that they produced contrasting type of signals after acute dose exposures). It is possible that level of adaptive response seen after initial damage is dependent on prior exposure history in the C57BL6 mice but this is not the case with Balb/c mice. Clearly there are different bystander signals being produced depending not only on genetics but also on treatment regime.
TABLE 1.2.
Growth Differences in Reporters given ITCM from Irradiated Balb/c Mice
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 |
|---|---|---|---|---|---|---|---|---|
| 0 Gy | 1 | 1 | 1 | 3 | 2 | 3 | 3 | 3 |
| 20 mGy | 1 | 1 | 1 | 3 | 2 | 3 | 3 | 3 |
| 2 Gy | 1 | 1 | 1 | 2 | 2 | 3 | 3 | 3 |
| (20 mGy + 2 Gy) 4 Hours Apart | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 |
| (20 mGy + 2 Gy) 24 Hours Apart | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| (20 mGy + 20 mGy + 20 mGy + 2 Gy) 48 Hours Apart | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
Individual colony area values were determined using automated algorithms performed on serial photographs of reporter colonies. This allowed for the tracking of colony area growth over the course of the incubation period. The averaged colony area values were compared using ANOVA (p<0.05) across each treatment group on each day of incubation. Numbers (1 through 4, where 1 represents the largest average colony area and 4 being the smallest average colony area) are designated to each treatment group so as to indicate a) statistically significant differences in colony area values on each day of incubation and b) which treatment posses the largest versus the smallest colonies (growth speed). For example, on days 1–3, average colony area for each treatment was statistically similar across all the treatments. However, by day 8, reporters exposed to ITCM from acutely irradiated (0 Gy, 20 mGy, and 2 Gy) Balb/c mice showed a similar decrease in average area growth values whereas the reporters exposed to ITCM from mice in the 24 hour group showed the largest area values.
Results also indicate that calcium induction in response to in vivo generated bystander signals also exhibit genotypic differences. Acutely irradiated C57BL6 mice caused enhanced cell killing that was also accompanied by transient calcium signaling, where as acutely irradiated Balb/c mice failed to induce a pro-apoptotic response in reporters despite the presence of transient calcium signaling (2 Gy Group). Interestingly, after day 3 of incubation, a significant decrease in colony growth was detected in this group of reporters (table 1.2). This suggests that the genetic environment of Balb/c mice does in fact allow for the production of bystander signals, however, these factors utilize calcium pulse signaling as a means to activate alternative cellular pathway(s) that may influence various biological endpoints. This type of variation in bystander signal production has been documented by Mothersill et al. (2005) which showed that irradiated C57BL/6 mice but not CBA/Ca mice produced bystander signals that induced calcium fluxes, loss of mitochondrial potential, and apoptosis in reporter HPV-G keratinocytes. Previously, the presence of a calcium flux has been associated with production of mitochondria-derived reactive oxygen species (Rego and Oliveira 2003), which are vital in the production of radiation induced bystander effects such as apoptosis (Lyng et al., 2000,Lyng et al., 2002a and 2006). Furthermore, increase in ROS levels have also been associated with DNA damage and induction of p53 levels (Hickman et al 1994, Prives and Hall, 2000, Azzam et al., 2001), which in turn regulates cell cycle progression ((Bygrave and Roberts 1995). Radiation has also been documented to induce nitrogen oxide synthase activity (nitric oxide production), which functions to activate epidermal growth factor receptors (EGFR) (Lee et al. 2008) that consequently play a central role in regulating cell division, death, and thus carcinogenesis (Johnston et al. 2006). As a result, the ability of a cell to actually undergo apoptosis as a result of calcium signaling depends on how downstream molecules such as p53 and NO interact with the surrounding environment. It may be that acutely irradiated Balb/c mice are generating signals that utilize calcium as a means for regulating cell cycle checkpoints and growth rates rather than apoptosis. In both types of mice, repeat exposures of radiation resulted in the persistent induction of calcium levels that corresponded with significantly excessive colony growth (table 1.1 and 1.2). In fact, Scholz et al. (2009) discusses various pathologies including carcinogenesis and metastasis that are associated with plasma hypocalcemia. This strongly suggests that stimulation of cell growth elicited by repeated exposures are in fact carcinogenic rather than adaptive in nature.
TABLE 1.1.
Growth Differences in Reporters given ITCM from Irradiated C57BL6 Mice
| Treatment | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|---|---|---|
| 0 Gy | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| 20 mGy | 2 | 2 | 1 | 3 | 4 | 4 | 4 | 4 | 4 |
| 2 Gy | 1 | 1 | 1 | 2 | 4 | 4 | 4 | 4 | 4 |
| (20 mGy + 2 Gy) 4 Hours Apart | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| (20 mGy + 2 Gy) 24 Hours Apart | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (20 mGy + 20 mGy + 20 mGy + 2 Gy) 48 Hours Apart | 2 | 3 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
Serial photography of reporter colonies was used to track area growth over the course of the incubation period. The averaged colony area value was compared using ANOVA (p<0.05) across each treatment group on each day of incubation. Numbers (1 through 4, where 1 represents the largest average colony area and 4 is the smallest average colony area) are designated so as to signify statistical significance as well as growth speed. Looking across the treatments on day 1 of incubation, the 2 Gy and 24 hour group show statistically similar reporter colony area measurements, which are also the largest colonies detected. However, the reporters in the 4 hour treatment group and the reporters exposed to ITCM from the unirradiated controls show the smallest area values, although they are not statistically similar.
Overall, this paper concludes that genetic background can modulate the type of bystander signal that is produced, and these signals may utilize other intracellular signaling mechanisms to induce various types of biological effects. Such variations in bystander responses, especially within in vivo systems are common, thus serving as a reminder of the complexity within and around a biological system. Clearly, more work is needed to fully understand the link between the nature of the bystander signal produced and its manifestation in terms of cellular response, and what that response means on a homeostatic level.
Acknowledgments
This research was made possible through the support of the NSERC Industrial Chairs Programme, the Canada Research Chairs Programme, the NOTE EU Integrated Project, The CANDU Owners Group and Atomic Energy Canada Ltd.
REFERENCES
- Azzam EI, deToledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. Direct Evidence for the participation of gap junction mediated intercellular communication in the transmission of damage signals from alpha particle irradiated to non-irradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besplug J, Burke P, Ponton A, Filowski J, Titov V, Kovalchuk I, Kovalchuk O. Sex and tissue specific differences in low dose radiation induced oncogenic signaling. Int J Radiat Biol. 2005;81:157–68. doi: 10.1080/09553000500103512. [DOI] [PubMed] [Google Scholar]
- Bygrave FL, Roberts HR. Regulation of cellular calcium through signaling cross-talk involves an intricate interplay between the actions of receptors, G-proteins, and second messengers. FASEB J. 1995;9:1297–1303. doi: 10.1096/fasebj.9.13.7557019. [DOI] [PubMed] [Google Scholar]
- Chen X, Oppenheim J, Howard O. Balb/c Mice Have More CD4+CD25+ T regulatory cells and Show Greater Susceptibility to Suppression of their CD4+CD25- responder T cells than C57BL/6 Mice. Journal of Leukocyte Biology. 2005;78:1–8. doi: 10.1189/jlb.0604341. [DOI] [PubMed] [Google Scholar]
- Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype –dependent bystander signaling. Can Res. 2008;68:450–6. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- Crawford DR, Davies JAK. Adaptive Response and Oxidative Stress. Environ Healt Perspec. 1994;102:25–28. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE. Alpha particle induced sister chromatid exchange in normal human lung fibroblasts; evidence for an extranuclear target. Radiat Res. 1996;145:260–267. [PubMed] [Google Scholar]
- Hickman AW, Jaramillo RJ, Lechner JF, Johnson N. Alpha particle induced p53 protein expression in a rat lung epithelial cell strain. Cancer Research. 1994;54:5797–5800. [PubMed] [Google Scholar]
- Hollowell JG, Littlefield LG. Chromosome aberrations induced by plasma from irradiated patients. J S C Med Assoc. 1967;63:437–442. [Google Scholar]
- Ikushima T, Aritomi H, Morisita J. Radioadaptive response: efficient repair of radiation induced DNA damage in adapted cells. Mutat. Res. 1996;358:193–198. doi: 10.1016/s0027-5107(96)00120-0. [DOI] [PubMed] [Google Scholar]
- Jackson JH, Gajewski IU, Schraufstatter PA, Hyslop AF, Furiarelli CG. Damage to the bases in DNA induced by stimulated human neutrophils. J Clin Investig. 1989;84:1644–1649. doi: 10.1172/JCI114342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Navaratbam S, Pitz MW, Maniate JM, Wiehec E, Baust H, Gingerich J, Skliris GP, Murphy LC, Marek Los. Targeting the EGFR pathway for cancer therapy. Current Medicinal Chemistry. 2006;13:1–10. doi: 10.2174/092986706779026174. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium alpha particle irradiation. Nature. 1992;355:738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Burke P, Besplug J, Slovack M, Filkowski J, Pogribny I. Methylation changes in muscle and liver tissues of male and female mice exposed to acute and chronic low dose X ray irradiation. Mutat Res. 2004a;548:75–81. doi: 10.1016/j.mrfmmm.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Ponton A, Filkowski J, Kovalchuk I. Dissimilar genome response to acute and chronic low dose radiation in male and female mice. Mutat Res. 2004b;550:59–72. doi: 10.1016/j.mrfmmm.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Lee HC, An S, Lee H, Woo SH, Jin HO, Seo SK, Choe TB, Yoo DH, Lee SJ, Hong YJ, Park MJ, Rhee CH, Park IC, Hong SI. Activation of epidermal growth factor receptor and its downstream signaling pathway by nitric oxide in response to ionizing radiation. Mol Can Res. 2008;6:996–1002. doi: 10.1158/1541-7786.MCR-08-0113. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Mayhugh BM, Qin Y, Trott K, Mendonca MS. Production of delayed death and neoplastic transformation in CGL cells by radiation induced bystander effects. Radiat Res. 2001;156:251–258. doi: 10.1667/0033-7587(2001)156[0251:poddan]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Kadhim M, Pocock DA, Papworth D, Stevens DL, Goodhead DT, Wright EG. Chromosomal Instability in the descendants of unirradiated surviving cells after alpha- particle irradiation. Proc Natl Acad Sci USA. 1998;95:5730–5733. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects. Oncogene. 2001;20:7085–95. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- Lorimore SA, Wright EG. Radiation induced genomic instability and bystander effects: related inflammatory type response to radiation induced stress and injury? A review. Int J Radiat Biol. 2003;19:15–25. [PubMed] [Google Scholar]
- Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoetic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008;68:8122–6. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Oxidative stress in cells exposed to low levels of Ionizing radiation. Biochem Soc Trans. 2000;29:350–353. doi: 10.1042/0300-5127:0290350. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosim. 2002a;99:162–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from progeny of irradiated cells: A possible mechanism for bystander induced genomic instability. Radiat Res. 2002b;157:365–70. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation induced bystander effects. Radiat Res. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- Maguire P, Mothersill C, McClean B, Seymour C, Lyng FM. Modulation of radiation responses by pre-exposure to irradiated cell conditioned medium. Radiat Res. 2007;167:485–492. doi: 10.1667/RR0159.1. [DOI] [PubMed] [Google Scholar]
- Mitchel RE, Burchart P, Wyatt H. A lower dose threshold for the in vivo protective adaptive response to radiation. Tumorigenesis in chronically exposed normal and Trp53 heterozygous C57BL/6 mice. Radiat Res. 2008;170:765–775. doi: 10.1667/RR1414.1. [DOI] [PubMed] [Google Scholar]
- Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Cell – cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- Mothersill EC, Kiaran JO’Malley, Murphy MD, Seymour BC, Lorimore AS, Wright GE. Identification and characterization of three subtypes of radiation response in normal human urothelial cultures exposed to ionizing radiation. Carcino. 1999;20:2273–2278. doi: 10.1093/carcin/20.12.2273. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Rea D, Wright E, Lorimore S, Murphy D, Seymour C, O’Malley K. Individual variation in the production of a bystander signal following irradiation of primary cultures of normal human urothelium. Carcino. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Lyng F, Seymour C, Maguire P, Lorimore S, Wright E. Genetic Factors Influencing Bystander Signaling in Murine Bladder Epithelium after Low-Dose Irradiation In Vivo. Radiation Research. 2005;163:391–399. doi: 10.1667/rr3320. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour B. Actions of Radiation on living cells in the post-bystander era. EXS. 2006a:159–177. doi: 10.1007/3-7643-7378-4_7. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour B. Radiation induced bystander effects: evidence for an adaptive response to low dose exposures? Dose Response. 2006b;4:283–290. doi: 10.2203/dose-response.06-111.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Seymour B. Radiation induced bystander effects and the DNA paradigm: An “out of field” perspective. Mut Res. 2006c;597:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Bucking C, Smith RW, Agnihotri N, Oneil A, Kilemade M, Seymour CB. Communication of radiation induced stress of bystander signals between fish in vivo. EnvironSciTechnol. 2006;40:6859–64. doi: 10.1021/es061099y. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Smith RW, Agnihotri N, Seymour CB. Characterization of a radiation induced stress response communicated in vivo between zebrafish. Environ Sci Technol. 2007;41:3382–7. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of Sister-Chromatid Exchanges by extremely Low Doses of Alpha Particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- O’Dowd C, Mothersill C, Cairns MT, Austin B, McClean B, Lyng FM, Murphy J. The release of bystander factor(s) from tissue explant cultures of Rainbow Trout (Onchorhynchus mykiss) after exposure to gamma radiation. Radiat Res. 2006;166:611–617. doi: 10.1667/RR0606.1. [DOI] [PubMed] [Google Scholar]
- Parsons WB, Watkins CH, Pease GL, Childs DS. Changes in sternal bone marrow following roentgen ray therapy to the spleen in chronic granulocytic leukemia. Cancer. 1954;7:170–180. doi: 10.1002/1097-0142(195401)7:1<179::aid-cncr2820070120>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol. 1998;74:793–8. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- Prise KM, Folkard M, Michael BD. Radiation induced bystander and adaptive responses in cell and tissue models. Dose Response. 2006;23:263–76. doi: 10.2203/dose-response.06-113.Prise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112–26. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Puck TH, Marcus PI. Action of X rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;10:1563–74. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Scholz DA, Purnell DC, Goldsmith RS, Smith LH, Lawrence R, Arnaud CD. Hypercalcemia and Cancer. A Can J for Clinicians. 2009;25:27–30. doi: 10.3322/canjclin.25.1.27. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Nakamura A, Kovalchuk O, Koturbash I, Mitchell SA, Marino SA, Brenner DJ, Bonner WM. DNA double strand breaks from bystander cells after microbeam irradiation of three dimensional human tissue models. Can Res. 2007;67:4295–302. doi: 10.1158/0008-5472.CAN-06-4442. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander killing environment. Radiat Res. 1997;149:256–262. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Development of an in vivo assay for detection of non targeted radiation effects. Dose Res. 2006;4:277–282. doi: 10.2203/dose-response.06-116.Seymour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto J. Tumor development in the rate induced by the blood of irradiated animals. Nature. 1962;195:1317–1318. doi: 10.1038/1951317a0. [DOI] [PubMed] [Google Scholar]
- Watson GE, Lorimore SA, Macdonald DA, Wright EG. Chromosomal instability in unirradiated cells induced in vivo by a bystander effect of ionizing radiation. Can Res. 2000;60:5608–5611. [PubMed] [Google Scholar]
- Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generation oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- Wright EG, Coates PJ. Untargeted effects of ionizing radiation: implications for radiation pathology. Mutat Res. 2006;597:119–32. doi: 10.1016/j.mrfmmm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Randers-Pehrson G, Waldren CA, Geard CR, Yu AL, Hei TK. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Hong M, Chai Y, Hei TK. Consequences of cytoplasmic irradiation: studies from microbeam. J Radiat Res. 2009;50:A59–65. doi: 10.1269/jrr.08120s. [DOI] [PMC free article] [PubMed] [Google Scholar]