Abstract
2D-NOE and 1H NMR chemical shift data obtained for the title oligonucleotides were compared with similar data previously reported [Broido et al. (1985) Eur. J. Biochem. 150, 117-128] for the unmodified "parent" structure, [d(GGAATTCC)]2. The spectroscopically detectable structural perturbations caused by replacement of phosphate oxygen with sulfur were mostly localized within the GsA moiety, and were greater for the Rp configuration wherein sulfur is oriented into the major groove of the B-helix. UV-derived Tm measurements gave the following order of stability for the duplexes in 0.4 M NaCl: unmodified (33.9 +/- 0.1 degrees C) approximately Sp-Sp (34.1 degrees C) greater than Rp-Rp (31.7 degrees C). The title compounds were prepared by a new and convenient synthetic route which utilized HPLC to separate the diastereomeric O-ethyl phosphorothioate precursors, (Rp)- and (Sp)-d[GG(S,Et)AATTCC], for subsequent de-ethylation by ammonia in water.
Full text
PDF





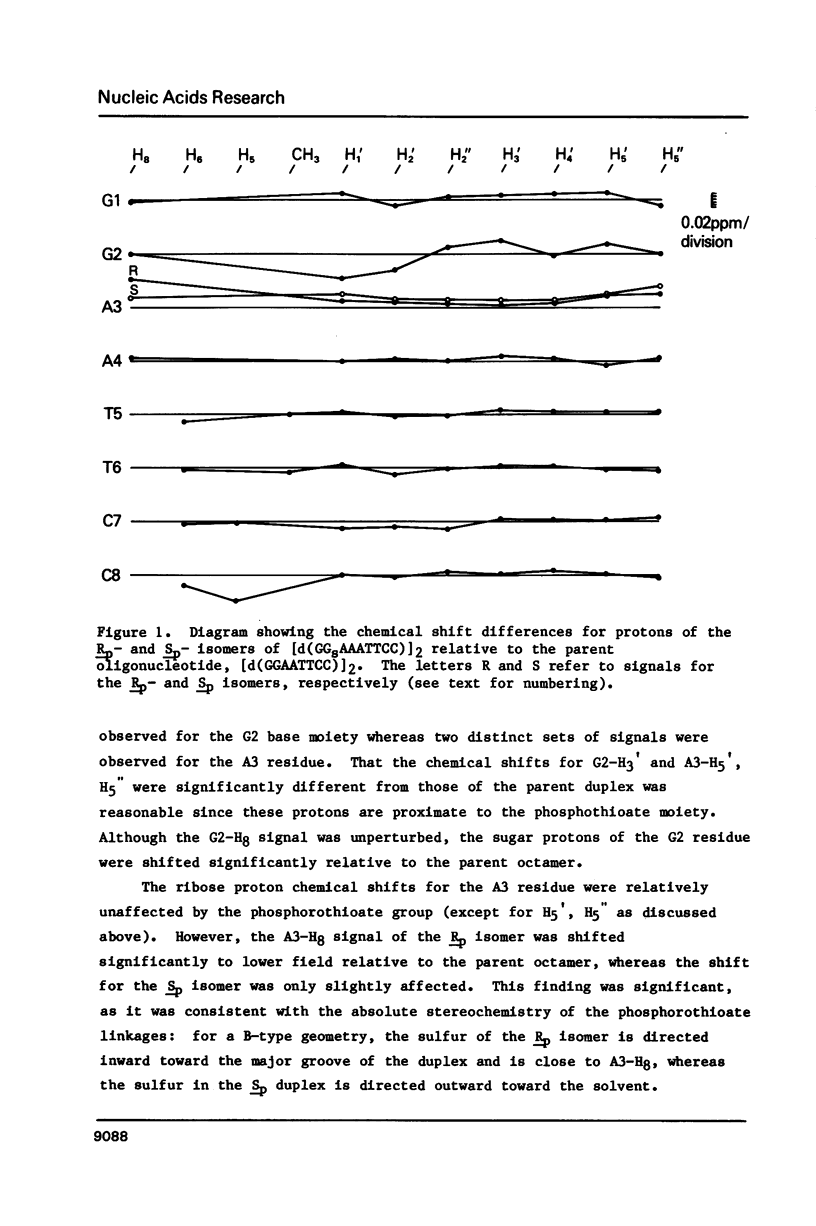



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan C. A., Gumport R. I. T4 RNA ligase catalyzed synthesis of base analogue-containing oligodeoxyribonucleotides and a characterization of their thermal stabilities. Nucleic Acids Res. 1985 Dec 20;13(24):8665–8684. doi: 10.1093/nar/13.24.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
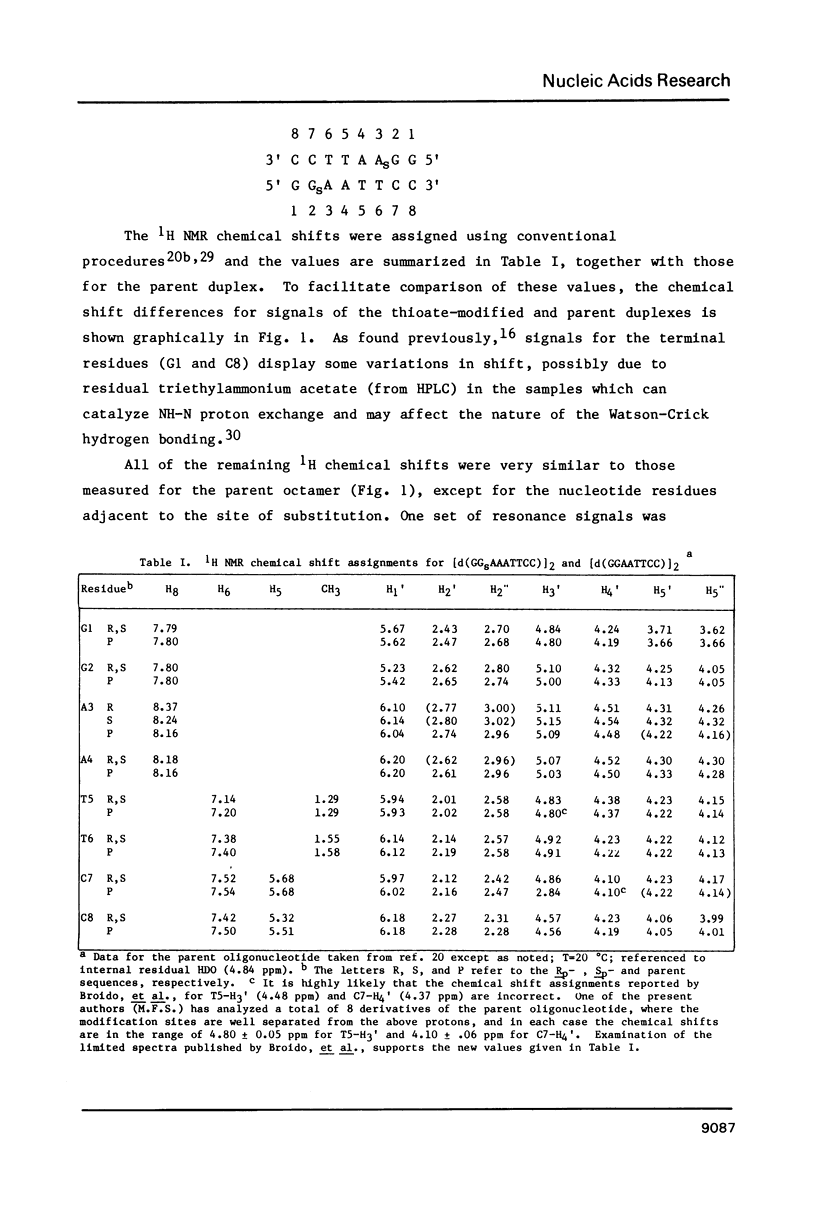
- Broido M. S., James T. L., Zon G., Keepers J. W. Investigation of the solution structure of a DNA octamer [d(GGAATTCC)]2 using two-dimensional nuclear Overhauser enhancement spectroscopy. Eur J Biochem. 1985 Jul 1;150(1):117–128. doi: 10.1111/j.1432-1033.1985.tb08996.x. [DOI] [PubMed] [Google Scholar]
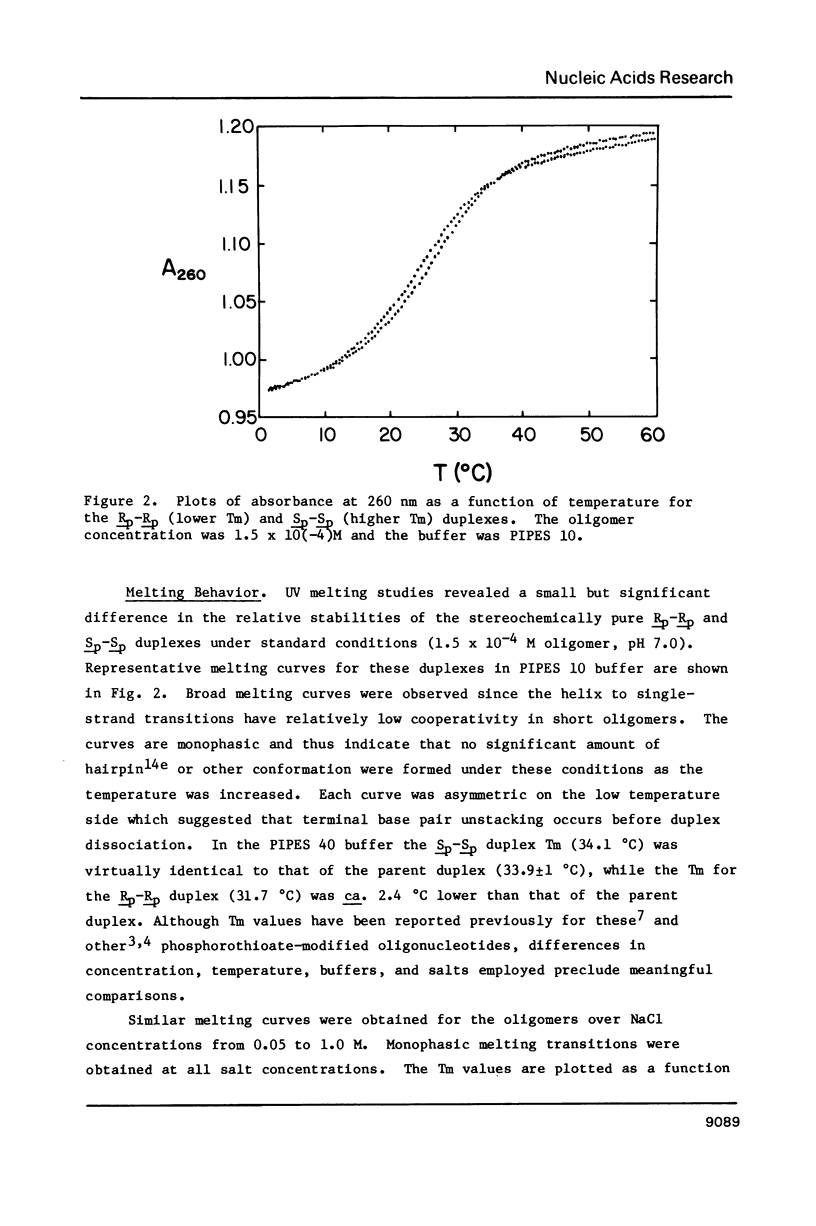
- Broido M. S., Zon G., James T. L. Complete assignment of the non-exchangeable proton nmr resonances of [d-(GGAATTCC)]2 using two-dimensional nuclear overhauser effect spectra. Biochem Biophys Res Commun. 1984 Mar 15;119(2):663–670. doi: 10.1016/s0006-291x(84)80301-0. [DOI] [PubMed] [Google Scholar]
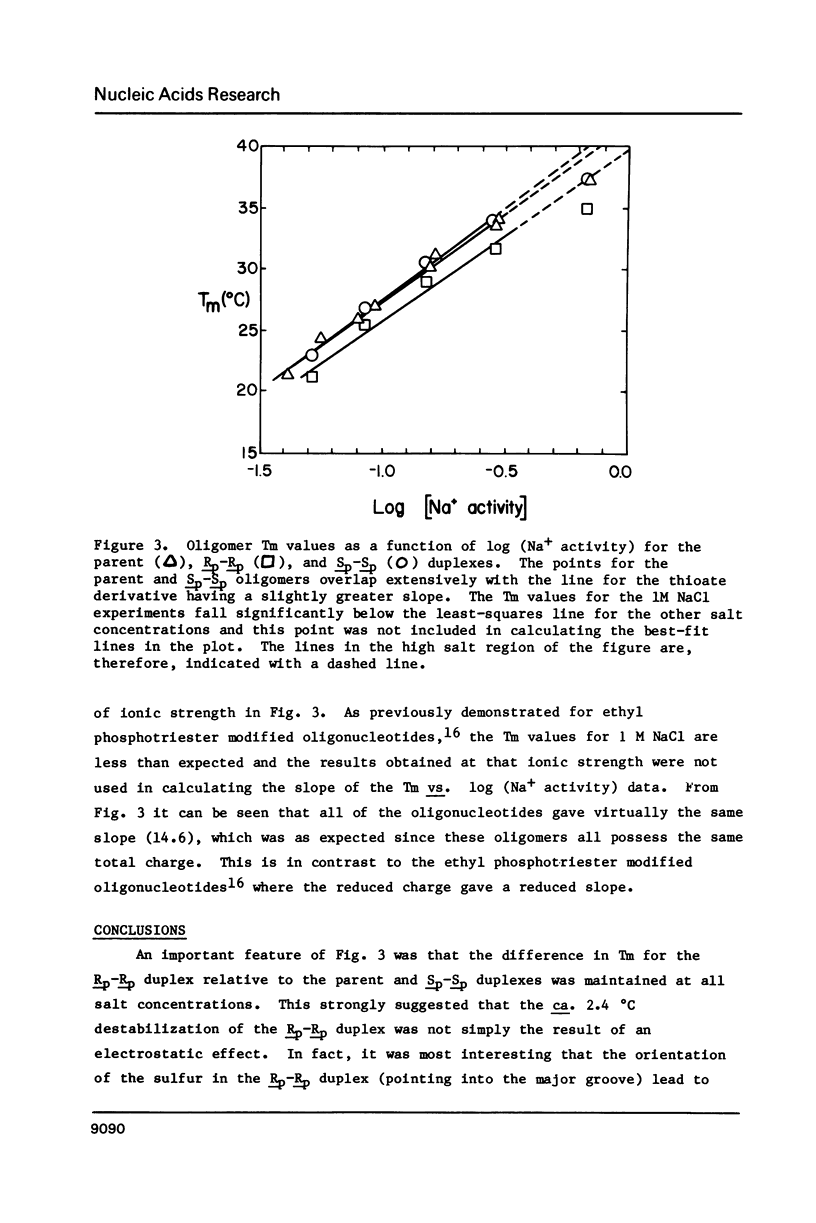
- Connolly B. A., Eckstein F., Pingoud A. The stereochemical course of the restriction endonuclease EcoRI-catalyzed reaction. J Biol Chem. 1984 Sep 10;259(17):10760–10763. [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Cosstick R., Eckstein F. Synthesis of d(GC) and d(CG) octamers containing alternating phosphorothioate linkages: effect of the phosphorothioate group on the B-Z transition. Biochemistry. 1985 Jul 2;24(14):3630–3638. doi: 10.1021/bi00335a035. [DOI] [PubMed] [Google Scholar]
- Dwyer-Hallquist P., Kézdy F. J., Agarwal K. L. Interaction of the HpaI endonuclease with synthetic oligonucleotides. Biochemistry. 1982 Sep 14;21(19):4693–4700. doi: 10.1021/bi00262a027. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Jovin T. M. Assignment of resonances in the phosphorus-31 nuclear magnetic resonance spectrum of poly[d(A-T)] from phosphorothioate substitution. Biochemistry. 1983 Sep 13;22(19):4546–4550. doi: 10.1021/bi00288a030. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Schryver B. B., Marsh Y. V. Stereoconfiguration markedly affects the biochemical and biological properties of phosphorothioate analogs of 2-5A core, (A2'p5')2A. J Biol Chem. 1986 May 5;261(13):5999–6003. [PubMed] [Google Scholar]
- Feigon J., Denny W. A., Leupin W., Kearns D. R. Proton nuclear magnetic resonance investigation of the conformation and dynamics in the synthetic deoxyribonucleic acid decamers d(ATATCGATAT) and d(ATATGCATAT). Biochemistry. 1983 Dec 6;22(25):5930–5942. doi: 10.1021/bi00294a037. [DOI] [PubMed] [Google Scholar]
- Frey P. A., Sammons R. D. Bond order and charge localization in nucleoside phosphorothioates. Science. 1985 May 3;228(4699):541–545. doi: 10.1126/science.2984773. [DOI] [PubMed] [Google Scholar]
- Gallo K. A., Shao K. L., Phillips L. R., Regan J. B., Koziolkiewicz M., Uznanski B., Stec W. J., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. V. Synthesis and absolute configuration of Rp and Sp diastereomers of an ethyl phosphotriester (Et) modified EcoRI recognition sequence, d[GGAA(Et)TTCC]. A synthetic approach to regio- and stereospecific ethylation-interference studies. Nucleic Acids Res. 1986 Sep 25;14(18):7405–7420. doi: 10.1093/nar/14.18.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goppelt M., Langowski J., Pingoud A., Haupt W., Urbanke C., Mayer H., Maass G. The effect of several nucleic acid binding drugs on the cleavage of d(GGAATTCC) and pBR 322 by the Eco RI restriction endonuclease. Nucleic Acids Res. 1981 Nov 25;9(22):6115–6127. doi: 10.1093/nar/9.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goppelt M., Pingoud A., Maass G., Mayer H., Köster H., Frank R. The interaction of the EcoRI restriction endonuclease with its substrate. A physico-chemical study employing natural and synthetic oligonucleotides and polynucleotides. Eur J Biochem. 1980 Feb;104(1):101–107. doi: 10.1111/j.1432-1033.1980.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Wentz B. A., Payne W. L., Jagow J. A., Zon G. DNA colony hybridization method using synthetic oligonucleotides to detect enterotoxigenic Escherichia coli: collaborative study. J Assoc Off Anal Chem. 1986 May-Jun;69(3):531–536. [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Cheng D. M., Miller P. S., Yano J., Ts'o P. O. Proton nuclear magnetic resonance studies on dideoxyribonucleoside methylphosphonates. Biochemistry. 1980 May 13;19(10):2122–2132. doi: 10.1021/bi00551a020. [DOI] [PubMed] [Google Scholar]
- Marugg J. E., van den Bergh C., Tromp M., van der Marel G. A., van Zoest W. J., van Boom J. H. Synthesis of phosphorothioate-containing DNA fragments by a modified hydroxybenzotriazole phosphotriester approach. Nucleic Acids Res. 1984 Dec 11;12(23):9095–9110. doi: 10.1093/nar/12.23.9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Ishino Y., Ibaraki K., Ikehara M. Recognition by restriction endonuclease EcoRI of deoxyoctanucleotides containing modified sugar moieties. Eur J Biochem. 1984 Mar 15;139(3):447–450. doi: 10.1111/j.1432-1033.1984.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L. Helix-coil transition of the self-complementary dG-dG-dA-dA-dT-dT-dC-dC duplex. Eur J Biochem. 1979 May 15;96(2):267–276. doi: 10.1111/j.1432-1033.1979.tb13037.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Kozlowski S. A., Gaffney B. L., Jones R. A. Structural studies of the O6meG.T interaction in the d(C-G-T-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986 Mar 11;25(5):1036–1042. doi: 10.1021/bi00353a013. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Romaniuk P. J., Eckstein F. Stereochemical course of DNA hydrolysis by nuclease S1. J Biol Chem. 1983 Feb 10;258(3):1758–1760. [PubMed] [Google Scholar]
- Romaniuk P. J., Eckstein F. A study of the mechanism of T4 DNA polymerase with diastereomeric phosphorothioate analogues of deoxyadenosine triphosphate. J Biol Chem. 1982 Jul 10;257(13):7684–7688. [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Seela F., Driller H. Palindromic oligonucleotides containing 7-deaza-2'-deoxyguanosine: solid-phase synthesis of d[(p)GG*AATTCC] octamers and recognition by the endodeoxyribonuclease EcoRI. Nucleic Acids Res. 1986 Mar 11;14(5):2319–2332. doi: 10.1093/nar/14.5.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec W. J., Zon G., Uznanski B. Reversed-phase high-performance liquid chromatographic separation of diastereomeric phosphorothioate analogues of oligodeoxyribonucleotides and other backbone-modified congeners of DNA. J Chromatogr. 1985 Jun 19;326:263–280. doi: 10.1016/s0021-9673(01)87452-5. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Zon G., Pastor R. W. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4469–4473. doi: 10.1073/pnas.83.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs J. W., Taylor D. A. Evidence for sequence-specific conformational changes in DNA from the melting temperatures of DNA phosphorothioate derivatives. Nucleic Acids Res. 1985 Aug 12;13(15):5707–5716. doi: 10.1093/nar/13.15.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Byrd R. A., Gallo K. A., Samson C. J., Zon G., Egan W. Nuclear magnetic resonance and circular dichroism studies of a duplex--single-stranded hairpin loop equilibrium for the oligodeoxyribonucleotide sequence d(CGCGATTCGCG). Nucleic Acids Res. 1985 Sep 11;13(17):6375–6386. doi: 10.1093/nar/13.17.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Powell C., Egan W., Byrd R. A., Wilson W. D., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. VI. NMR and UV spectroscopic studies of ethyl phosphotriester (Et) modified Rp-Rp and Sp-Sp duplexes, (d[GGAA(Et)TTCC])2. Nucleic Acids Res. 1986 Sep 25;14(18):7421–7436. doi: 10.1093/nar/14.18.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Sequence-specific recognition of DNA: assignment of nonexchangeable proton resonances in the consensus Pribnow promoter DNA sequence by two-dimensional NMR. Biochemistry. 1984 May 8;23(10):2262–2268. doi: 10.1021/bi00305a027. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Stereochemistry of the guanyl nucleotide binding site of transducin probed by phosphorothioate analogues of GTP and GDP. Biochemistry. 1985 Dec 31;24(27):8094–8101. doi: 10.1021/bi00348a039. [DOI] [PubMed] [Google Scholar]


