Abstract
CD40 ligand (CD40L or CD154) is regulated at the posttranscriptional level by an activation-induced process that results in a highly stable transcript at extended times of T cell activation. Transcript stability is mediated by polypyrimidine tract-binding protein (PTB)-containing complexes (Complex I and II) that bind to three adjacent CU-rich sequences within the 3′ untranslated region (3′UTR). To assess the role of PTB in the expression and distribution of CD40L mRNA, PTB was targeted using shRNA in both primary T cells and a T cell line that recapitulates the stability phase of regulated CD40L mRNA decay. PTB knockdown resulted in a marked decrease in the mRNA stability that resulted in lowered CD40L surface expression. PTB was also critical for appropriate distribution of CD40L mRNA between the nucleus and cytoplasm and in the cytoplasm between the cytosol and the translating polysomes. The activation-induced formation of PTB-specific RNP complexes was observed only with cytoplasmic and not nuclear PTB indicating functional differences in the protein defined by cellular localization. Finally, we observed that cytoplasmic and nuclear PTB isoforms were differentially modified relative to each other and that the changes in cytoplasmic PTB were consistent with activation-induced phosphorylation. Together this work suggests that distinctly modified PTB regulates CD40L expression at multiple steps by 1) retaining CD40L mRNA in the nucleus, 2) directly regulating mRNA stability at late times of activation and 3) forming a ribonuclear complex that preferentially associates with translating ribosomes thus leading to an enhanced level of CD40L protein.
Introduction
The interaction of CD40 ligand (CD40L, CD154), expressed primarily on CD4+ T cells, with CD40 on antigen presenting cells (APCs) is a critical event resulting in the activation of select pathways in both innate and adaptive immunity (1-3). Early work on CD40-CD40L interactions underscored the critical role of this receptor-ligand pair in humoral immunity through the initiation of signaling pathways required for B cell proliferation, antibody class switching, somatic hypermutation, memory cell development, enhanced expression of costimulatory molecules and survival (4-7). Subsequent work revealed the importance of CD40L in the priming of CD4+ T cells and enhanced innate responses through the direct interaction with CD40 expressed on macrophage and dendritic cells (DCs). Recent work has also supported a role for CD40 signaling in augmenting TOLL-like receptor (TLR) responses leading to the enhanced secretion of multiple cytokines and chemokines by a variety of cell types (1, 7).
The near constitutive nature of CD40 expression on APCs necessitates that regulation of signaling pathways occurs primarily through modulated CD40L expression. Although CD40L transcription is rapidly induced following T cell activation, posttranscriptional processes also have a critical role in regulating CD40L gene expression throughout T cell activation (reviewed in (8)). Since lymphocyte activation is characterized by transitions between different checkpoints, diversification at the level of mRNA stability provides a unique mechanism to specifically regulate the expression of a number of responding genes (8, 9). The stability of the CD40L message in both human and mouse CD4+ T cells significantly increases with activation either through prolonged signaling through the TCR or via exposure to PMA/ion (10, 11). Transcript stability is mediated by the two complexes (Complex I and II) that bind to cis-acting elements within the 3′ untranslated region (UTR) of the CD40L transcript (8, 12, 13). The major binding protein in Complex I and II is the polypyrimidine tract-binding protein (PTB) or hnRNP I which belongs to a family of ribonuclear proteins displaying diverse roles in multiple aspects of RNA metabolism (14). PTB shuttles between the nucleus and cytoplasm and is important for regulating alternative splicing, IRES-mediated translation initiation and enhanced stability of a number of transcripts (15). Cellular localization and/or interactions with specific factors appear to be critical to the specificity of PTB function (15, 16), which is consistent with its role in CD40L stabilization where two additional RNA binding proteins, nucleolin and hnRNP L, have been identified as additional components of Complex I/II that interact both with the RNA and PTB (17-19). Although the relationship between PTB and CD40L mRNA stability has been firmly established, how PTB-mediated regulated decay influences the expression of CD40L protein through T cell activation is less clear.
In this study the effect of downregulating PTB on CD40L mRNA expression and distribution was analyzed both in an activated T cell line (Jurkat/D1.1) and in primary CD4+ T cells. We found that PTB-mediated regulation of CD40L mRNA stability had a considerable effect on levels of surface CD40L in both primary CD4+ T cells and cell lines. In addition, PTB influenced the cellular distribution of CD40L transcript between the nucleus and cytoplasm as well as within the polysomal cytoplasmic fraction. Finally, PTB was differentially modified as a function of its distribution within the nucleus and cytoplasm and in response to stimulation, suggesting a link between T cell activation, PTB modification and CD40L expression.
Materials and Methods
Antibodies and cell lines
The anti-human-PTB mAb (ATCC number: CRL-2501) was purified as previously described (20). Anti-β-actin (C-11), Anti-PARP, and anti-nucleolin (C23 antibody 4E2) mAbs was purchased from Santa Cruz Biotechnologies. Anti-S6 ribosomal protein (54D2) mAb was purchased from Cell Signaling. Anti-DDK (Flag antibody 4C5) mAb was purchased from Origene. Anti-human-CD154 (24-31) was purchased from Biolegend. The Jurkat/D1.1 cell line was obtained from Dr. S. Lederman (Columbia University, NY) and cultured as previously described (12). The Flag-PTB-Jurkat/D1.1 line was generated by the stable expression of the vector pCDNA.3 expressing PTB-1 downstream of the Flag epitope.
Short hairpin RNA (shRNA) knockdown and analysis of PTB
The pLV-CTRL and pLV-PTB together with virus production techniques have been previously described (21). Briefly, the following pairs of primers U6-shPTB: 5′-GAT CCA ACT TCC ATC ATT CCA GAG AAC TTG CTT CTT CTC TGG AAT GAT GGA AGT TTT TTT TGG AAA-3′ (forward) and 5′-AGC TTT TCC AAA AAA AAC TTC CAT CAT TCC AGA GAA GAA GCA AGT TCT CTG GAA TGA TGG AAG TTG-3′ (reverse); and U6-shCTRL: 5′-GAT CCA ATC AGA CGT GGA CCA GAA GAG AGA TCT TCT GGT CCA CGT CTG ATT TTT TTT GGA AA-3′ (forward) and 5′-AGC TT TTC CAA AAA AAA TCA GAC GTG GAC CAG AAG ATC TCT CTT CTG GTC CAC GTC TGA TTG-3′ (reverse) were cloned into the HindIII and EcoRI sites of the pSilencer2.1-U6 hygro (Ambion). The sequence containing the U6 promoter and shRNA was PCR amplified using the primer set pLVTHM: 5′-CCA TCG ATG GAG CTT TTC CAA AAA AAA CTT-3′ (forward) and 5′-CAG AAA GGT GAC CCC TTA AGC TTC TAG AAG-3′ (reverse). These PCR products were cloned into the pLVTHM vector (Addgene plasmid 12247).
Viral particles were packaged by transfection of 293T cells with pLVTHM-U6-shPTB and pLVTHM-U6-CTRL together with the virus packaging plasmids psPAX2 (Addgene plasmid 12260) and pCI-VSVG (Addgene plasmid 1733), both obtained from D. Trono (Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland), using Fugene. A total of 5×106 Jurkat/D1.1 cells were incubated with lentivirus for 24 h followed by replacement of the conditioned media with RPMI-Complete (RPMI 1640 media supplemented with 10% heat inactivated fetal bovine serum (FBS), 50U/mL penicillin, 50 μg/mL streptomycin and 1mM L-glutamine at 37°C) and 48 h post-infection cells were checked for GFP expression by flow cytometry.
Infection of primary human CD4+ T cells
PBMCs were isolated from freshly drawn blood and CD4+ T isolated using Miltenyi beads. Concentrated control (pLV-CTRL)and PTB (pLV-PTB) lentiviruses were co-cultured with 1 × 106 CD4+ T cells with 0.1 μg/ml phytohemagglutinin (PHA) (Calbiochem) and 100U/mL rIL-2 in RPMI-Complete. Cells were expanded by adding 100 U/ml rIL-2 at 3 days and 50 U/mL rIL-2 every other day following. At 10 days post-activation the cells were placed into RPMI Complete media minus rIL-2 and 3 to 4 days later cells were activated for 2 h with 1ng/mL of PMA and 1μg/mL ionomycin. Cells were stained with anti-CD40L mAb and analyzed by flow cytometry. Intracellular surface mobilization assay was performed as previously described (22). Briefly, CD4+ T cells were infected and prepared as above. Thirty min prior to activation cells were treated with 10 μg/ml cycloheximide. Activation was with 1μg/ml ionomycin, 10 ng/ml PMA with 10 μg/ml cylcoheximide for 30 min.
Steady state expression and decay analyses of the CD40L mRNA
RNA stability was measured by incubating 5 × 106 Jurkat/D1.1 cells with 50 μg/ml 5,6-Dichlorobenzimidizole 1-β-D-ribofuranoside (DRB) and aliquots collected every 30 min for 2 h. Approximately 1ug of RNA was reverse transcribed and real-time PCR performed on an ABI HT7000 PCR Cycler. For quantification of CD40L and the β-actin transcripts the following primer sets were used: CD40L: 5′-TTC CAC CCT GTC CCT ATC TC-3′ (forward) and 5′-CTG GAA ACA ATG GAG ACT GC-3′ (reverse), β-actin: 5′-GCA TCC TCA CCC TGA AGT A-3′ (forward) and 5′-TGT GGT GCC AGA TTT TGT CC-3′ (reverse).
Cell Fractionation and Protein Immunoblots
Total, nuclear and cytoplasmic extracts were prepared as previously described (21) and analyzed using immunoblotting with anti-PTB antibodies at a dilution of 1:3000; and anti-Flag (DDK) antibodies, anti-S6 ribosomal protein antibodies, anti-actin antibodies and anti-nucleolin antibodies at a dilution of 1:1000. Horseradish peroxidase-conjugated secondary antibodies were used for detection by ECL. To obtain the non-polysomal and polysomal proteins and RNA, cell fractionation was performed essentially as previously described (23).
RNA Electromobility Shift Assay (RNA-EMSA) and RNA immunoprecipitation
Isolation of CD4+ T cells, CD3 activation of T cells and preparation of cytoplasmic fractions and RNA-EMSAs were carried out as previously described (18). To analyze the CD40L mRNA bound by PTB, 5 × 106 Jurkat/D1.1 cell equivalents of cytoplasmic and nuclear extracts were incubated O/N at 4°C with Agarose-A/G beads bound to anti-PTB antibodies in NT2 Buffer (50mM Tris (pH 7.4), 150mM NaCl, 1mM MgCl2 and 0.05% NP-40). The beads were washed 6× with NT2 buffer and resuspended in 100 μl NT2 buffer with 0.1% SDS and 30μg of Proteinase K, followed by incubation at 55 °C for 3 h. RNA was extracted from the supernatant, reverse transcribed and the cDNA amplified by PCR.
2D Gel Electrophoresis (2D GE) and Phosphatase Assay
Cytoplasmic (20 μg) and nuclear (5 μg) extracts from differentially activated CD4+ T cells were dialyzed against DeStreak Rehydration Solution (GE Healthcare) for 2-4 hours. Isoelectric focusing (IEF) was carried out on samples using 7 cm Immobiline DryStrip Gels with immobilized pH gradient range 6–11. IEF was performed in an IPGphor according to the protocols provided. Following IEF, strips were equilibrated in 50 mM Tris–HCl, pH 8.8, 6 M urea, 2% SDS, 30% glycerol with 1% DTT for 15 min, followed by 15 min in the same buffer without DTT but with 4% iodoacetamide. Equilibrated strips were run through 4-20% linear gradient polyacrylamide gels, transferred to PVDF membranes and immunoblotted with anti-PTB antibodies. Twenty micrograms from Jurkat/D1.1 cytoplasmic extracts were mock-treated or treated with 800U Lambda Phosphatase in 20 μL final volume of 50mM HEPES (7.5), 100mM NaCl, 2mM DTT, 0.01% Brij 35, 2mM MnCl2, and 1× PIC for 4 h. The samples were subsequently dialyzed and focused as described.
Results
PTB is critical for maintaining steady state levels of CD40L mRNA
To determine the effect of attenuated PTB expression on surface CD40L levels, Jurkat/D1.1 cells expressing a highly stable CD40L transcript were infected with a modified pLVTHM-U6 lentiviral vector expressing GFP and either an shRNA against PTB (pLV-PTB) or a scrambled-sequence control (pLV-CTRL). Infection efficiency was consistently between 70 and 100% (GFP positive) using both viruses (data not shown). To control for off target effects, a Jurkat/D1.1 subclone was generated that stably expressed FLAG-tagged PTB-1 from a cDNA lacking the siRNA target sequence within the 3′UTR. This subclone was similarly infected with pLV-PTB and pLV-CTRL viruses and analyzed in parallel with the infected, non-complemented populations. Two days following infection, Jurkat/D1.1 and Flag-PTB-Jurkat/D1.1 cytoplasmic and nuclear fractions were prepared and analyzed for PTB expression using immunoblotting blotting. Cells expressing shPTB had decreased PTB in both fractions with a particularly sharp drop in the cytoplasmic level (20% of shCTRL), which may reflect a preferential distribution of PTB in the nucleus prior to its redistribution into the cytoplasm. In contrast, PTB expression in Flag-PTB-shPTB Jurkat cells was 70% and 60% of that observed for the Flag-PTB-shCTRL cytoplasmic and nuclear fractions, respectively (Fig. 1A). Overall the steady-state levels of CD40L mRNA were reduced more than 50% in cells expressing shPTB, while cells complemented with Flag-tagged PTB had higher (Flag-PTB-shCTRL) or equal (Flag-PTB-shPTB) levels of transcript to the parent cell line (Fig. 1B).
Fig. 1. PTB directly regulates the stability of the CD40L transcript.
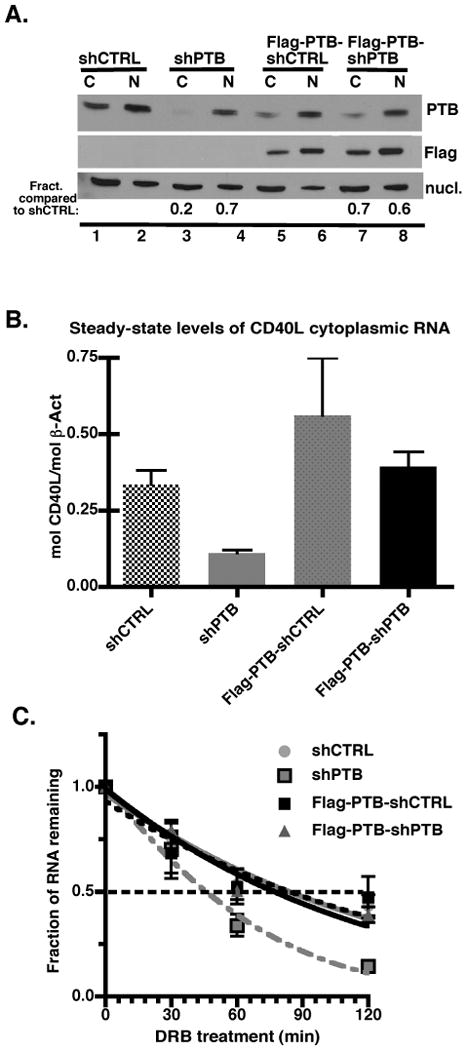
(A). Extracts were prepared from 2 × 104 cell equivalents of Jurkat/D1.1 (lanes 1-4) or Flag-PTB-Jurkat/D1.1 (lanes 5-8) infected with pLV-CTRL (lanes 1, 2, 5, and 6) or pLV-PTB (lanes 3, 4, 7 and 8) and assayed for PTB expression by immunoblotting with anti-PTB mAb. Membranes were stripped and re-hybridized with anti-Flag and anti-nucleolin antibodies. Numbers represent the fraction of PTB in the indicated lanes relative to the appropriate CTRL lane. (B). Steady-state levels of CD40L mRNA were determined by quantitative PCR for Jurkat/D1.1 and Flag-PTB-Jurkat/D1.1 cells infected with either pLV-CTRL or pLV-PTB. Results were normalized to β-actin levels and the average plus the SEM of three independent experiments are presented. (C). To determine the effect of PTB on the decay rate of CD40L mRNA, quantitative PCR was performed on mRNA isolated from infected Jurkat/D1.1 or Flag-PTB-Jurkat/D1.1 cells exposed to the transcriptional inhibitor DRB (50 ug/ml) for 0, 30 min, 60 min and 120 min. Results were normalized to β-actin levels determined at the same time period. Findings represent the Average plus the SEM of three independent experiments.
To confirm that the change in PTB levels affected the decay rate of CD40L mRNA, infected Jurkat/D1.1 cells were treated with the transcriptional inhibitor, 5,6-Dichlorobenzimidizole 1-β-D-ribofuranoside (DRB) and decay measured over a two-hour time course (Fig. 1C). In cells infected with the pLV-PTB virus the CD40L mRNA decayed with a t1/2 of approximately 45 min, which represented a 50% decrease in stability compared to cells infected with control virus. Assessment of Flag-tagged PTB-expressing cells expressing either shPTB or shCTRL revealed degradation profiles very similar to that seen with cells expressing the control shRNA confirming that the Flag-tagged PTB was complementing the decay deficiency seen with shPTB.
CD40L surface expression is regulated by PTB
We next analyzed the effect of PTB on CD40L protein expression in the different Jurkat/D1.1 populations by comparing the Mean Fluorescence Intensity (MFI) 2 days following infection with virus. Jurkat/D1.1 cells expressing shPTB were found to have approximately 65% less CD40L on the surface than control cells (MFI = 314.5 versus 104.5, Fig. 2A, left panel). Analysis of the Flag-PTB-Jurkat populations showed that the clonal Flag-PTB-Jurkat/D1.1 cells expressed an overall lower level of surface CD40L compared to the parent Jurkat/D1.1 cells (right panel). However, the difference in MFI between the shCTRL and shPTB was still evident but greatly reduced to 32% indicating that PTB was influencing the surface expression of CD40L (right panel).
Fig. 2. PTB regulates CD40L surface expression in both Jurkat and Primary T cells.

(A). CD40L expression (x-axis) of Jurkat/D1.1 (left panel) or Flag-PTB-Jurkat/D1.1 cells (right panel) infected with either pLV-CTRL (grey line) or pLV-PTB (black line). The MFI are given for the individual populations and results are representative of 5 independent experiments. (B). Expression of GFP (x-axis) and CD40L (y-axis) was monitored in primary CD4+ T cells infected with pLV-CTRL (top graphs) or pLV-PTB (bottom graphs). Cells were either non-activated (left graphs) or activated (right graphs) with PMA/ion for 2 hours prior to analysis. Within a specific graph, uninfected cells are present in the left quadrants and infected cells are in the right quadrants. Numbers in each quandrant represent MFI for representative populations (C). CD4+ T cells infected with either pLV-CTRL (top graphs) or pLV-PTB (bottom graphs) were treated with (10 ug/ml) cycloheximide for 30 min prior to analysis for CD40L expression without activation (left graphs) or following activation with PMA/ion for 30 min (right graphs). Cells in left quandrants are uninfected and those in the right quandrants are infected. Numbers in each quandrant represent MFI for representative populations.
Because Jurkat/D1.1 cells have an activated phenotype and constitutively express PTB-containing stability complexes, we sought to confirm our findings using primary CD4+ T cells in which the effect of PTB down regulation could be assessed in both resting and activated cells. To this end, primary CD4+ T cells were infected with either virus and surface expression monitored following PMA/ion activation, conditions that promote a highly stable CD40L transcript (10, 24). Examination of CD40L expression within the whole population allowed for PTB function to be analyzed in infected (GFP+) and uninfected (GFP-) cells under identical conditions of activation. Specifically, the non-activated and activated T cells infected with pLV-CTRL (upper graphs) showed similar levels of CD40L expression between infected and non-infected cells (right versus left quadrants). In contrast, cells infected with pLV-PTB (lower graphs) expressed reduced amounts of CD40L in both the non-activated and activated populations (upper right quadrants) compared to the non-infected populations (upper left quadrants) (Fig. 2B). Over several experiments we observed an approximate 9% decrease in expression in non-activated/shPTB infected cells and a 25% decrease in expression in activated/shPTB infected cells compared to uninfected or shCTRL infected cells (Table I).
Table I. PTB is critical for CD40L expression in both resting and activated CD4+ T cells.
| Virus | MFI (CD40L) | % Δ in MFI (Uninfected to infected in same population)a | |
|---|---|---|---|
| Uninfected | Infected | ||
| shCTRL | |||
| Non-Activated | 33.9+/- 0.10 | 35.32+/-1.2 | 4.2% |
| Activated | 245.7+/-40.3 | 260.6+/-29.1 | 6.1% |
| shPTB | |||
| Non-Activated | 43.1+/-4.3 | 39.33+/-3.06 | -8.7% |
| Activated | 271.3+/-24.9 | 204.5+/-17.1 | -24.6% |
|
| |||
| w/cycloheximide | |||
|
| |||
| shCTRL | |||
| Non-Activated | 38.95+/-0.15 | 40.0+/-0.22 | +2.7% |
| Activated | 46.4+/-0.4 | 52.1+/-0.7 | +12.3% |
| shPTB | |||
| Non-Activated | 38.3+/-0.15 | 36.1+/-1.3 | -5.7% |
| Activated | 46.9+/-1.65 | 38.4+/-1.3 | -18.1% |
Primary CD4+ T cells were infected either pLVTHM-shCTRL or pLVTHM-shPTB virus in the presence of IL-2 and PHA. 10 days later cells were either untreated (top rows) or treated with cycloheximide for 30 min (bottom rows). Cells were then left unactivated for 2 h or activated with PMA/ion for 2 h (top rows) or 30 min (bottom rows) and analyzed for GFP (infected) and CD40L (activated) expression. The MFI are the average of 3 (top) and 2 (bottom) independent experiments and the numbers in the right column represent the percent change in the average MFI between the uninfected and infected subpopulations in the same culture.
Finally, we asked whether PTB influenced the expression of preformed CD40L in CD4+ T cells by pretreating cells with cycloheximide and measuring the upregulation of CD40L on the surface following activation. Again, a large decrease in the MFI of CD40L expression was observed in T cells infected with pLV-PTB both in the resting and activated states (Fig. 2C and Table I). Whereas these data support a role for PTB stabilization of CD40L mRNA during T cell activation, they also clearly indicate that PTB influences CD40L expression at all times independent of activation-state.
The distribution of CD40L mRNA between the nuclear and cytoplasmic fractions and within the polysome non-polysome fraction is PTB dependent
To identify an additional role for PTB in CD40L expression we used our shRNA expression system to analyze the compartmentalization of CD40L mRNA under restricted and normal PTB expression. Nuclear and cytoplasmic extracts were prepared from virus infected-Jurkat/D1.1 cells or -Flag-PTB-Jurkat/D1.1 cells and cytoplasmic extracts further fractionated into non-polysomal (S130 and free ribosomes) and polysomal fractions. As shown in Fig. 3A, in cells expressing shCTRL, approximately 60% the CD40L transcript was found in the nucleus and 40% associated with the cytoplasm. In contrast, downregulating PTB resulted in a reversal of how the CD40L mRNA was distributed with 45% and 55% found in the nuclear and cytoplasmic fractions, respectively. Evaluation of the Flag-tagged cells revealed a pattern of CD40L mRNA close to that seen with shCTRL confirming that PTB plays a role in the appropriate distribution of the CD40L transcript between the nucleus and cytoplasm.
Fig. 3. PTB is actively involved in the distribution of CD40L mRNA between the nucleus and cytoplasm.
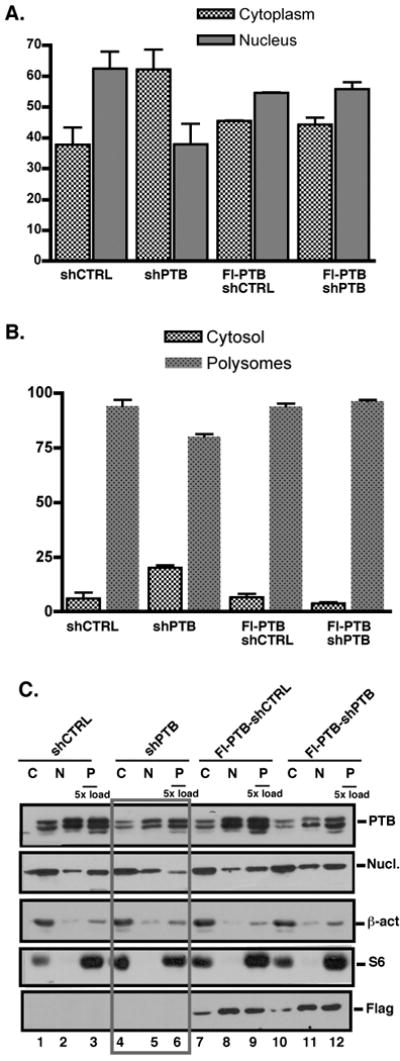
(A). Levels of CD40L mRNA levels in the cytoplasm (grey bars) and nucleus (black bars) of pLV-CTRL and pLV-PTB infected Jurkat/D1.1 and Flag-PTB-Jurkat/D1.1 cells as determined by quantitative PCR. Results represent the Average and SEM of 3 independent experiments. (B) Quantitative PCR analysis of CD40L mRNA from the non-polysomal (white bars) and polysomal (black bars) fractions of pLV-CTRL- and pLV-PTB-infected Jurkat/D1.1 cells and Flag-PTB-Jurkat/D1.1. (C). Western blot analysis using cytoplasmic (2 × 103 cell equivalents), nuclear (2 × 103 cell equivalents) and polysomal (1 × 104 cell equivalents) extracts of Jurkat/D1.1 (lanes 1-6) and Flag-PTB-Jurkat/D1.1 (lanes 7-12) cells infected with pVL-PTB or pVL-CTRL were analyzed for expression of PTB and nucleolin in the different cellular fractions by Western blotting. Membranes were re-probed with antibodies against β-actin and the cytoplasmic ribosomal protein S6 to validate the efficient separation of nuclear, cytoplasmic and polysomal fractions. The membrane was further hybridized with anti-Flag antibodies to confirm expression of the Flag-PTB in the different fractions. Boxed lanes show the effect of PTB downregulation on the different proteins, in particular PTB and nucleolin.
Further analysis of the distribution of CD40L transcripts in the cytoplasm revealed that the vast majority associated with translating polysomes however, under conditions of reduced PTB there was a 20% increase in association with the non-polysomal fraction (Fig. 3B). This skewing was observed in the presence or absence of cycloheximide (CHX) but was significantly more pronounced with CHX treatment (data not shown). Infection of Flag-PTB cultures revealed no difference in the distribution of CD40L between the non-polysomal and polysomal fractions in cells infected with either pLV-CTRL or pLV-PTB.
To extend these findings we analyzed the expression of PTB protein in the nuclear, cytoplasmic and polysomal fractions of cells expressing shPTB and found decreased levels in all three fractions (Fig. 3C, lanes 4-6). Because nucleolin is a second component of Complex I, we also examined its distribution under conditions where PTB levels were reduced. Surprisingly, we observed that much less nucleolin was associated with the polysomes under conditions of targeted PTB (compare lanes 3 and 6) and this decrease in polysome-associated nucleolin was not observed when PTB was complemented by Flag-PTB (compare lanes 9 and 12). This finding strongly suggests that the association of nucleolin with the translating polysomes is dependent, in part on PTB expression.
Complex I binding is distinct in cytoplasmic and nuclear fractions from resting and activated CD4+ T cells
The presence of substantial levels of PTB in both the nucleus and cytoplasm compelled us to ask whether PTB from both fractions complexed to CD40L mRNA. To this end, binding experiments were set up with a probe spanning the CD40L stability element (Xba-Hae) and hybridized with nuclear and cytoplasmic extracts from Jurkat/D1.1 cells and differentially activated CD4+ T cells. We found that strong Complex I binding was observed in both fractions from Jurkat/D1.1 cells (Fig. 4A, lanes 3-5). Similarly, a high level of binding activity was observed in the nuclear fractions from resting and activated T cells from all time periods (lanes 6-9). In contrast and as previously observed, only primary T cells stimulated for 48 h, and not resting or early activated cells, contained a measurable level of complex binding (lanes 10-13). The specific requirement for PTB in Complex I binding was demonstrated by blocking Complex I formation with the addition of anti-PTB antibodies to the binding reaction (lane 15).
Fig. 4. CD40L mRNA stability complexes are constitutively present in the nucleus and only form in the cytoplasm with prolonged CD4+ T cell activation.
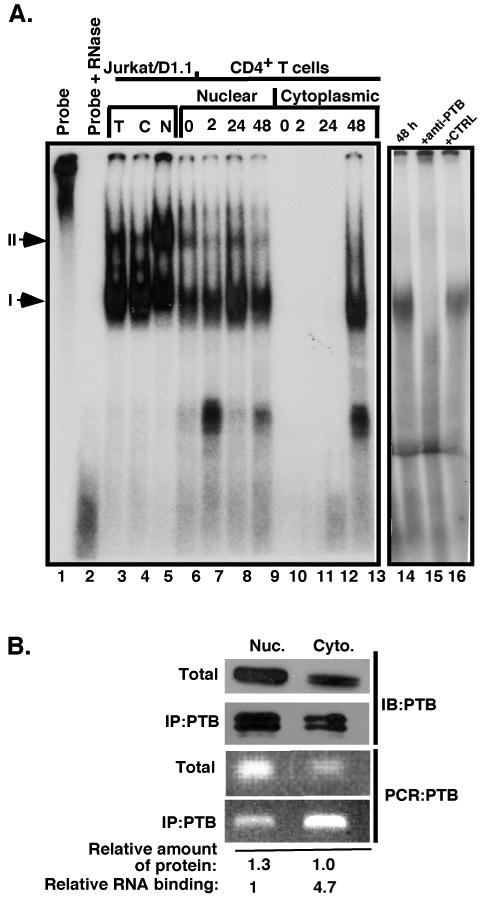
(A) Binding reactions were carried out with the full length CD40L 3′UTR stability element (nucleotides 1300-1609) that was uniformly labeled with 32P-rUTP and either total extract (lane 3), nuclear (lanes 5-9) or cytoplasmic (lanes 4, 10-13) extracts from Jurkat/D1.1 cells (lanes 3-5), resting CD4+ T cells (lanes 6 and 10) and activated CD4+ T cells for the indicated times with anti-CD3 plate-bound antibodies (lanes 7-9 and 11-13). Lane 1, Probe alone; lane 2, probe with RNase alone. CD4+ T cell extract stimulated for 48 h with anti-CD3 mAb (lanes 14-16) was hybridized with probe alone (lane 14) or with probe and anti-PTB mAb (lane 15) or isotype control Ab (lane 16) added to the reaction prior to hybridization to determine presence of PTB in complex. Arrows indicate the location of Complexes I and II. (B). Jurkat/D1.1 nuclear and cytoplasmic extracts from 5 × 106 cell equivalents were separated by SDS-PAGE (top panel) or immunoprecipitated with anti-PTB antibodies and separated by SDS-PAGE (second panel) and immunoblotted with anti-PTB antibodies. RNA was purified from both total and immunoprecipitated samples and used in RT-PCR (lower two panels). The relative amount of protein and level of RNA binding was quantified and indicated under the corresponding samples.
To further confirm that both nuclear and cytoplasmic PTB binds CD40L mRNA and to quantify the binding relative to levels of PTB, analysis of CD40L mRNA was carried out using RNA-immunoprecipitation with anti-PTB antibodies and Jurkat/D1.1 nuclear and cytoplasmic extracts. Following antibody incubation with mAb cross-linked agarose beads, one-half of the eluate was used in Western blotting to analyze PTB binding and the other half was used to isolate RNA for RT-PCR. As shown in Fig. 4B, the pattern of PTB was similar between the total and immunoprecipitated fractions. However, the majority of CD40L mRNA binding activity was associated with the cytoplasmic fraction as evidenced by the amount released from the complex. This was unexpected since the levels of both PTB and CD40L mRNA were significantly higher on a per cell basis in the nuclear fraction. Together these results confirm that the binding of nuclear PTB to CD40L mRNA occurs in the absence of activation and is present in CD4+ T cells at all times following activation. However based on our in vivo binding, PTB-CD40L binding activity is highest in the cytoplasmic fraction. Thus, the complexing of CD40L mRNA with PTB in CD4+ T cells is influenced by both the subcellular localization of complex components as well as by signals mediating T cell activation.
Cytoplasmic PTB is modified in response to activation
The lack of Complex I binding activity in the cytoplasmic fraction from resting and early activated CD4+ T cells could be explained by either the lack of PTB in the cytoplasm or the inability of cytoplasmic PTB to bind transcript due to the absence of specific modifications. To distinguish between these two possibilities cytoplasmic and nuclear extracts were prepared from resting and CD3-activated CD4+ T cells and analyzed by immunoblotting for PTB expression. As predicted from our binding studies, PTB was present in all nuclear fractions over the activation time course (Fig. 5A, lanes 6-8). Surprisingly, PTB was also readily present in the cytoplasmic fractions of unstimulated, 2 h- and 48 h-stimulated cells (lanes 2-4). This finding suggested that the lack of PTB-CD40L mRNA complexing in resting and early-activated T cells was not due to an absence of cytoplasmic PTB but to a potential modification in PTB that favored complex formation.
Fig. 5. PTB from the cytoplasm and nucleus is differentially modified and mediated by activation.

(A). Cytoplasmic (lanes 1-4) and nuclear (lanes 5-8) extracts were prepared from Jurkat/D1.1 cells (lanes 1 and 5) and CD4+ T cells that were unstimulated (lanes 2 and 6) or stimulated with anti-CD3 mAb for 2 h (lanes 3 and 7) or 48 h (lanes 4 and 8). PTB expression was assessed by SDS-PAGE and immunoblotting with anti-PTB antibodies. Membranes were stripped and re-hybridized with antibodies against S6 and PARP (lower two panels) to verify the separation of cytoplasmic and nuclear fractions, respectively. (B). Two dimensional gel electrophoresis (2DGE) was carried out across a pH gradient of 6-11 using cytoplasmic (left panels) and nuclear extracts (right panels) from CD4+ T cells activated for 0, 2, 24 and 48 h with anti-CD3 mAb. Black arrows indicate the major isoforms in each fraction. (C). Cytoplasmic extract from Jurkat/D1.1 cells was either left untreated or treated with 800 U lambda phosphatase for 4 h. prior to separation by 2DGE across a pH gradient of 6-11. PTB was visualized by immunoblotting.
To examine if PTB was differentially modified in response to activation, nuclear and cytoplasmic extracts were prepared from CD4+ T cells stimulated for different time periods and separated using two dimensional gel electrophoresis (2DGE). Following Western blotting with anti-PTB antibodies, we found that the pattern of nuclear and cytoplasmic PTB isoforms was clearly different (Fig. 5B). Specifically, in resting T cells, there were several cytoplasmic isoforms that were spread over a wide pH range and the major bands shifted to the positive pole upon activation. In contrast, the PTB isoforms in the nucleus were more closely aligned, were shifted more toward the negative pole, and did not change position over the activation time course.
To test whether phosphorylation was a factor in the different cytoplasmic isoforms, extract from Jurkat/D1.1 cells was either untreated or treated with phosphatase prior to subjection to 2DGE. As shown in Fig. 5C, we observed distinct shift in the PTB isoform to the negative pole after phosphatase treatment indicating that phosphorylation accounts for some if not all the isoform modifications in this specific cytoplasmic fraction. Together these findings provide compelling evidence that phosphorylation of cytoplasmic PTB isoforms is linked to cellular activation and that the population in the nucleus and cytoplasm are differentially modified suggesting they represent distinct functional subsets.
Discussion
The synergy of transcriptional and posttranscriptional processes is a hallmark of dynamically regulated immune-specific genes that ensures the rapid adjustment of critical inflammatory molecules in response to pathogenic challenges (reviewed in (25-27)). Accordingly, appropriate steady-state levels of CD40L are defined by a still unique post-transcriptional control mechanism involving ribonuclear complexing at defined regions within the 3′ UTR region at discrete times of T cell activation (8). Previous work identified a role for PTB in CD40L mRNA stability and our current work extends these findings by demonstrating that PTB regulates CD40L mRNA decay, which directly impacts the surface expression of the protein. Importantly, examination of the distribution of CD40L mRNA revealed that optimal PTB levels are required for maintaining appropriate levels of both the cytoplasmic pool as well as the polysomal subpopulation. Therefore, these findings highlight a role for PTB in CD40L expression beyond its role in regulating transcript stability.
A large body of work has focused on understanding ARE-regulated mRNA decay, a mechanism utilized by a vast number of genes involved in the inflammatory response (i. e. TNF-α, GM-CSF, IL-2, and IL-10) and results in the rapid turnover of ARE-containing transcripts (28-31). Conversely, a program of posttranscriptional regulation that further extends the t1/2 of relatively long-lived transcripts represents an energy efficient mechanism to sustain expression of a particular protein (32). The PTB-dependent phase of CD40L expression falls into this second type of regulation and coincides with processes that occur at more advanced stages of the humoral response, such as germinal center formation and the generation of differentiated plasma cells and memory B cells (33). Similar to what has been shown with BCR signaling (34-38), duration and quality of CD40 signals appear to directly affect differentiation outcomes of antigen-selected B cells as well as other APCs (34, 39-43). Thus, the level and duration of CD40 signaling is critical for fate decisions in responding populations and PTB is directly linked to these decisions by its ability to regulate CD40L expression at extended times of T cell activation.
The fact that PTB-containing complexes are constitutively active in the nucleus but active only at late times of activation in the cytoplasm is consistent with the different subsets of complexes being functionally distinct. It has previously been shown that PTB acts as a chaperone for nuclear-associated viral RNAs transported to the nuclear pore (44, 45). If PTB functions in a similar way and is required to bring CD40L mRNA to the nuclear pore for export, one prediction would be that reducing levels of nuclear PTB would result in an increased accumulation of nuclear CD40L mRNA. However, our findings indicate that decreasing nuclear PTB results in an increase in cytoplasmic CD40L transcripts, suggesting that PTB functions to retain CD40L mRNA in the nucleus and that when PTB nuclear levels are reduced, there is an influx of CD40L mRNA into the cytoplasm. This RNA may be highly susceptible to degradation especially under conditions where PTB is limiting. These results complement other findings showing that shuttling of PTB between the nucleus and cytoplasm is independent of RNA binding (46).
Our finding that decreased levels of PTB also resulted in reduced association of CD40L mRNA with the polysomal fraction suggests that another function of PTB is to enhance the translational efficacy of specific transcripts. This finding is consistent with the known role of PTB in viral and endogenous IRES translation (15) as well as the fact that binding of hnRNPL, which is a component of Complex II that binds to both PTB and Site C in the stability element (18), alters the translation of the CD40L transcript (19). The fact that reducing PTB in the cell also resulted in a decreased level of nucleolin associated with the polysomes suggests a requirement for interaction between these two factors in translation; a finding extending previous reports of PTB and nucleolin interactions during posttranscriptional processes (17, 47).
From our data we hypothesize that levels of PTB regulate crucial nuclear and cytoplasmic events leading to optimal CD40L expression (Fig. 6). Specifically, at early times of T cell activation when transcriptional levels of RNA are at their highest, nuclear-specific PTB complexes retain only a portion of the CD40L message in the nucleus whereas the vast majority is transported into the cytoplasm in the absence of complex. While the turnover of this “naked” transcript is rapid, the high level of transcription along with the release of preformed protein stores (22, 48) lead to significant levels of CD40L expressed on the cell surface. With continued activation, there are specific modifications of cytoplasmic PTB that result in Complex I/II-CD40L RNA ribonuclear complexes in the cytoplasm. Precedence for functional modifications comes from work on both PTB and other RNA binding proteins. Whereas, the nucleocytoplasmic distribution of PTB has been linked to direct PKA-dependent phosphorylation of Ser16 (49, 50) the stability of many ARE-regulated transcripts appear to be controlled by signaling through p38 MAPK-specific phosphorylation events that result in the cellular redistribution of specific RNA binding proteins including tritetraprolin (TTP) (9). Thus, pathways involved in mRNA transport, localization, turnover and potentially translation intersect with PTB expression and ultimately these PTB-mediated events may be highly dependent on pathways induced by T cell activation.
Fig. 6. Working model of PTB-mediated regulation of CD40L expression.
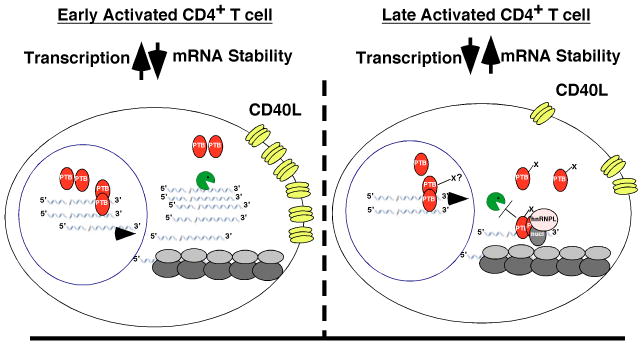
CD40L expression during a time course of CD4+ T cell activation can be divided into an early period (2-12 h) and late period (beyond 24 h) with respect to CD40L RNA turnover. During the early stages of activation (left panel) high CD40L surface expression is transcription-dependent and coincident with a rapid decay rate of the message. At early time points, nuclear, but not cytoplasmic PTB is capable of forming complexes with CD40L RNA. With extended periods of activation (right panel), transcription is decreased and cytoplasmic PTB becomes both modified and competent to form Complex I (along with nucleolin and hnRNP-L). It is at this point that the CD40L mRNA is stabilized by PTB-containing complexes. In addition, the ribonuclear complex containing CD40L RNA preferentially associates with translating polysomes together resulting in both increased RNA stability and protein expression.
Acknowledgments
We thank Lauren Martin for generating the PTB-FLAG construct and Frank Sinquett for excellent technical assistance.
References
- 1.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 3.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 5.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 6.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 7.van Kooten C. Immune regulation by CD40-CD40-l interactions - 2; Y2K update. Front Biosci. 2000;5:D880–693. doi: 10.2741/kooten. [DOI] [PubMed] [Google Scholar]
- 8.Vavassori S, Covey LR. Post-transcriptional regulation in lymphocytes: the case of CD154. RNA Biol. 2009;6:259–265. doi: 10.4161/rna.6.3.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353–359. doi: 10.1038/ni1584. [DOI] [PubMed] [Google Scholar]
- 10.Ford GS, Barnhart B, Shone S, Covey LR. Regulation of CD154 (CD40 ligand) mRNA stability during T cell activation. J Immunol. 1999;162:4037–4044. [PubMed] [Google Scholar]
- 11.Vavassori S, Shi Y, Chen CC, Ron Y, Covey LR. In vivo post-transcriptional regulation of CD154 in mouse CD4+ T cells. Eur J Immunol. 2009;39:2224–2232. doi: 10.1002/eji.200839163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnhart B, Kosinski PA, Wang Z, Ford GS, Kiledjian M, Covey LR. Identification of a complex that binds to the CD154 3′ untranslated region: implications for a role in message stability during T cell activation. J Immunol. 2000;165:4478–4486. doi: 10.4049/jimmunol.165.8.4478. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton BJ, Genin A, Cron RQ, Rigby WF. Delineation of a novel pathway that regulates CD154 (CD40 ligand) expression. Mol Cell Biol. 2003;23:510–525. doi: 10.1128/MCB.23.2.510-525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhury A, Chander P, Howe PH. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: Focus on hnRNP E1's multifunctional regulatory roles. RNA. 2010;16:1449–1462. doi: 10.1261/rna.2254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawicka K, Bushell M, Spriggs KA, Willis AE. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem Soc Trans. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 16.Auweter SD, Allain FH. Structure-function relationships of the polypyrimidine tract binding protein. Cell Mol Life Sci. 2008;65:516–527. doi: 10.1007/s00018-007-7378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh K, Laughlin J, Kosinski PA, Covey LR. Nucleolin is a second component of the CD154 mRNA stability complex that regulates mRNA turnover in activated T cells. J Immunol. 2004;173:976–985. doi: 10.4049/jimmunol.173.2.976. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin J, Oghlidos S, Porter JF, Matus-Nicodemos R, Sinquett FL, Marcelli V, Covey LR. Functional analysis of a tripartite stability element within the CD40 ligand 3′ untranslated region. Immunology. 2008;124:368–379. doi: 10.1111/j.1365-2567.2007.02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton BJ, Wang XW, Collins J, Bloch D, Bergeron A, Henry B, Terry BM, Zan M, Mouland AJ, Rigby WF. Separate cis-trans pathways post-transcriptionally regulate murine CD154 (CD40 ligand) expression: a novel function for CA repeats in the 3′-untranslated region. J Biol Chem. 2008;283:25606–25616. doi: 10.1074/jbc.M802492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin D, Zhang L, Wang R, Radvanyi L, Haudenschild C, Fang Q, Kehry MR, Shi Y. Ligation of CD28 in vivo induces CD40 ligand expression and promotes B cell survival. J Immunol. 1999;163:4328–4334. [PubMed] [Google Scholar]
- 21.Porter JF, Vavassori S, Covey LR. A polypyrimidine tract-binding protein-dependent pathway of mRNA stability initiates with CpG activation of primary B cells. J Immunol. 2008;181:3336–3345. doi: 10.4049/jimmunol.181.5.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koguchi Y, Thauland TJ, Slifka MK, Parker DC. Preformed CD40 ligand exists in secretory lysosomes in effector and memory CD4+ T cells and is quickly expressed on the cell surface in an antigen-specific manner. Blood. 2007;110:2520–2527. doi: 10.1182/blood-2007-03-081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerner RS, Seiser RM, Zheng T, Lager PJ, Reedy MC, Keene JD, Nicchitta CV. Partitioning and translation of mRNAs encoding soluble proteins on membrane-bound ribosomes. RNA. 2003;9:1123–1137. doi: 10.1261/rna.5610403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez A, Mozo L, AbelGayo, Zamorano J, Gutierrez C. Requirement of a second signal via protein kinase C or protein kinase A for maximal expression of CD40 ligand. Involvement of transcriptional and posttranscriptional mechanisms. Eur J Immunol. 1997;27:2822–2829. doi: 10.1002/eji.1830271112. [DOI] [PubMed] [Google Scholar]
- 25.Clark AR, Dean JL, Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546:37–44. doi: 10.1016/s0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]
- 26.Clark A. Post-transcriptional regulation of pro-inflammatory gene expression. Arthritis Res. 2000;2:172–174. doi: 10.1186/ar83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoecklin G, Anderson P. Posttranscriptional mechanisms regulating the inflammatory response. Adv Immunol. 2006;89:1–37. doi: 10.1016/S0065-2776(05)89001-7. [DOI] [PubMed] [Google Scholar]
- 28.Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- 29.Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol. 2009;10:281–288. doi: 10.1038/ni.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 31.Kruys V, Marinx O, Shaw G, Deschamps J, Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989;245:852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- 32.Russell JE, Morales J, Liebhaber SA. The role of mRNA stability in the control of globin gene expression. Prog Nucleic Acid Res Mol Biol. 1997;57:249–287. doi: 10.1016/s0079-6603(08)60283-4. [DOI] [PubMed] [Google Scholar]
- 33.Guzman-Rojas L, Sims-Mourtada JC, Rangel R, Martinez-Valdez H. Life and death within germinal centres: a double-edged sword. Immunology. 2002;107:167–175. doi: 10.1046/j.1365-2567.2002.01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson LD, Durell BG, Vogel LA, O'Connor BP, Cascalho M, Yasui T, Kikutani H, Noelle RJ. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J Clin Invest. 2002;109:613–620. doi: 10.1172/JCI14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connor BP, Vogel LA, Zhang W, Loo W, Shnider D, Lind EF, Ratliff M, Noelle RJ, Erickson LD. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J Immunol. 2006;177:7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benson MJ, Erickson LD, Gleeson MW, Noelle RJ. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr Opin Immunol. 2007;19:275–280. doi: 10.1016/j.coi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson LD, Vogel LA, Cascalho M, Wong J, Wabl M, Durell BG, Noelle RJ. B cell immunopoiesis: visualizing the impact of CD40 engagement on the course of T cell-independent immune responses in an Ig transgenic system. Eur J Immunol. 2000;30:3121–3131. doi: 10.1002/1521-4141(200011)30:11<3121::AID-IMMU3121>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart R, Wei W, Challa A, Armitage RJ, Arrand JR, Rowe M, Young LS, Eliopoulos A, Gordon J. CD154 tone sets the signaling pathways and transcriptome generated in model CD40-pluricompetent L3055 Burkitt's lymphoma cells. J Immunol. 2007;179:2705–2712. doi: 10.4049/jimmunol.179.5.2705. [DOI] [PubMed] [Google Scholar]
- 41.Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J Exp Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat Med. 2004;10:540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 43.Luft T, Maraskovsky E, Schnurr M, Knebel K, Kirsch M, Gorner M, Skoda R, Ho AD, Nawroth P, Bierhaus A. Tuning the volume of the immune response: strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004;104:1066–1074. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- 44.Zuany-Amorim C, Hastewell J, Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat Rev Drug Discov. 2002;1:797–807. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]
- 45.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamath RV, Leary DJ, Huang S. Nucleocytoplasmic shuttling of polypyrimidine tract-binding protein is uncoupled from RNA export. Mol Biol Cell. 2001;12:3808–3820. doi: 10.1091/mbc.12.12.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu H, Li W, Noble WS, Payan D, Anderson DC. Riboproteomics of the hepatitis C virus internal ribosomal entry site. J Proteome Res. 2004;3:949–957. doi: 10.1021/pr0499592. [DOI] [PubMed] [Google Scholar]
- 48.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie J, Lee JA, Kress TL, Mowry KL, Black DL. Protein kinase A phosphorylation modulates transport of the polypyrimidine tract-binding protein. Proc Natl Acad Sci U S A. 2003;100:8776–8781. doi: 10.1073/pnas.1432696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma S, Liu G, Sun Y, Xie J. Relocalization of the polypyrimidine tract-binding protein during PKA-induced neurite growth. Biochim Biophys Acta. 2007;1773:912–923. doi: 10.1016/j.bbamcr.2007.02.006. [DOI] [PubMed] [Google Scholar]


