Abstract
The adolescent brain is particularly vulnerable to the effects of alcohol, with intoxications at this developmental age often producing long-lasting effects. The present study addresses the effects of a single acute ethanol exposure on GAP-43 and BDNF gene expression in neurons in the cerebellum and hippocampus of adolescent rats. Male postnatal day 23 (P23) Sprague-Dawley rats were exposed to ethanol vapors for two hours and after a recovery period of two hours, the cerebellum and hippocampus were harvested and samples were taken for blood alcohol concentration (BAC) determinations. We found that this exposure resulted in a mean BAC of 174 mg/dl, which resembles levels in human adolescents after binge drinking. Analyses of total RNA and protein by qRT-PCR and western blotting, respectively, revealed that this single ethanol exposure significantly decreased the levels of GAP-43 mRNA and protein in the cerebellum but increased the levels of mRNA and protein in the hippocampus. BDNF mRNA and protein levels were also increased in the hippocampus but not in the cerebellum of these animals. In situ hybridizations revealed that GAP-43 and BDNF mRNA levels were primarily increased by alcohol exposure in hippocampal dentate granule cells and CA3 neurons. Overall, the reported alterations in the expression of the plasticity-associated genes GAP-43 and BDNF in juvenile rats are consistent with the known deleterious effects of binge drinking on motor coordination and cognitive function.
Keywords: GAP-43, BDNF, ethanol, hippocampus, cerebellum, juvenile rats
INTRODUCTION
Alcohol consumption begins in the second decade of life, typically in early adolescence, at 13–14 years of age (Faden, 2006). Recent national surveys (National Survey on Drug Use and Health, NSDUH, 2008) indicate that there is a significant increase in the rates of both alcohol use and binge drinking between 12 and 21 years of age (Substance Abuse and Mental Health Services Administration, SAMHSA, 2009). Binge drinking can be defined as reaching a blood alcohol level (BAL) of greater than 80 mg/dl within one hour of consumption or consuming more than 5 drinks per occasion and about one fifth of the individuals in the 12–21 age group report at least one binge drinking episode per month (SAMHSA, 2009). One of the most obvious effects of binge drinking in humans is the impairment of motor activity and coordination, which depends on the function of the cerebellum. The cerebellum continues to develop during childhood and throughout adolescence, making this region susceptible to the effects of alcohol (White and Swartzwelder, 2004). In addition, alcohol’s effects on learning and memory are known to be more severe in adolescents than in adults. For instance, adolescent rats exhibited more alcohol-induced impairments in the Morris water maze task than mature animals (Markweise et al., 1998). In humans, subjects in their early twenties were shown to be more vulnerable to alcohol induced memory impairments in a variety of memory tasks than those in their late twenties (Acheson et al., 1998). These studies suggest that younger rodent or human brains have a heightened vulnerability to alcohol induced effects.
In this study, we examined the effect of binge drinking in the adolescent brain by focusing on the expression of two plasticity-associated proteins, growth-associated protein-43 (GAP-43) and brain-derived neurotrophic factor (BDNF). GAP-43 is a neuron specific phosphoprotein that plays a major role in initial development and remodeling of neural connections (Benowitz and Routtenberg, 1997; Perrone-Bizzozero and Tanner, 2006). Levels of GAP-43 mRNA are highest in the hippocampus in the first postnatal week and then decrease by 90% over the next few weeks (Cantallops and Routtenberg, 1999; Bolognani et al., 2007). Expression of GAP-43 is also developmentally regulated in the cerebellum with similar reductions in levels of this mRNA in maturing cerebellar granule cells (Console-Bram et al., 1996). The developmental decrease of GAP-43 gene expression from adolescence to adult suggests a role of this protein in the maturation of both the hippocampus and cerebellum, which could be affected by binge alcohol consumption. Two studies addressed the effects of alcohol exposure on GAP-43 expression in the hippocampus of adult (Kim et al., 2006) and aging rats (Casoli et al., 2002); however, no studies have addressed alcohol-induced changes in this protein during adolescence until now.
BDNF has been shown to influence neuronal survival, neurite outgrowth and maintenance of structural and neurochemical phenotype of a variety of neurons including cerebellar and hippocampal neurons (Gao et al., 1995; Lindholm et al., 1997). In the hippocampus, BDNF mRNA is also developmentally regulated with highest expression at P14 in dentate granule cells and CA3 neurons and lower in CA1 pyramidal cells (Sathanoori et al., 2004). BDNF is critical for synaptic changes such as hippocampal LTP (Bramham and Messaoudi, 2005) and for learning and adaptive behaviors (Tyler et al., 2002) via its activity-dependent secretion (Aicardi et al., 2004). Interesting, not only are GAP-43 and BDNF co-regulated in a variety of conditions including brain injury, temporal lobe epilepsy and schizophrenia (Miyake et al., 2002; Murray et al., 2000; Proper et al., 2000; Paz et al., 2006) but also infusions of BDNF increase GAP-43 expression during nerve regeneration (Kobayashi et al., 1997; Klocker et al., 2001; Song et al., 2008). Furthermore, it has been shown that the neurotrophic actions of BDNF are blocked in the absence of GAP-43 (Gupta et al., 2009), suggesting that GAP-43 acts downstream of BDNF and is an integral component of its biological function.
Based upon the role of GAP-43 and BDNF in developmental and adult plasticity and the known deleterious effects of alcohol on these processes, we hypothesized that acute ethanol exposure could alter the levels of these proteins in the hippocampus and cerebellum of adolescent rats. Consistent with this idea, we found alterations in GAP-43 and BDNF gene expression in both regions from ethanol exposed juvenile rats. Interestingly, the changes were region-specific, with the cerebellum showing decreased expression of GAP-43 and BDNF and the hippocampus increased expression of these proteins. These results suggest that alcohol affects brain function during adolescence in part by interfering with the normal remodeling of synaptic connections characteristic of this developmental period.
MATERIALS AND METHODS
Alcohol Exposure Paradigm
Male Sprague-Dawley rats (post-natal day 23, P23, Harlan, Indianapolis, IN) were housed two per cage in a temperature-controlled room and had ad libitum access to both standard food and water. Animals were kept on a normal 12h light and dark cycle. All procedures were approved by the IACUC in compliance with NIH’s guidelines for Animal Care and Use. Rats were exposed to ethanol vapors in inhalation chambers. This paradigm consists of exposing adolescent rats to ethanol vapors by placing animals in transparent sealed chambers (La Jolla Research Inc, La Jolla, CA) connected to an intake hose that continuously delivers ethanol vapor via a heating flask that receives a constant drip of liquid ethanol (200 proof, Decon Labs, King of Prussia, PA) controlled by a regulating peristaltic pump. An exhaust hose connected to a vacuum line continuously removes the ethanol vapor. Cages held 2 rats each and were exposed to air or air plus ethanol. Both control and ethanol treated experiments were started at the same time of the day (11:00am) and the ethanol vapor in the chamber was monitored every half-hour.
Determination of Ethanol levels
Animals were anesthetized with isoflurane (Sigma-Aldrich, St. Louis, MO) and sacrificed by decapitation 2hr after exposure ended. Trunk blood samples were collected into tubes containing heparin and the concentrations of ethanol in plasma were determined by a standard alcohol dehydrogenase-based assay (Galindo and Valenzuela, 2006). A standard ethanol concentration curve was generated utilizing control plasma from non-ethanol exposed rats. Samples were exposed to an enzyme reaction assay involving the reduction of NAD+ (NAD, Roche Diagnostics Corporation, Indianapolis, IN) to NADH and oxidation of ethanol by alcohol dehydrogenase (ADH, Roche Diagnostics Corporation, Indianapolis, IN). NADH absorbance was read at a wavelength of 340 nm. The mean blood alcohol levels (BAC) at the time of euthanasia (2 hr after the end of exposure) were 174 mg/dl (37.8 mM). Based on the report of Rivier and Lee (2001) using the same vapor chamber paradigm, peak BAC levels during the exposure were likely 25% higher. Preliminary work based on a small number of animals agrees with this estimate (data not shown).
Tissue Preparation for Biochemical assays
Whole brains and cerebella were removed and hippocampi were dissected on ice, quickly frozen on dry ice and stored at −80ºC for later use. The cerebellum and hippocampus were processed as described by Tanner et al., 2004. Briefly, tissues were homogenized with a glass/ Teflon homogenizer in ice-cold buffered sucrose solution (20mM Tris-Hcl, pH 7.4, 0.32M sucrose, 2 mM EDTA, 2 mM EGTA, 0.3 mM PMSF, 0.2 mM sodium vanadate, 50 mM sodium fluoride, 2μg/mL leupeptin, and 2μg/ul aprotinin.). The homogenate was then centrifuged at 1000 X g for 4 min to remove the nuclear fraction and the supernantant saved. Protein determination was performed as previously described (Bradford, 1976) using bovine serum albumin (Bio-Rad, Hercules, CA) as the standard.
RNA isolation and Quantitative RT- PCR
Quantitative reverse transcription PCR (qRT-PCR) was performed as previously described (Bullock, et al., 2008, 2009). Briefly, RNA was extracted from the tissue using Tri-Reagent (Sigma-Aldrich, St. Louis, MO). The integrity of samples was validated using a Bioanalyzer 2100 (Agilent Technologies; Santa Clara, CA) and used only if the RNA integrity number was >8. cDNA was synthesized using 2 μg of total RNA, and was performed using Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Exon spanning primer pairs (Operon; Huntsville, AL) specific to GAP-43, β-actin and BDNF were designed with Primer Express 3.0 (Applied Biosystems) and validated using the NCBI primer BLAST software. The following primers were used for GAP-43 (Forward 5’-AGCCAAGGAGGAGCCTAAAC, Reverse 5’-CTGTCGGGCACTTTCCTTAG), BDNF (Forward 5’ GACTCTGGAGAGCGTGAAT, Reverse 5’ CCACTCGCTAATACTGTCAC) and β-actin (Forward 5’-CTCTTCCAGCCTTCCTTCCT, Reverse 5’-AGGAGCCAGGGCAGTAATCT). qRT-PCR reactions were run on an Applied Biosystems 7300 or 7500 Fast real time PCR machine. Gene expression levels in all samples were examined using SYBR® Green (Applied Biosystems; Foster City, CA). Dissociation curves of all SYBR® Green primer pairs revealed no evidence of dimerization. Also primers for genes of interests were validated against β-actin and found to be within optimal amplification values (validation curve slopes <|0.1|). Samples were run in triplicate in three separate plates and compared to β-actin on the same plate. The relative levels of expression of each mRNA were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Western Blot analysis
Cerebellar and hippocampal samples containing 1 μg or 5 μg, respectively, of protein were resolved in 10% polyacrylamide gels, transferred to PVDF membranes and incubated with a polyclonal antibody against GAP-43 (Benowitz et al, 1988) or a phospho-specific GAP-43 antibody (Millipore Corp., Billerica, MA) overnight at 4ºC as described by Tanner et al., 2004. A similar protocol was used to measure the levels of mature BDNF protein in the tissue using the N-20 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:100 dilution. Bound antibodies were detected using HRP-conjugated secondary antibody and enhanced chemiluminescence (Millipore). Blots were scanned and band intensities were quantified using NIH ImageJ (Rasband, 1997). The optical density of each band was corrected by β-actin (Cell-Signaling Technologies, Danvers, MA).
Quantitative In situ hybridization
The whole brain was flash-frozen in isopentane at −40ºC, sectioned on a cryostat and processed for in situ hybridization, as previously described (Bolognani et al., 2006). Briefly, sections (10um) were hybridized overnight at 55ºC (GAP-43) or 42ºC (BDNF), with 1.5 × 106cpm/75ul of specific 35S-labeled antisense riboprobe for GAP-43 mRNA or 0.5 × 106cpm/75ul of specific 35S-labeled antisense oligonucleotide for BDNF mRNA, respectively. After hybridization, sections were treated with RNAse A, washed with Standard Saline Citrate (SSC) solutions containing 10 mM DTT, and finally dehydrated with increasing concentrations of ethanol. Dried slides were exposed to Kodak Biomax MR film (Eastman Kodak, Rochester, NY, USA). Densitometric analysis of the film was performed using ImageJ.
GAP-43 Immunohistochemistry
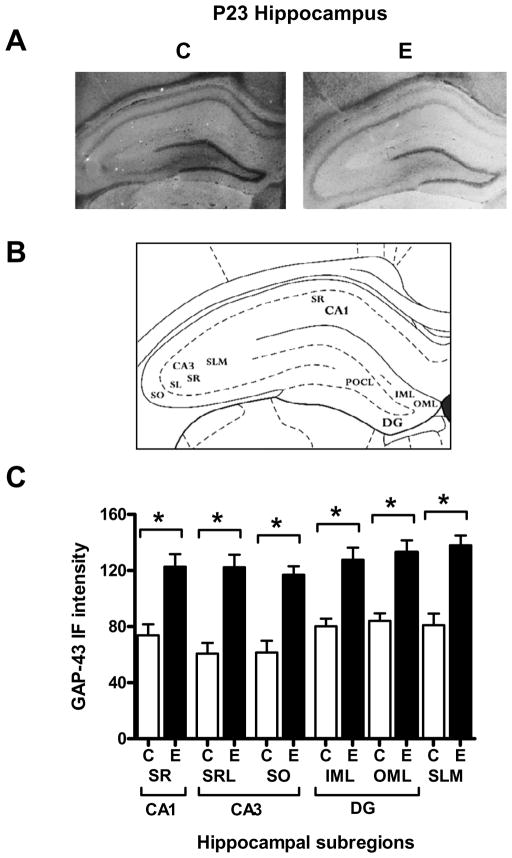
Slides prepared as described above were incubated for 1 h in 10% normal horse serum followed by overnight incubation at 4 ºC with a sheep anti-GAP-43 antibody (1:500; Benowitz et al., 1988). Sections were then washed in phosphate-buffered saline containing 0.1% Tween-20 and 300 mM NaCl, to decrease background staining (Benowitz et al, 1989) followed by incubation with fluorescent secondary antibodies (donkey-anti-sheep Alexa Fluor 488, 1:250) for two hours at room temperature. After washing in 1xPBST, slides were mounted in SlowFade Gold antifade reagent (Invitrogen Corporation, Carlsbad, California). Slides were examined on an Olympus BX60 microscope and images captured with an Olympus DP71 camera using the same exposure times (typically 2–3 sec) for control and experimental slides. Images were converted to grayscale using Adobe PhotoShop Elements, and regions were analyzed using ImageJ. At least three adjacent sections were analyzed from 6 control and 6 ethanol exposed rats. Images from the dorsal hippocampus were analyzed blindly by two independent observers and levels of GAP-43 immunofluorescence in all the hippocampal subregions shown in Figure 5 were averaged for each animal.
Figure 5. Immunohistochemical analysis of levels of GAP-43 protein in the hippocampus of control and ethanol exposed P23 rats.
[A] Representative IHC images of GAP-43 protein in control (C) and acute ethanol exposed (E) P23 rats, both taken using a 2.5 sec exposure. [B] Diagram of the analyzed hippocampal subregions. [C] Densitometric quantitation of immunofluorescence intensities in each hippocampal subregion. n=6. *p=0.05
Statistical analyses
Statistical analyses of the effect of alcohol on gene expression in each brain region and developmental age were accomplished using GraphPad Prism (v. 4.0). Statistical comparisons between sample sets were made using an unpaired two-tailed t-test.
RESULTS
Effects of a single ethanol exposure on GAP-43 gene expression in the hippocampus and cerebellum of juveniles rats
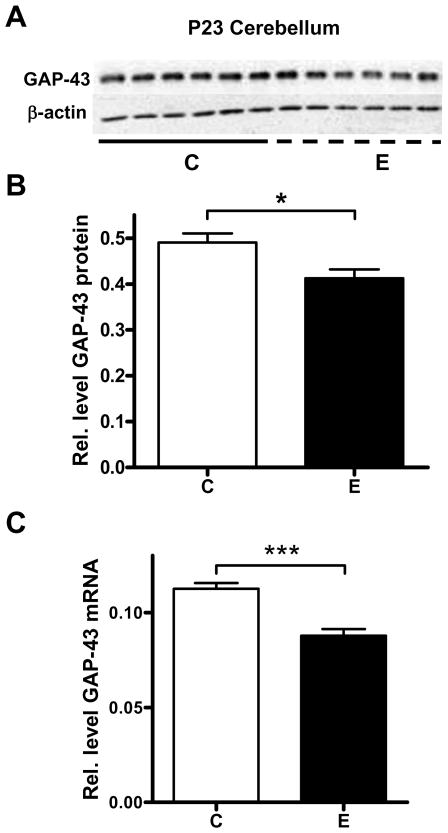
It has been previously determined that GAP-43 mRNA levels in developing cerebellar granule cells are decreased by GABA receptor agonists and glutamate receptor antagonists (Console-Bram et al., 1998). To measure whether this pattern could be replicated with two hours of an acute ethanol exposure, juvenile rats were exposed to ethanol vapors in sealed chambers for 2 hours, followed by a recovery period of 2 hours, after which they had an average BAL of 174 ±10 mg/dl (n = 9). Controls animals were exposed to air in the same type of chambers for the same length of time. Western blot analysis (Fig. 1A, B) revealed that acute ethanol exposure significantly decreased the relative amount of GAP-43 protein in the cerebellum. GAP-43 mRNA levels also significantly decreased after exposure to ethanol (Fig. 1C). Subsequent studies measured GAP-43 expression in the hippocampus of the same groups of animals. Surprisingly, we found that in contrast to the cerebellum, acute ethanol exposure increased GAP-43 mRNA and protein levels in the whole hippocampus (Fig. 2A-C).
Figure 1. Decreased GAP-43 gene expression in the cerebellum of alcohol exposed P23 rats.
[A] Representative western blot of GAP-43 protein levels in the cerebellum of P23 rats exposed to air (control, C) and ethanol vapors (E). [B] GAP-43 protein levels were normalized to β-actin, n=6, *p=0.0177. [C] qRT-PCR analysis of GAP-43 mRNA levels in whole cerebellum of control (C) and acute ethanol exposed (E) P23 rats; mRNA levels were normalized to β-actin, n=6, ***p=0.0003
Figure 2. Increased GAP-43 gene expression in the hippocampus of alcohol exposed P23 rats.
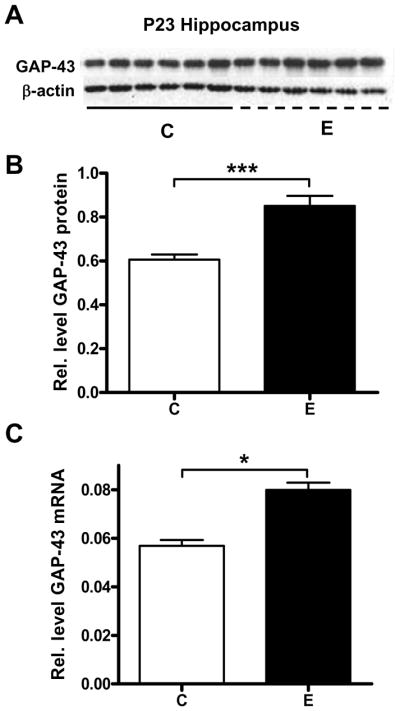
[A, B] Western blot analysis of GAP-43 protein levels in whole hippocampus of control (C) and acute ethanol exposed (E) P23 rats, normalized to β-actin, n=6, ***p=0.0007. [C] qRT-PCR analysis of GAP-43 mRNA levels in whole hippocampus between air control (C) and acute ethanol exposed (E) P23 rats, normalized to β-actin, n=6, *p=0.0250.
Since the effect of ethanol in the hippocampus was rather unexpected, we decided to validate these observations using a second group of animals. In addition, given the significance of PKC-dependent phosphorylation in GAP-43 function (Routtenberg et al., 2000) and the previous findings that this process is affected by alcohol (Perrone-Bizzozero et al., 1998; Kim et al., 2006), we also examined if GAP-43 phosphorylation was affected by the acute ethanol exposure. In agreement with the data shown in Figure 1, the levels of total GAP-43 protein increased in the hippocampus of ethanol exposed rats (Fig. 3A, B). Levels of phosphorylated GAP-43 were not significantly affected by ethanol exposure in the hippocampus (Fig. 3C). Moreover, the ratio of phosphorylated/total GAP-43 was not significantly different between the ethanol and control groups (Fig. 3D), suggesting that the main effect of the acute ethanol exposure is on the levels of expression rather than phosphorylation of GAP-43.
Figure 3. Increases in GAP-43 protein levels but not its phosphorylation state in the hippocampus of alcohol exposed P23 rats.
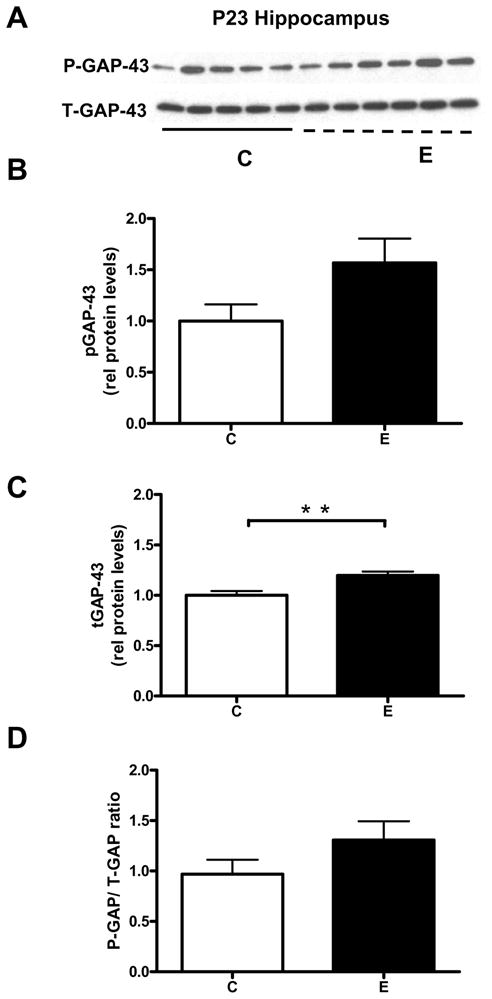
[A] Western blot analysis of total GAP-43 protein levels (T-GAP-43) and levels of the PKC-phosphorylated protein (P-GAP-43) in the hippocampus of control (C) and acute ethanol exposed (E) P23 rats. Levels of T-GAP-43 [B], P-GAP-43 [C] and ratios of T-GAP-43 over p-GAP-43 [D], n=6, **p=0.007.
To investigate and localize the increases in GAP-43 mRNA and protein, we performed in situ hybridization (ISH) and immunofluorescence studies on hippocampal sections. As postnatal development progresses, GAP-43 transcript expression is known to decrease in the CA1, CA3 and DG of the hippocampus, and almost completely disappears in dentate granule cells in adulthood. In agreement with previous studies (Meberg and Routtenberg, 1991; Bolognani et al., 2007), GAP-43 mRNA levels in dentate granule cells are very low at P23 (Fig. 4A control). A quantitative analysis of the different hippocampal subregions (Fig. 4B) revealed a significant increase in GAP-43 mRNA levels in the CA3 and the DG of ethanol exposed animals (CA3, p=0.0203; DG, p=0.0258). In contrast to the GAP-43 mRNA, which is primarily localized to cell bodies, GAP-43 protein is localized to axons and presynaptic terminals, allowing us to map the fibers with increased levels of this protein by immunofluorescence (Figs. 5A, B). Consistent with the results of the GAP-43 ISH, alcohol exposed rats had increased GAP-43 protein levels in fibers and terminals from DG and CA3 neurons (Fig. 5C), with an additional increase in the CA1 region, the molecular layer of the dentate gyrus and the stratum lacunosum moleculare. As GAP-43 protein is not only present in the intrinsic DG-CA3-CA1 hippocampal circuitry but also in afferent fibers from other brain regions including the entorhinal cortex and septum (Benowitz et al, 1988; Tanner et al, 2008), our results suggest that GAP-43 expression in these regions is affected by alcohol exposure as well.
Figure 4. GAP-43 in situ hybridization in hippocampal subregions of control and alcohol exposed P23 rats.
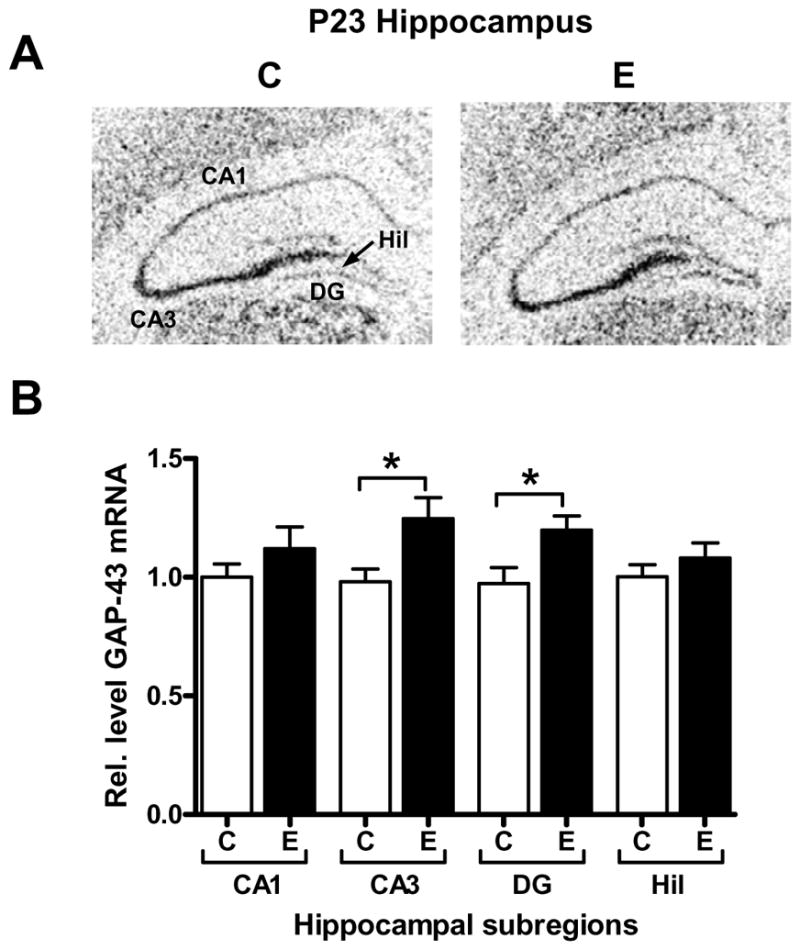
[A] Representative ISH images of GAP-43 mRNA in control (C) and acute ethanol exposed (E) P23 rats. [B] Densitometric quantitation of GAP-43 mRNA levels in CA1, CA3, dentate gyrus (DG), and hilus (Hil), n=6; CA1, *p=0.0203, DG, *p=0.0258.
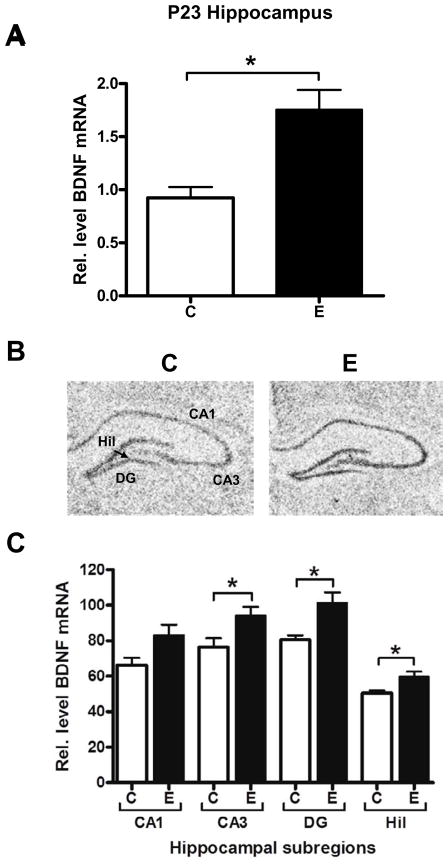
Single ethanol exposure increases BDNF mRNA and protein in the hippocampus
Given that GAP-43 gene expression is regulated by neuronal activity during hippocampal development (Cantallops and Routtenberg, 1999), our findings suggest that ethanol may increase the activity of hippocampal neurons. To investigate this further, we evaluated the effect of ethanol on the expression of BDNF, another activity regulated gene that is co-regulated with GAP-43 in many conditions. We found significant increases in BDNF mRNA in the hippocampus of alcohol exposed P23 rats (Fig. 6A). In contrast to the hippocampus, BDNF mRNA levels in the cerebellum of the same animals were not affected by this acute exposure (data not shown). In situ hybridization localized the increases in BDNF mRNA in the CA3, DG and the hilus (Fig. 6B, CA3 p=0.0318, DG p=0.0320, Hil p=0.0245). These findings are consistent with the changes in GAP-43 mRNA levels, suggesting that the activity of hippocampal neurons is increased after the single ethanol exposure. Furthermore, samples were also analyzed by western blots to evaluate whether that the changes in BDNF mRNA levels resulted in increased expression of BDNF protein. As shown in Figure 7, alcohol exposed rats had increased levels of the mature (14 kDa) form of BDNF relative to control animals, demonstrating the increased BDNF mRNA levels observed in alcohol exposed rats were translated and processed into protein.
Figure 6. Increased BDNF mRNA levels in the hippocampus of alcohol exposed P23 rats.
[A] qPCR analysis of BDNF mRNA levels in whole hippocampus between air control (C) and acute ethanol exposed (E) P23 rats, normalized to β-actin, n=6, *p=0.0311, [B, C] In situ hybridization analysis and localization of BDNF mRNA to hippocampal subregions, CA1, CA3, dentate gyrus (DG), and hilus (Hil), of air control, (C) and acute ethanol exposed (E) P23 rats, n=6; CA3 *p=0.0318, DG *p=0.0320, Hil *p=0.0245.
Figure 7. Increased BDNF protein levels in the hippocampus of alcohol exposed P23 rats.
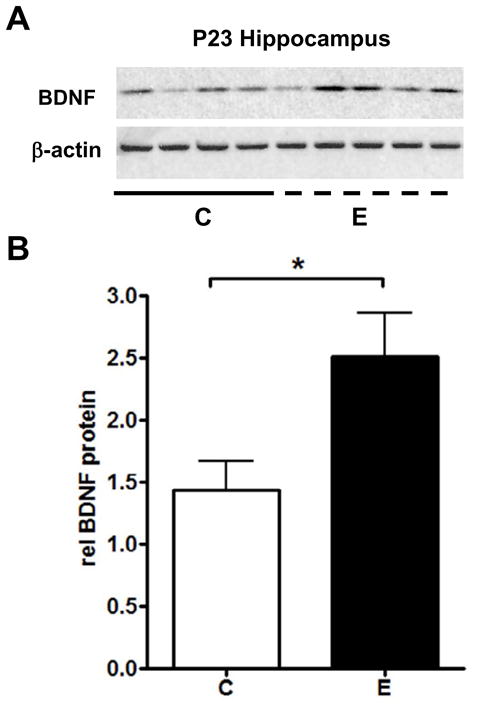
[A] Western blot analysis of mature BDNF protein levels. [B] BDNF protein levels were normalized to β-actin, n=6, *p=0.0434
DISCUSSION
Alcohol is the drug most frequently used by teenagers in the United States. Alcohol intoxication affects brain function in adolescents differently than adults, often producing more severe and persistent effects (Markwiese et al., 1998; Spear and Varlinskaya, 2005; White and Swartzwelder, 2005). Adolescent exposure to alcohol is also known to significantly increase voluntary alcohol intake in adult rats (Pascual et al., 2009; Acevado et al., 2010). Unfortunately, early to late adolescence is also the period when the incidence of both alcohol use and binge alcohol drinking rises in the US, making this a serious national health issue (NSDUH, 2008). At the cellular level, adolescence is characterized by substantial synaptic pruning and remodeling (Rakic et al., 1994). Therefore, in this study we examined the effect of an acute binge drinking-like exposure on the juvenile brain by focusing on the levels of expression of two proteins associated with synaptic plasticity, GAP-43 and BDNF. We found that acute alcohol exposure decreased expression of GAP-43 and BDNF in the cerebellum suggesting that these changes may contribute to the motor coordination alterations that are associated with acute intoxication. Intriguingly, we found the opposite effect on the expression of both proteins in the hippocampus, suggesting a potential mechanism by which alcohol intoxication may lead to learning and memory deficits in the adolescent brain.
In humans, a binge ethanol exposure can be defined as an ethanol exposure that raises the blood ethanol content to 80 mg/dl or higher (Courtney and Polich, 2009). In a recent pediatric emergency room study, the mean BAL of adolescent patients admitted for treatment of acute ethanol intoxication was ~185 ± 55mg/dl, with 75 percent of these cases being described as “moderate” intoxication without need for hospitalization (n=104, mean age 16.2 years; Sanz Marcos et al., 2009). Thus, the mean BAC of the ethanol exposed animals in this study, ~174 ± 10 mg/dl, which was sufficient to impair motor coordination in our animal model (data not shown), meets the criteria for being a binge ethanol exposure in humans and falls within the range of a probable “moderate” intoxication on an equivalent human pediatric setting.
The decreases in GAP-43 expression in the cerebellum of rats exposed to acute binge-like alcohol exposure are consistent with previous reports indicating that acute ethanol exposure increases GABAergic transmission to cerebellar granule neurons and also to Purkinje neurons in cerebellar slices from juvenile rats (reviewed in Botta et al., 2007). Inhibition of ionotropic glutamate receptors may be an alternative mechanism underlying the decrease in GAP-43 expression (Console-Bram et al., 1998). However, slice studies have shown that acute ethanol exposure, at subanesthetic concentrations, minimally affects basal ionotropic glutamate receptor-mediated transmission in cerebellar granule and Purkinje neurons (Mameli et al., 2008; Belmeguenai et al., 2008; Carta et al., 2006; Offenhauser, 2006). Therefore, the increase in GABAergic inhibition is more likely to be responsible for the decrease in cerebellar GAP-43 expression. GAP-43 is a high turnover protein that is susceptible to proteosomal degradation (Denny, 2004; DeMoliner et al., 2005). GAP-43 gene expression is rapidly regulated by changes in mRNA stability, which is controlled by the interaction of the RNA-binding protein HuD with highly conserved sequences in the 3’ UTR of the mRNA. The increase in GABAergic input to cerebellar neurons is likely to cause a decrease in Ca2+ influx through voltage-gated Ca2+ channels and a decrease in PKC-dependent phosphorylation, which is required for HuD binding to the GAP-43 mRNA (Pascale et al., 2005). The ultimate outcome is a decrease in mRNA levels and GAP-43 protein synthesis, leading to an imbalance between production and degradation of this protein in favor of the latter.
In contrast to the cerebellum, acute ethanol exposure increased GAP-43 expression in the hippocampus of juvenile rats. This effect was quite unexpected considering that GAP-43 expression in the hippocampus increases with neuronal activity (Cantallops and Routtenberg, 1999; Meberg et al., 1993) and acute ethanol exposure has been demonstrated to decrease hippocampal neuronal activity, which could be, in part, mediated by an increase in GABAergic transmission and a decrease in glutamatergic transmission (Ryabinin et al., 1997, for reviews see Weiner and Valenzuela, 2006 and Siggins et al., 2005). However, in vivo electrophysiological studies have shown that acute ethanol exposure can excite hippocampal neurons under some conditions (Grupp, 1980; Newlin et al., 1981). This paradoxical excitatory effect of ethanol could be mediated by the inhibition of the kainate receptor-dependent excitatory drive of hippocampal GABAergic interneurons (Carta et al., 2003), which would cause a disinhibition of CA1 pyramidal neurons, increasing GAP-43 expression in these cells. Alternatively, ethanol could initially inhibit neuronal activity in hippocampal neurons but these could develop acute tolerance to this action of ethanol, leading to a compensatory increase in neuronal excitability. It should be noted, however, that acute ethanol exposure significantly increased GAP-43 protein levels but not mRNA levels in CA1 pyramidal neurons, suggesting that disinhibition of these neurons may alter GAP-43 translation. In contrast, in the CA3 region and the dentate gyrus both GAP-43 mRNA and protein were increased in alcohol exposed animals. The mechanism responsible for this effect remains to be elucidated but it may involve increased stabilization of GAP-43 mRNA via interactions with the RNA-binding protein HuD, as we previously described in HuD overexpressor mice (Bolognani et al., 2006). Interestingly, we found that ethanol exposure also increased the expression of BDNF, another activity-regulated gene, in the CA3 region, dentate gyrus and hilus. Since BDNF has been shown to increase GAP-43 levels (Kobayashi et al., 1997; Klocker et al., 2001; Song et al., 2008), it is conceivable that the observed increases in BDNF protein levels in the hippocampus may, in part, mediate the effect of ethanol on GAP-43 expression. Finally, as indicated above, the changes in BDNF expression could be also related to a compensatory mechanism after acute alcohol exposure.
In general agreement with our findings in adolescent rats, we previously showed that fetal alcohol exposure increased GAP-43 expression in the hippocampus of adult rat offspring (Perrone-Bizzozero et al., 1998). Likewise, another report demonstrated that alcohol exposure increased the levels of GAP-43 in the hippocampus of aging rats (Casoli et al., 2002). However, a different study using a single i.p. injection of a high dose of alcohol reported a decrease in GAP-43 expression and phosphorylation in the hippocampus of adult rats (Kim et al., 2006). Under our experimental conditions, we observed an increase in GAP-43 expression with no change in phosphorylation levels. One possible explanation for these distinct findings is that the effect of ethanol on GAP-43 expression varies with the developmental age of the animal. Indeed, we found no change in GAP-43 mRNA when post-pubescent (P37) rats were treated using the same vapor chamber exposure paradigm (unpublished observation). A similar age-dependent effect was observed for BDNF in the hippocampus, where we found alcohol-induced increases at P23 but alcohol-induced decreases at P37 (unpublished observation). Furthermore, these results are also consistent with another recent study (Miki et al., 2008) that reported a similar alcohol-induced increase in BDNF mRNA in P16 and P20 rats and a decrease in P60 animals.
While the causes and consequences of the differential effects of ethanol on GAP-43 and BDNF expression in the hippocampus vs. the cerebellum are unknown, one possible explanation for our findings is that the increases in GAP-43 and BDNF gene expression in hippocampal tissue may be a neuroprotective response to alcohol-induced tissue damage. GAP-43 and BDNF expression are known to be altered after certain types of neuronal insult. For example, GAP-43 expression is increased in the granule cell layer of the dentate gyrus following kainic acid induced seizures (Meberg et al., 1993; McNamara and Lenox, 2000), indicating a possible response to cell damage. Likewise, BDNF levels also increase in response to epileptic seizures (Isackson et al., 1991; Murray et al., 2000). As GAP-43 has been recently shown to be integral to the neuroprotective ability of BDNF (Gupta et al., 2009), it is conceivable that the observed changes in GAP-43 gene expression are part of the BDNF-mediated neuroprotective response to ethanol cytotoxicity in the hippocampus. In general agreement with this idea are the findings of McGough et al. (2004) indicating that acute exposure of neurons to ethanol increases BDNF expression in the dorsal striatum, which is thought to be part of a homeostatic response that opposes the development of alcohol addiction. The distinct responses observed in the cerebellum may be due to the fact that this tissue is generally more resistant to glutamate excitotoxicity than the hippocampus and neocortex (Wu et al., 2005) and the rats used is our study were older than the critical period (P4-P6) to alcohol-induced cerebellar damage (Hamre and West, 1993; Thomas et al., 1998, Siler-Masiglio et al., 2006). Supporting this idea, it is known that adolescents are more sensitive to the cognitive impairments induced by alcohol than to its effects on motor coordination and sedation (Markweise et al., 1998; White et al., 2002; White, et al., 2005).
In conclusion, we have demonstrated that a single acute ethanol exposure can have opposite effects on the expression of GAP-43 and BDNF in the hippocampus and cerebellum of juvenile rats. Given that these proteins play a central role in the refinement and maintenance of neuronal circuits, the mechanisms responsible for these actions of ethanol should be further investigated. Studies should also explore whether acute ethanol exposure affects GAP-43 and BDNF expression in other brain regions and if these effects are age dependent. These studies may ultimately result in the development of novel therapeutic interventions against alcohol-induced neurotoxicity.
Acknowledgments
We thank Dr. Martina Rosenberg for her help in some of the experiments. This work was supported by T32 grant AA011336 (VVK, NPB and CFV); and RO1 grants AA14973 and AA15614 (CFV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo MB, Molina JC, Nizhnikov ME, Spear NE, Pautassi RM. High ethanol dose during early adolescence induces locomotor activation and increases subsequent ethanol intake during late adolescence. Dev Psychobiol. doi: 10.1002/dev.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C. Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse. J Neurophysiol. 2008;100:3167–3174. doi: 10.1152/jn.90384.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI, Bird ED. Localization of the growth-associated phosphoprotein GAP-43 (B-50, F1) in the human cerebral cortex. J Neurosci. 1989;9:990–995. doi: 10.1523/JNEUROSCI.09-03-00990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognani F, Tanner DC, Merhege M, Deschenes-Furry J, Jasmin B, Perrone-Bizzozero NI. In vivo post-transcriptional regulation of GAP-43 mRNA by overexpression of the RNA-binding protein HuD. J Neurochem. 2006;96:790–801. doi: 10.1111/j.1471-4159.2005.03607.x. [DOI] [PubMed] [Google Scholar]
- Bolognani F, Tanner DC, Nixon S, Okano HJ, Okano H, Perrone-Bizzozero NI. Coordinated expression of HuD and GAP-43 in hippocampal dentate granule cells during developmental and adult plasticity. Neurochem Res. 2007;32:2142–2151. doi: 10.1007/s11064-007-9388-8. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol. 2007;41:187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bullock WM, Bolognani F, Botta P, Valenzuela CF, Perrone-Bizzozero NI. Schizophrenia-like GABAergic gene expression deficits in cerebellar Golgi cells from rats chronically exposed to low-dose phencyclidine. Neurochem Int. 2009;55:775–782. doi: 10.1016/j.neuint.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Routtenberg A. Activity-dependent regulation of axonal growth: posttranscriptional control of the GAP-43 gene by the NMDA receptor in developing hippocampus. J Neurobiol. 1999;41:208–220. [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci USA. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors. J Neurosci. 2006;26:1906–1912. doi: 10.1523/JNEUROSCI.4430-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casoli T, Di Stefano G, Fattoretti P, Delfino A, Solazzi M, Bertoni-Freddari C. Effects of ethanol on GAP-43 levels in hippocampus and cerebellum of aged rats. Ann NY Acad Sci. 2002;973:313–316. doi: 10.1111/j.1749-6632.2002.tb04658.x. [DOI] [PubMed] [Google Scholar]
- Console-Bram LM, Baird DH, Fitzpatrick-McElligott SG, McElligott JG. Modulation of GAP-43 mRNA by GABA and glutamate in cultured cerebellar granule cells. Brain Res. 1998;783:316–325. doi: 10.1016/s0006-8993(97)01386-3. [DOI] [PubMed] [Google Scholar]
- Console-Bram LM, Fitzpatrick-McElligott SG, McElligott JG. Distribution of GAP-43 mRNA in the immature and adult cerebellum: a role for GAP-43 in cerebellar development and neuroplasticity. Brain Res Dev Brain Res. 1996;95:97–106. doi: 10.1016/0165-3806(96)00079-x. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moliner KL, Wolfson ML, Perrone Bizzozero N, Adamo AM. Growth-associated protein-43 is degraded via the ubiquitin-proteasome system. J Neurosci Res. 2005;79:652–660. doi: 10.1002/jnr.20388. [DOI] [PubMed] [Google Scholar]
- Denny JB. Growth-associated protein of 43 kDa (GAP-43) is cleaved nonprocessively by the 20S proteasome. Eur J Biochem. 2004;271:2480–2493. doi: 10.1111/j.1432-1033.2004.04179.x. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30:1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Galindo R, Valenzuela CF. Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol. 2006;40:111–118. doi: 10.1016/j.alcohol.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WQ, Zheng JL, Karihaloo M. Neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) act at later stages of cerebellar granule cell differentiation. J Neurosci. 1995;15:2656–2667. doi: 10.1523/JNEUROSCI.15-04-02656.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp LA. Biphasic action of ethanol on single units of the dorsal hippocampus and the relationship to the cortical EEG. Psychopharmacology (Berl) 1980;70:95–103. doi: 10.1007/BF00432377. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Mishra R, Kusum S, Spedding M, Meiri KF, Gressens P, Mani S. GAP-43 is essential for the neurotrophic effects of BDNF and positive AMPA receptor modulator S18986. Cell Death Differ. 2009;16:624–637. doi: 10.1038/cdd.2008.188. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Choi KM, Ku BM, Mun J, Joo Y, Han JY, Kim YH, Roh GS, Kang SS, Cho GJ, Choi WS. Acute ethanol administration decreases GAP-43 and phosphorylated-GAP-43 in the rat hippocampus. Brain Res. 2006;1112:16–25. doi: 10.1016/j.brainres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Klocker N, Jung M, Stuermer CA, Bahr M. BDNF increases the number of axotomized rat retinal ganglion cells expressing GAP-43, L1, and TAG-1 mRNA--a supportive role for nitric oxide? Neurobiol Dis. 2001;8:103–113. doi: 10.1006/nbdi.2000.0329. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Hamner S, Zirrgiebel U. Neurotrophins and cerebellar development. Perspect Dev Neurobiol. 1997;5:83–94. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–917. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Lenox RH. Differential regulation of primary protein kinase C substrate (MARCKS, MLP, GAP-43, RC3) mRNAs in the hippocampus during kainic acid-induced seizures and synaptic reorganization. J Neurosci Res. 2000;62:416–426. doi: 10.1002/1097-4547(20001101)62:3<416::AID-JNR12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Routtenberg A. Selective expression of protein F1/(GAP-43) mRNA in pyramidal but not granule cells of the hippocampus. Neuroscience. 1991;45:721–733. doi: 10.1016/0306-4522(91)90284-u. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Gall CM, Routtenberg A. Induction of F1/GAP-43 gene expression in hippocampal granule cells after seizures [corrected] Brain Res Mol Brain Res. 1993;17:295–299. doi: 10.1016/0169-328x(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Miki T, Kuma H, Yokoyama T, Sumitani K, Matsumoto Y, Kusaka T, Warita K, Wang ZY, Hosomi N, Imagawa T, et al. Early postnatal ethanol exposure induces fluctuation in the expression of BDNF mRNA in the developing rat hippocampus. Acta Neurobiol Exp (Wars) 2008;68:484–493. doi: 10.55782/ane-2008-1714. [DOI] [PubMed] [Google Scholar]
- Miyake K, Yamamoto W, Tadokoro M, Takagi N, Sasakawa K, Nitta A, Furukawa S, Takeo S. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 2002;935:24–31. doi: 10.1016/s0006-8993(02)02420-4. [DOI] [PubMed] [Google Scholar]
- Murray KD, Isackson PJ, Eskin TA, King MA, Montesinos SP, Abraham LA, Roper SN. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J Comp Neurol. 2000;418:411–422. doi: 10.1002/(sici)1096-9861(20000320)418:4<411::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Newlin SA, Mancillas-Trevino J, Bloom FE. Ethanol causes increases in excitation and inhibition in area CA3 of the dorsal hippocampus. Brain Res. 1981;209:113–128. doi: 10.1016/0006-8993(81)91175-6. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, Govoni S, Alkon DL, Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKC alpha-dependent pathway. Proc Natl Acad Sci USA. 2005;102:12065–12070. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paz RD, Andreasen NC, Daoud SZ, Conley R, Roberts R, Bustillo J, Perrone-Bizzozero NI. Increased expression of activity-dependent genes in cerebellar glutamatergic neurons of patients with schizophrenia. Am J Psychiatry. 2006;163:1829–1831. doi: 10.1176/ajp.2006.163.10.1829. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Isaacson TV, Keidan GM, Eriqat C, Meiri KF, Savage DD, Allan AM. Prenatal ethanol exposure decreases GAP-43 phosphorylation and protein kinase C activity in the hippocampus of adult rat offspring. J Neurochem. 1998;71:2104–2111. doi: 10.1046/j.1471-4159.1998.71052104.x. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Tanner D. GAP-43 in Neural Development and Plasticity. In: Lajtha A, Lim R, editors. The Handbook of Neurochemistry and Molecular Neurobiology. Neuroactive Proteins and Peptides. 3. Ch 15. Vol. 9. Springer, Plenum Press; New York: 2006. [Google Scholar]
- Proper EA, Oestreicher AB, Jansen GH, Veelen CW, van Rijen PC, Gispen WH, de Graan PN. Immunohistochemical characterization of mossy fibre sprouting in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2000;123:19–30. doi: 10.1093/brain/123.1.19. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2008. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rivier C, Lee S. Effect of repeated exposure to alcohol on the response of the hypothalamic-pituitary-adrenal axis of the rat: II. Role of the length and regimen of alcohol treatment. Alcohol Clin Exp Res. 2001;25:106–111. [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci USA. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Sanz Marcos N, Arias Constanti V, Trenchs Sainz de la Maza V, Curcoy Barcenilla AI, Matali Costa J, Luaces Cubells C. Acute ethanol intoxication in a paediatric emergency department. Ann Pediatr (Barc) 2009;70:132–136. doi: 10.1016/j.anpedi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Sathanoori M, Dias BG, Nair AR, Banerjee SB, Tole S, Vaidya VA. Differential regulation of multiple brain-derived neurotrophic factor transcripts in the postnatal and adult rat hippocampus during development, and in response to kainate administration. Brain Res Mol Brain Res. 2004;130:170–177. doi: 10.1016/j.molbrainres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Siler-Marsiglio KI, Madorsky I, Pan Q, Paiva M, Neeley AW, Shaw G, Heaton MB. Effects of acute ethanol exposure on regulatory mechanisms of Bcl-2-associated apoptosis promoter, bad, in neonatal rat cerebellum: differential effects during vulnerable and resistant developmental periods. Alcohol Clin Exp Res. 2006;30:1031–1038. doi: 10.1111/j.1530-0277.2006.000126.x. [DOI] [PubMed] [Google Scholar]
- Song XY, Li F, Zhang FH, Zhong JH, Zhou XF. Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One. 2008;3:e1707. doi: 10.1371/journal.pone.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H–36, HHS Publication No. SMA 09–4434. Office of Applied Studies; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Tanner DC, Githinji AW, Young EA, Meiri K, Savage DD, Perrone-Bizzozero NI. Fetal alcohol exposure alters GAP-43 phosphorylation and protein kinase C responses to contextual fear conditioning in the hippocampus of adult rat offspring. Alcohol Clin Exp Res. 2004;28:113–122. doi: 10.1097/01.ALC.0000106308.50817.B3. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann NY Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wu X, Jiang X, Marini AM, Lipsky RH. Delineating and understanding cerebellar neuroprotective pathways: potential implication for protecting the cortex. Ann NY Acad Sci. 2005;1053:39–47. doi: 10.1196/annals.1344.004. [DOI] [PubMed] [Google Scholar]