Abstract
The feeling of “body ownership” may be experimentally investigated by perceptual illusions. The “rubber hand illusion” (RHI) leads human subjects to experience an artificial hand as their own. According to functional imaging, the ventral premotor cortex (PMv) plays a key role in the integration of multisensory inputs allowing the “incorporation” of the rubber hand into body representation. However, causal structure–function relationships can only be obtained by lesion studies.
Here, we tested the RHI in 70 stroke patients and in 40 age-matched healthy controls. Additionally, asomatognosia, the unawareness of one's own body parts, was assessed in a subgroup of 64 stroke patients. Ischemic lesions were delineated on diffusion-weighted magnetic resonance images and normalized. Right-hemispheric lesions were mirrored across the midline. Voxels that might be essential for RHI and/or somatognosia were defined by voxel-based lesion-symptom mapping. Probabilistic diffusion tractography was used to identify tracts passing through these voxels.
Contralesional rubber hand illusion failure (RHIF) was observed in 18 (26%) of 70 stroke patients, an additional ipsilesional RHIF in seven of these patients. RHIF-associated lesion voxels were located subcortically adjacent to the insula, basal ganglia, and within the periventricular white matter. Tractography revealed fiber tract connections of these voxels with premotor, parietal, and prefrontal cortex. Contralesional asomatognosia was found in 18 (28%) of 64 stroke patients. In contrast to RHIF, asomatognosia-associated lesion voxels showed no connection with PMv.
The results point to a role of PMv and its connections in mediating changes in the sense of limb ownership driven by multisensory stimulation.
Introduction
The feeling of ownership of our limbs is a fundamental aspect of self-consciousness. The distinction between “self” and “environment” starts to develop on the first day of life (Rochat and Striano, 2000; Rochat, 2010). Any organism exploring objects in the environment also gains implicit knowledge about the self as perceiver and actor (Rochat and Striano, 2000). In other words, exploring the environment by means of vision, touch, and proprioception may unconsciously calibrate one's own body and coinstantaneously generate bodily self-identification as a “byproduct” (Rochat and Striano, 2000; van der Kamp and Savelsbergh, 2000). Knowledge about which cortical areas are involved in the formation of a feeling of “body ownership” is of considerable neurobiological interest, but also of clinical relevance. Loss of awareness of one's body, asomatognosia, is frequently observed in, for instance, stroke victims, who may perceive all or part of their body as being strange, transformed, or even totally alien (Babinski, 1914).
Various perceptual illusions in healthy subjects (e.g., Lackner, 1988; Ramachandran and Hirstein, 1998; Botvinick and Cohen, 1998) have been used to investigate the neurobiological underpinnings of the feeling of “bodily self.” Studies based on the application of these illusions lend support to the hypothesis that sensory systems may deal with intermodal perceptual correlation by applying an universal Bayesian logic (Ramachandran and Hirstein, 1998). One such paradigm is the “rubber hand illusion” (RHI) (Botvinick and Cohen, 1998). Subjects acquire feelings of body ownership of a rubber hand placed in front of them if the viewed rubber hand and the subject's covered own hand are repeatedly stricken simultaneously. In a functional imaging study, presence of the RHI was associated with activity in bilateral premotor cortex and frontal operculum (Ehrsson et al., 2004). A number of studies and neurocognitive models of RHI-associated body ownership point to ventral premotor cortex (PMv) as the place of body-related sensory integration whose activity is linked to the feeling of ownership during the RHI (Ehrsson et al., 2005; Makin et al., 2008; Tsakiris, 2010). Apart from its roles, for instance, in motor control or event anticipation, PMv is an ideal candidate for the multisensory representation of one's own body as it is anatomically connected to visual and somatosensory areas in the posterior parietal cortex and to frontal motor areas (Rizzolatti et al., 1998). However, it remains an open question whether concurrent multisensory experience alone may be sufficient to generate a feeling of ownership for the rubber hand, or whether such novel ownership feelings depend on assimilation of the multisensory experience to a reference model of representations of the body built from prior experience and innate body representations (Tsakiris, 2010). In other words, there might be a kind of “native” body representation that is congruent with the physical body until it is challenged by an intersensory discrepancy. This discrepancy may result, for instance, from tool use or from experimental manipulation such as the rubber hand illusion. Assuming an involvement of PMv in the multisensory integration mediating the referral of touch from the real hand to the rubber hand (Makin et al., 2008; Tsakiris, 2010), we used a lesion analysis in stroke patients to test the following hypotheses: (1) RHI failure (RHIF) is associated with lesions to PMv or its connections. (2) In patients with asomatognosia, but without RHIF, PMv or its connections are not affected.
Materials and Methods
The protocol conformed to the principles of the declaration of Helsinki and was approved by the Ethics Committee of the Medical Faculty at the University of Würzburg. All patients and control subjects gave their written informed consent. Unless stated otherwise, all values are given as means ± SD.
Subjects and clinical investigation.
Seventy stroke patients aged between 21 and 86 years (59.5 ± 13.4 years; 25 female, 45 male; 66 right handed, 4 ambidextrous) with slight to moderate stroke symptoms following circumscribed unilateral left (n = 37) or right (n = 33) hemispheric brain lesions were recruited from the stroke unit at the Department of Neurology, University of Würzburg. Patients were eligible for the study if the following inclusion criteria were met: (1) age between 18 and 90 years, (2) acute ischemic stroke within 1–7 d after symptom onset, (3) no previous strokes or other structural brain alterations, (4) retained capacity to perceive light touch on the affected hand without difficulty (mild sensory impairment was permitted), and (5) sufficient cognitive and linguistic abilities to understand and complete the questionnaire (slight aphasia was permitted). Thorough neurological examination included a test of extrapersonal (clock drawing) and personal spatial neglect (hand-reaching task) (Azouvi et al., 2002). Handedness was assessed by a modified version of the Edinburgh Inventory, which ranges from −20 for strong left handedness to +20 for strong right handedness. Subjects with handedness scores from −12 to +12 were classified as ambidextrous. Additionally, 40 age-matched healthy controls (59.6 ± 15.0 years; 22 female, 18 male; 35 right handed, 4 ambidextrous, 1 left handed) were included.
Rubber hand illusion.
The subject's real hand was hidden out of view in a shoebox exhibiting 10 cm × 8 cm open windows on both sides. A realistic, life-sized, gender-matched artificial hand was placed on the top of the box in an anatomically plausible position, and the gap between the trunk of the artificial hand and the subject's upper arm was covered with a towel. The subject was sitting with eyes fixed on the fake hand while the experimenter used two interconnected small paintbrushes to stroke the artificial hand and the subject's hidden hand, synchronizing the timing of the brushing as closely as possible (Botvinick and Cohen, 1998). The brushstrokes were small and brisk and applied to the dorsal surface of the index, middle, and ring fingers at a frequency range of 0.5–3 Hz. After 2 min, subjects completed a two-part questionnaire that requested an open-ended description of their experience and asked them to affirm or deny the occurrence of nine specific perceptual effects by reporting −−−, −−, or − (denial), 0 (not able to affirm or deny), or +, ++, or +++ (affirmation) (Botvinick and Cohen, 1998; Ehrsson et al., 2004). These reports were transferred to values from 1 (−−−) to 7 (+++) for further analysis. Subjects with an affirmation (score 5, 6, or 7) of question number (No) 3 (“I felt as if the rubber hand were my hand”) were defined to experience the RHI at the respective hand; otherwise, an RHIF was diagnosed. In each subject, both hands were tested sequentially in randomized order, and subjects completed one questionnaire for each hand immediately after it had been tested.
Asomatognosia.
In a subgroup of 64 patients, the presence of asomatognosia was assessed by the question, “At what percentage does your hand currently belong to yourself?” The question was asked while the patient was sitting in a chair in comfortable position. It was answered for each hand separately on a visual analog scale ranging from 0 to 100%. To avoid any influence of the RHI on the rating, asomatognosia was always assessed before RHI testing.
MRI protocol.
Stroke patients received an MRI scan 4.3 ± 3.0 d after onset of stroke. All studies were performed on a clinical 1.5 T MRI unit (Siemens Symphony). MRI protocol included diffusion-weighted imaging (DWI), which was performed with a single-shot echo planar imaging spin echo sequence [with 3 diffusion encoding gradients along orthogonal axes at b = 1000 s/mm2 and 1 B0 acquisition with no diffusion weighting; repetition time (TR), 4600 ms; echo time (TE), 137 ms; field of view (FOV), 250 × 250 mm; matrix, 128 × 128 pixels; slice thickness, 6 mm; gap, 1.2 mm, 2 averages]. DWI has proven to be very sensitive for the detection of acute infarcts and shows high accuracy in predicting final infarct size (Ricci et al., 1999; Schaefer et al., 2002).
Lesion analysis.
Ischemic lesions were identified by DWI. Patients with bilateral brain lesions or with tumors, and those in whom MRI scans revealed no DWI restriction, were excluded. At the individual level, preprocessing consisted of (1) nonbrain removal, and (2) Eddy Current Correction, using tools from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain's Software Library implemented in FSL (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). Thereafter, regions displaying diffusion restriction in each of the three gradient directions were drawn manually on the transverse slices as regions of interest (ROIs). Each individual 3D mask was coregistered to the corresponding structural image in native space, and structural images were transformed to the structural standard space of the MNI152 template (supplied with FSL) by full affine registration. The same transformation matrices used for structural-to-standard transformations were then used for ROI-to-standard space transformations. All registrations were performed using an intermodal registration tool based on the correlation ratio (Jenkinson and Smith, 2001). In the end, there was one three-dimensional, spatially standardized lesion mask for each patient.
Group analysis.
The statistical process performed in voxel-based lesion–symptom mapping (VLSM) (Bates et al., 2003) consists of the following steps: At each voxel of the spatially standardized MR images, patients are divided into two groups according to whether they did or did not have a lesion affecting that voxel. Behavioral scores are then compared for these two groups with a t test, yielding a single-tailed p value for each voxel. This results in color-coded VLSM maps that represent voxels where patients with lesions show a significantly different behavioral score from those whose lesions spared that voxel at an α level of 0.001 after correction for multiple comparisons using the false discovery rate (Curran-Everett, 2000). Software to perform VLSM operates on Matlab (Mathworks, 2002) and is freely available online (http://crl.ucsd.edu/vlsm). VLSM analysis was run once for each behavioral score, i.e., experience of contralesional RHI, experience of ipsilesional RHI, and contralesional somatognosia, each resulting in a probability map. Additionally, VLSM was run for the scores of questions No 4–9, which served as control statements.
Probabilistic diffusion tractography.
To trace cortical connections of fiber bundles that were interrupted by the ischemic subcortical lesions, significant voxels yielded by VLSM analysis served as seed areas for probabilistic diffusion tractography based on a dataset of 12 age-matched healthy control subjects aged between 44 and 74 years (56.7 ± 9.5 years). Diffusion-weighted images (axial slice thickness, 72 × 2 mm; matrix, 128 × 104; FOV, 256 × 208 mm; giving a voxel size of 2 × 2 × 2 mm; 60 (isotropically distributed) diffusion directions; b = 1000 s/mm2; 1.5 T Siemens Sonata scanner) were processed using FMRIB's Diffusion Toolbox (Behrens et al., 2003; Smith et al., 2004). We skull stripped (Smith, 2002) diffusion-weighted, T1-weighted (fast low-angle shot; TR = 12 ms; TE = 5.65 ms; flip angle = 19°; voxel size, 1 × 1 × 1 mm) and MNI standard brain template images and performed affine registration (Jenkinson and Smith, 2001) to derive transformation matrices among the three spaces.
We fitted a multifiber diffusion model (Behrens et al., 2007) that estimates probability distributions on the direction of one or more fiber populations at each brain voxel. Probabilistic tractography was then performed from seed voxels by tracing streamline samples through these probabilistic distributions on fiber direction. For all tractography, we generated 25,000 streamline samples from each seed voxel to build up a connectivity distribution. The number of these samples passing through each brain voxel is interpreted as proportional to the probability of connection to the seed voxel. Tractography outputs from each individual subject were thresholded and binarized to include only those voxels through which at least 250 streamline samples passed. Thresholded, binarized outputs were then overlaid across subjects and converted to population probability maps in which voxel values represent the proportion of the population in whom the tract is present at that voxel.
Results
Stroke topography and clinical features
An overlay of the lesions of all included patients (n = 70) is shown in supplemental Figure 1 (available at www.jneurosci.org as supplemental material). A maximum number of overlapping lesions was found in basal ganglia and periventricular white matter, reflecting ischemic strokes within areas supplied by the medial cerebral artery, whose territory is most frequently affected in ischemic stroke. Ischemic lesions were mainly found in insular cortex, putamen, postcentral and precentral cortical regions, and thalamus.
While two of the patients had moderate extrapersonal neglect according to the clock-drawing task (Azouvi et al., 2002), no patient showed signs of personal spatial neglect in the hand-reaching task.
Rubber hand illusion failure after stroke
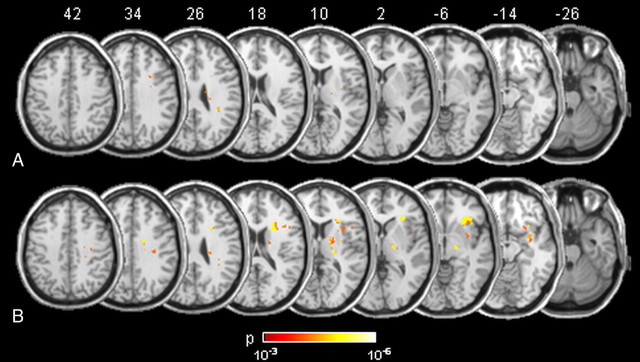
Contralesional RHIF was observed in 18 (26%) out of 70 stroke patients. Nine patients with contralesional RHIF had right hemispheric and nine had left hemispheric strokes. White matter lesions that were significantly associated with contralesional RHIF as outlined by VLSM (total number of 71 voxels) are shown in Figure 1A. An additional ipsilesional RHIF was found in seven of the patients with contralesional RHIF (three with right, four with left hemispheric stroke). Ipsilesional RHIF was associated with white matter lesions similar to the regions found for contralesional RHIF, though considerably more widespread (total number of 738 voxels) (Fig. 1B). When VLSM was run for the scores of the control statements (No 4–9), no significant lesion voxels were obtained. In the healthy control group (n = 40), three subjects (7.5%) reported unilateral and one (2.5%) reported bilateral RHIF (p = 0.047 as compared to stroke patients, χ2 test). The questionnaire results reporting nine specific perceptual effects of the RHI experiment (from Botvinick and Cohen, 1998) are shown in supplemental Figure 2 (available at www.jneurosci.org as supplemental material).
Figure 1.
Probability maps of lesion voxels significantly associated with contralesional RHIF (A) and ipsilesional RHIF (B). The level of probability is illustrated by colors. Stereotaxic space (MNI152) z coordinates of the transverse sections are given.
Probabilistic diffusion tractography—rubber hand illusion failure
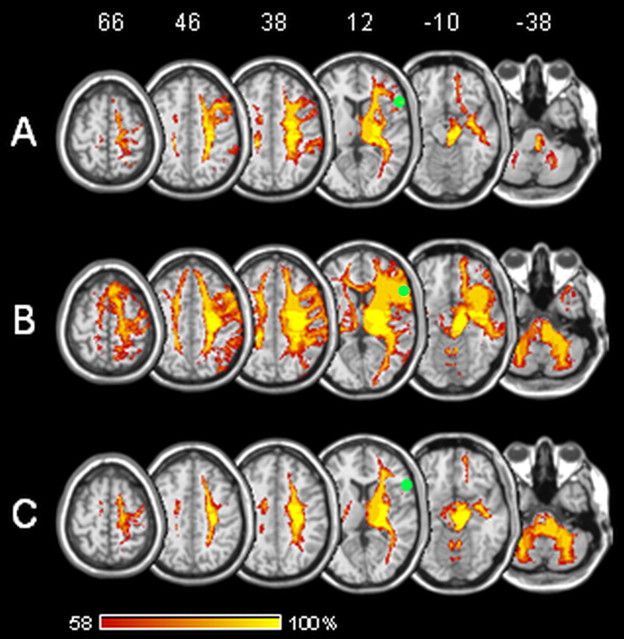
Voxels significantly associated with RHIF were located within white matter fiber tracts. Probabilistic diffusion tractography was used to test their connections to cortical areas that might be relevant for the RHI. From seeds generated out of VLSM analysis, there were consistent and strong connections to PMv and frontal operculum. Moreover, paths were generated up to parietal and prefrontal cortex and down into thalamus, basal ganglia, and cerebellum (Fig. 2A,B).
Figure 2.
Diffusion tractography based on a dataset of 12 healthy control patients. Seed areas were voxels significantly associated with contralesional (A) and ipsilesional (B) rubber hand illusion failure and contralesional asomatognosia (C). The grouped tracts are in standard (MNI) space. Voxel values represent the percentage of subjects in whom a path passed through the respective voxel. Green sphere, Premotor area associated with rubber hand illusion in Ehrsson et al. (2004). Stereotaxic space (MNI152) z coordinates of the transverse sections are given.
Asomatognosia after stroke
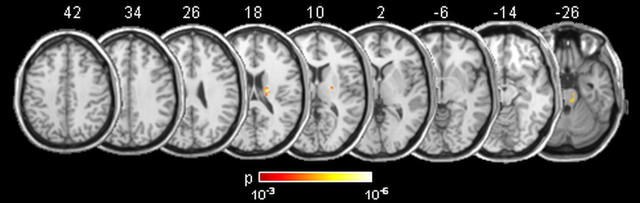
Spontaneous asomatognosia of the contralesional hand was found in 18 (28%) out of 64 stroke patients, but in none of the healthy control subjects. Twelve of the patients with asomatognosia had right, and six had left, hemispheric strokes. Lesion voxels within the subcortical white matter that showed significant association with contralesional asomatognosia (total number of 114 voxels) are shown in Figure 3.
Figure 3.
Probability maps of lesion voxels significantly associated with contralesional asomatognosia. The level of probability is illustrated by colors. Stereotaxic space (MNI152) z coordinates of the transverse sections are given.
Probabilistic diffusion tractography—asomatognosia
To test connections of voxels significantly associated with asomatognosia to cortical areas with potential relevance for self recognition, we used probabilistic diffusion tractography. From seeds generated out of VLSM analysis, there were connections to parietal cortex, prefrontal cortex, and frontal operculum and down into thalamus, basal ganglia, and cerebellum, but not to PMv (Fig. 2C).
Discussion
The present study mapped lesion location associated with the RHI in acute stroke patients to examine the role of PMv in generating the illusory feeling of hand ownership and asomatognosia.
Brain regions associated with RHIF were located exclusively subcortically. In particular, PMv did not emerge from the lesion-mapping approach. To interpret this finding, it is important to realize that the lesion-mapping approach has inherent limitations in stroke patients: (1) The likelihood of alerting patients to possible stroke varies widely across affected brain regions and might be highest if motor, somatosensory, or visual deficits are present. This results in a bias toward patients with deficits in motor or sensory capacities and might lower the detection rate of strokes within secondary cortical areas. (2) The probability of lesions is not distributed equally across the brain, but depends on the vascular architecture and differential vulnerability of brain regions (Rorden and Karnath, 2004). (3) Patients with aphasia and severe deficits of tactile perception and attention were excluded from participation in the study. Therefore brain regions associated with these deficits, such as Broca's area (which is located in the vicinity of premotor cortex), Wernicke's area, and primary somatosensory cortex, were unlikely to be associated with RHIF. Together, these considerations imply that the lesion method applied here may have low or absent sensitivity for certain brain regions. However, it may be highly specific as it demonstrates unequivocally that disruption of certain brain regions is causally associated with a behavioral phenotype (Rorden and Karnath, 2004).
The fact that premotor cortex did not emerge from the lesion analysis does not exclude the possibility that processing activity in PMv plays a decisive role in RHI. However, detecting this role might rather depend on the analysis of projecting fibers. Tractography analysis of fiber projections through the lesion voxels revealed consistent connections with unilateral PMv from voxels associated with failure of the RHI. This finding supports the hypothesis that PMv plays a causal role in the RHI and that lesions to PMv or its connections should be associated with RHIF (Ehrsson et al., 2004). Though RHI was associated with bilateral activation of premotor cortex in the fMRI study by Ehrsson et al. (2004), the absence of transcallosal projections to homologous PMv from RHIF-associated lesion voxels in the present study suggests that disruption of one PMv might suffice for failure of the illusion. Furthermore, since RHIF was found after lesions to either hemisphere, there is no hemispheric dominance in generating the RHI.
Somatognosia, the continuous awareness about one's own body parts, seems to be based on a variety of distributed systems built of specifically interconnected brain areas rather than one specialized region (Berlucchi and Aglioti, 2010). Analyses of the functional anatomy of asomatognosia have implicated brain regions including the parietal (So and Schaüble, 2004), temporoparietal (Arzy et al., 2006a), insular (Baier and Karnath, 2008), and premotor (Arzy et al., 2006b) cortices. Lesions associated with loss of awareness about one's body parts should be expected to overlap with those disrupting feelings of body ownership in the RHI paradigm. Indeed, the connections of asomatognosia-associated lesion voxels were partially congruent with those found for RHIF. However, we found two important dissociations between RHIF and asomatognosia: Behaviorally, RHIF was present in similar percentages of stroke patients with and without contralesional asomatognosia; in particular, a number of stroke patients with impairment of the ability to perceive their real hand as belonging to them easily integrated the plastic hand as their own. Anatomically, as outlined above, the fiber tracts affected by ischemic lesions in RHIF involved connections to PMv, which were not implicated in the functional anatomical analysis of the asomatognosia group.
The fact that somatognosia, unlike RHI, may be robust toward lesions of fiber connections with PMv, has important implications for understanding the role of PMv in body ownership. Somatognosia and RHI differ behaviorally, among other features, by the time scale of the perceptual experience, which is extended in the former, but highly dynamic and short-lived in the latter. We consider it likely that this behavioral difference holds a clue to explaining the anatomical dissociation, regarding the role of PMv. In line with a neurocognitive model of body ownership during the RHI (Tsakiris, 2010), we propose that there is a sequence of critical comparisons between current perception and preexisting models of the body. While visual and postural properties of the hand are matched in distinct other brain regions, PMv is active during the comparison between the vision of touch and the felt touch and the respective reference frames, i.e., body-related multisensory integration preceding the onset of illusory body-ownership feeling. We thus consider PMv necessary for the detection of concurrent multisensory events challenging body representation, and for the resolution of a potential conflict with current body representation by assimilation of the latter. Lesion to the network sustaining continual body ownership experience might not necessarily block induction of the RHI as long as connections with PMv are intact. Lesions to premotor areas, including PMv, have been shown to be associated with denial of hemiparesis following right-hemispheric stroke (Berti et al., 2005). This finding may indicate that the supramodal role of PMv is not restricted to the sensory domain but also relates to integration of action plans with actions.
There are a number of limitations to this study. First, RHIF and asomatognosia were assessed only in a subgroup of the stroke patients. Second, the RHI was not assessed with objective measures, but only with questionnaires. Another limitation results from the MRI protocol: We used DWI to visualize ischemic lesions at an early stage. DWI is particularly sensitive for the detection of hyperacute infarcts (Chong et al., 1998; Schaefer et al., 2002) and is able to predict final infarct volume (Schaefer et al., 2002), but areas that appear intact are not necessarily functioning normally, as a result of abnormal perfusion or deafferentation and diaschisis. This may result in an underestimation of the “functional size” of the ischemic lesions and consecutively in an underestimation of the seed areas used for tractographic analysis. Finally, the fact that we acquired high angular resolution diffusion data for tractography in a separate group of healthy control subjects and not in the stroke patients could be seen as a limitation. However, this approach was taken as the aim of the tractography was to determine the fiber pathways that would normally traverse through lesioned tissue. Interpretation of tractography in patients with brain lesions is challenging and would not have provided a straightforward test of the specific hypotheses considered here.
Altogether, our findings are consistent with a role of PMv in dynamic assimilation of body representation following multisensory challenge. Although feelings of ownership can be manipulated easily by experimental procedures or simply by tool use, they might not be updated continuously. One strong prediction arising from our conclusions is that recovery from asomatognosia depends on the functional intactness of (at least one) PMv.
Footnotes
This work was supported by the state of Bavaria.
References
- Arzy S, Thut G, Mohr C, Michel CM, Blanke O. Neural basis of embodiment: distinct contributions of temporoparietal junction and extrastriate body area. J Neurosci. 2006a;26:8074–8081. doi: 10.1523/JNEUROSCI.0745-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzy S, Overney LS, Landis T, Blanke O. Neural mechanisms of embodiment: asomatognosia due to premotor cortex damage. Arch Neurol. 2006b;63:1022–1025. doi: 10.1001/archneur.63.7.1022. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, Chokron S, Leclercq M, Marchal F, Martin Y, De Montety G, Olivier S, Perennou D, Pradat-Diehl P, Prairial C, Rode G, Siéroff E, Wiart L, Rousseaux M. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski J. Contribution a l'étude des troubles mentaux dans l'hémiplégie organique (anosognosie) Rev Neurol. 1914;27:845–848. [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39:486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti SM. The body in the brain revisited. Exp Brain Res. 2010;200:25–35. doi: 10.1007/s00221-009-1970-7. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, Castiglioni I, Vallar G, Paulesu E. Shared cortical anatomy for motor awareness and motor control. Science. 2005;309:488–491. doi: 10.1126/science.1110625. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Chong J, Lu D, Aragao F, Singer MB, Schonewille WJ, Silvers A, Tuhrim S, Atlas SW. Diffusion-weighted MR of acute cerebral infarction: comparison of data processing methods. AJNR Am J Neuroradiol. 1998;19:1733–1739. [PMC free article] [PubMed] [Google Scholar]
- Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Lackner JR. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain. 1988;111:281–297. doi: 10.1093/brain/111.2.281. [DOI] [PubMed] [Google Scholar]
- Makin TR, Holmes NP, Ehrsson HH. On the other hand: dummy hands and peripersonal space. Behav Brain Res. 2008;191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Ricci PE, Burdette JH, Elster AD, Reboussin DM. A comparison of fast spin-echo, fluid-attenuated inversion-recovery, and diffusion-weighted MR imaging in the first 10 days after cerebral infarction. AJNR Am J Neuroradiol. 1999;20:1535–1542. [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rochat P. The innate sense of the body develops to become a public affair by 2–3 years. Neuropsychologia. 2010;48:738–745. doi: 10.1016/j.neuropsychologia.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Rochat P, Striano T. Perceived self in infancy. Infant Behav Dev. 2000;23:513–530. [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Schaefer PW, Hunter GJ, He J, Hamberg LM, Sorensen AG, Schwamm LH, Koroshetz WJ, Gonzalez RG. Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR Am J Neuroradiol. 2002;23:1785–1794. [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- So EL, Schaüble BS. Ictal asomatognosia as a cause of epileptic falls: simultaneous video, EMG, and invasive EEG. Neurology. 2004;63:2153–2154. doi: 10.1212/01.wnl.0000145628.38030.3e. [DOI] [PubMed] [Google Scholar]
- Tsakiris M. My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- van der Kamp J, Savelsbergh G. Action and perception in infancy. Infant Behav Dev. 2000;23:237–251. [Google Scholar]