Abstract
Study Objectives:
To estimate the prevalence of and identify sociodemographic risk factors for sedative medication use in the general Canadian population, and to examine the association between sedative medication use and body mass index (BMI).
Design:
Cross-sectional study
Setting:
Canadian population
Participants:
Participants from the 1994-2003 Canadian national health surveys, the National Population Health Survey (NPHS) and the Canadian Community Health Survey (CCHS). For the 2003 CCHS, n = 134,072, ages 12-80+ years.
Interventions:
Not applicable
Measurements and Results:
The overall prevalence of sedative medication use in Canada in 2003 was 5.5%, having more than doubled since 1994. Notable rises in sedative medication use have occurred among men, non-elderly, and obese individuals. After adjusting for potential sociodemographic and health status confounders, including psychiatric comorbidities, the odds of sedative use were significantly greater among morbidly obese (BMI ≥ 35 kg/m2) men (OR = 1.89, 95%CI = 1.02-3.53) and underweight (BMI < 18.5 kg/m2) women (OR = 2.11, 95%CI = 1.26-3.53).
Conclusions:
The use of sedative medications has substantially risen among the general Canadian population, and among particular population subgroups. The greater odds of sedative medication use found among morbidly obese men may reflect the presence of underlying obstructive sleep apnea, which may in turn serve to explain in part the known relationship between sedative medications and mortality. The increase in sedative medications coupled with their known adverse health associations raises potential public health concerns.
Citation:
Vozoris NT; Leung RS. Sedative medication use: prevalence, risk factors, and associations with body mass index using population-level data. SLEEP 2011;34(7):869-874.
Keywords: Sedative, body mass index, epidemiology
INTRODUCTION
Longitudinal general population-level estimates of sedative medication use are lacking. The few Canadian studies on sedative medication use that do exist are single-point cross-sectional,1–3 and are limited to specific regions of the country,2–5 elderly individuals,1,3–5 or users of benzodiazepines only.1,3–5 Single-point cross-sectional estimates of sedative medication use are available for several other countries,2,6–10 but longitudinal data on sedative use is minimal.11 While gender and age disparities with respect to sedative use have been documented,2,7,11,12 trends over time, and other potential risk factors that may underlie sedative medication use (such as, socioeconomic status, cigarette and alcohol consumption), are poorly understood.
Understanding the scope and nature of sedative medication use is important because sedative use has been associated with increased mortality,13,14 although this has not been consistently demonstrated.1,15–17 Cancer Prevention Study data of over 1.1 million individuals in the US revealed that use of prescription sedatives (not limited to any specific medication type) of greater or equal to 30 times per month was associated with an approximately 25% increased risk of mortality over a 6-year follow-up period in both men and women.13 This association was independent of 32 sociodemographic and health status covariates, including sleep duration and various cardiorespiratory diseases.13 The increased risk of falls and fractures,18,19 car accidents,20,21 and poisonings22,23 among users of sedatives may in part serve to explain the sedative-mortality association. There is also data showing that sedatives are used with increased frequency among individuals with obstructive sleep apnea,24,25 which is in turn associated with increased mortality.26,27 However, these data are based on small, selected populations and potential confounders were not controlled for. If indeed sedatives are used with increased frequency in sleep apnea, this may also serve in part to explain the increased mortality associated with sedatives. Obesity is considered to be a major risk factor for obstructive sleep apnea,28–31 with the prevalence of obstructive sleep apnea among obese individuals being as high as 71%.32
The objectives of this study were estimate the prevalence of and identify sociodemographic risk factors for sedative medication use in the general Canadian population. We also examined the association between sedative use and body mass index (BMI), which is an important risk factor for obstructive sleep apnea.
METHODS
Data Sources
This study was conducted with data from Canada’s national health surveys, the National Population Health Survey (NPHS) and the Canadian Community Health Survey (CCHS), the latter of which supplanted the NPHS in 2000. The NPHS and the CCHS were undertaken by Statistics Canada to collect cross-sectional self-reported sociodemographic and health data every 2 years on a nationally representative sample of 98% of the Canadian population aged 12 years and older. A detailed description of the survey designs and methodologies appears elsewhere.33,34 Three cycles of the NPHS were completed (1994, 1996, 1998) before the CCHS began to be administered. Information on sedative medication use was collected by all 3 cycles of the NPHS, and the first two cycles of the CCHS (2000/01, 2003). Information on sedative medication use was not collected by the CCHS beyond 2003. See Appendix for the sample sizes and overall response rates for these surveys. The analyses of sedative medication use risk factors and association with BMI were conducted specifically with the 2003 CCHS, since this was the most recent national health survey cycle available, and because unlike previous cycles, it collected information on health-professional diagnosed self-reported mood and anxiety disorders, allowing us to control for their potentially confounding effects.
Variable Definitions
Both the NPHS and CCHS included the following question: In the past month, did you take sleeping pills? While potential examples of sleeping pills were provided, responses were not limited to any specific medication types, and potentially included both prescription and over-the-counter sedatives. The response rate to this question was very high among all survey cycles (e.g., 98.8% for the 2003 CCHS). Respondents who did not know or did not state their sedative medication use status were excluded from the analysis (e.g., 1.2% of respondents for the 2003 CCHS).
All sociodemographic data were collected via self-report. Presence of mood and anxiety disorders were based on self-reported health-professional diagnosis of the medical condition that had lasted, or was expected to last, for 6 months or longer. BMI was based on self-reported height and weight, and BMI classification followed World Health Organization cutoffs.35 Self-reported BMI has been previously found to have high accuracy with objectively measured BMI.36,37 Analysis of 2005 CCHS data, which collected self-reported and objectively measured BMI on a randomly selected subsample of survey participants, also showed high correlations between the self-reported and measured BMI (Pearson correlation coefficient = 0.89, P < 0.0001). Respondents who did not know or did not state their height and weight were excluded from the analysis involving BMI (14% of respondents for the 2003 CCHS).
Statistical Analysis
All analyses were performed on SAS version 9.1.3. The overall prevalence, and the prevalence of sedative medication use stratified by gender, age group, and obesity status, was calculated for each of the national health survey cycles. Using the 2003 CCHS, multiple logistic regression was used to identify independent sociodemographic risk factors for sedative medication use. Logistic regression was also used to calculate the unadjusted odds of sedative use across BMI groups using the 2003 CCHS. All the sociodemographic variables listed in Table 1, including self-reported health professional diagnosed mood and anxiety disorders, were then included in a second regression model to calculate the odds of sedative use after adjusting for these potential confounders. Given known gender differences in BMI and sedative medication use,2,7,11,12 the regression analysis were reported separately by gender as well.
Table 1.
Odds of sedative medication use across various sociodemographic factors using the 2003 CCHS sample
| Adjusted OR (95% CI)* | P-value | |
|---|---|---|
| Sex | ||
| Male | 1.00 | |
| Female | 1.40 (1.17-1.67) | < 0.01 |
| Province | ||
| Ontario | 0.88 (0.72-1.08) | 0.21 |
| British Columbia | 1.00 | |
| Age group | ||
| 12-19 | 0.61 (0.40-0.92) | 0.02 |
| 20-39 | 1.00 | |
| 40-59 | 1.36 (1.05-1.77) | 0.02 |
| 60+ | 1.75 (1.29-2.37) | 0.01 |
| Race | ||
| Caucasian | 1.00 | |
| Visible minority | 1.30 (0.96-1.75) | 0.09 |
| Immigrant | ||
| Yes | 0.95 (0.77-1.17) | 0.64 |
| No | 1.00 | |
| Income level | ||
| Lowest | 0.92 (0.62-1.39) | 0.71 |
| Lower middle | 0.96 (0.74-1.35) | 0.99 |
| Middle | 0.95 (0.77-1.19) | 0.69 |
| Upper middle | 1.00 | |
| Highest | 0.95 (0.77-1.19) | 0.67 |
| Major income source | ||
| Employment | 1.00 | |
| Welfare | 1.74 (1.12-2.68) | 0.01 |
| Seniors’ benefits | 1.60 (1.26-2.02) | < 0.01 |
| Other | 1.46 (0.97-2.18) | 0.07 |
| Highest education attained | ||
| < Secondary | 1.04 (0.74-1.46) | 0.83 |
| Secondary | 0.96 (0.68-1.37) | 0.84 |
| Some post-secondary | 1.00 | |
| Post-secondary | 0.98 (0.72-1.33) | 0.89 |
| Smoking status | ||
| Never smoker | 1.00 | |
| Former smoker | 1.42 (1.15-1.76) | < 0.01 |
| Current smoker | 1.61 (1.27-2.05) | < 0.01 |
| Alcohol drinking status | ||
| Never drinker | 1.00 | |
| Non-daily drinker | 0.94 (0.77-1.16) | 0.58 |
| Daily drinker | 1.11 (0.80-1.54) | 0.53 |
| Health professional-diagnosed mood disorder | ||
| Yes | 3.46 (2.58-4.64) | < 0.01 |
| No | ||
| Health professional-diagnosed anxiety disorder | ||
| Yes | 1.82 (1.31-2.53) | < 0.01 |
| No | 1.00 |
Adjusted for all other variables listed in table.
The CCHS uses a complex sampling design, employing stratification and multi-stage clustering. To account for the unequal probabilities of selecting respondents, all results were weighted using the survey sample weights provided. All sample weights were re-scaled prior to each analysis by dividing the original weight by the average weight of respondents included in the specific analysis, according to Statistics Canada guidelines.38 To account for the effects of stratification and clustering on variance estimates, all confidence intervals were calculated using bootstrap resampling techniques with bootstrap weights created by Statistics Canada. Ethics approval was granted by the University of Toronto Office of Research Ethics.
RESULTS
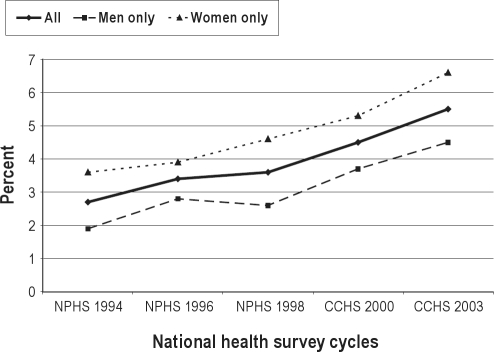
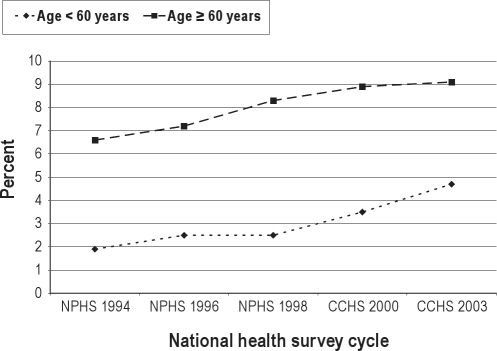
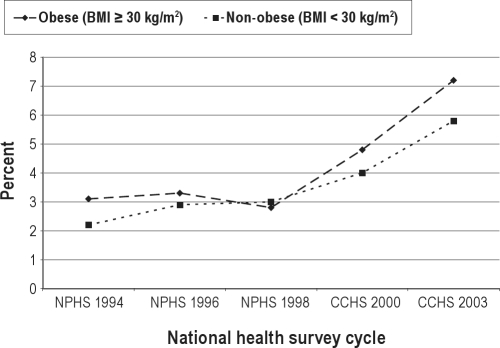
Of the 20,745 survey participants who were asked and gave a response to the question on sedative medication use in the 2003 CCHS, 1,192 individuals or 5.5% responded affirmatively to using sedatives within the past month. Between 1994-2003, the overall prevalence of sedative use in Canada has more than doubled (Figure 1). Although sedative use has consistently been higher among Canadian women, the relative percentage change in sedative use between 1994-2003 was greater among men (1.9% to 4.5%, or 200% increase) than women (3.6% to 6.6%, or 83% increase). While sedative use has been consistently higher among individuals aged 60 years and older versus individuals aged less than 60 years, the relative percentage change in sedative use was greater in the latter age group (1.9% to 4.7%, or 147% increase) than the former (6.6% to 9.1%, or 38% increase) (Figure 2). Up to 2000, the prevalence of sedative use was similar among obese and non-obese Canadians. However, starting in 2000, sedatives were used with increasing frequency among obese individuals versus non-obese individuals (7.2% versus 5.8% in 2003, respectively; Figure 3). Independent risk factors for sedative medication use were female gender, older age group, individuals supported by welfare and seniors’ benefits, current and former smokers, and individuals with health-professional diagnoses of mood and anxiety disorders (Table 1).
Figure 1.
Prevalence of sedative medication use among Canadian national health survey participants between 1994-2003, stratified by gender.
Figure 2.
Prevalence of sedative medication use among Canadian national health survey participants between 1994-2003, stratified by age group.
Figure 3.
Prevalence of sedative medication use among Canadian national health survey participants between 1994-2003, stratified by obesity status.
The odds of sedative medication use among BMI groups use followed a U-shaped distribution, with significantly greater odds among underweight (BMI < 18.5 kg/m2) individuals and among obese (BMI ≥ 30 kg/m2) and morbidly obese (BMI ≥ 35 kg/m2) individuals (Table 2). The associations between sedative medication use and underweight and morbid obesity remained significant even after controlling for the 12 covariates listed in Table 1. After stratifying the analysis by gender, the odds of sedative medication use were significantly greater only among women who were underweight and among men who were morbidly obese.
Table 2.
Odds of sedative medication use across BMI groups, stratified by gender, using the 2003 CCHS sample
| BMI (kg/m2) | Unadjusted OR (95% CI) with P-value for all | Adjusted OR (95% CI) with P-value for all | Adjusted OR (95% CI) with P-value for men | Adjusted OR (95% CI) with P-value for women |
|---|---|---|---|---|
| < 18.5 | 1.84 (1.15-2.95), P = 0.01 | 1.91 (1.21-3.04), P < 0.01 | 0.96 (0.03-29.23), P = 0.98 | 2.11 (1.26-3.53), P < 0.01 |
| 18.5-24.9 | 1.00 | 1.00 | 1.00 | 1.00 |
| 25-29.9 | 1.12 (0.93-1.36), P = 0.23 | 1.06 (0.88-1.29), P = 0.53 | 1.09 (0.79-1.51), P = 0.60 | 1.06 (0.83-1.34), P = 0.65 |
| 30-34.5 | 1.33 (1.03-1.72), P = 0.03 | 1.22 (0.93-1.60), P = 0.15 | 1.20 (0.76-1.92), P = 0.43 | 1.23 (0.89-1.72), P = 0.21 |
| ≥ 35 | 1.72 (1.21-2.45), P < 0.01 | 1.47 (1.02-2.12), P = 0.04 | 1.89 (1.02-3.53), P = 0.04 | 1.29 (0.84-2.00), P = 0.25 |
Adjusted models include sex, age group, province of residence, income level, major household income source, immigration status, racial group (Caucasian versus visible minority), highest education level attained, smoking status, alcohol drinking status, health-professional diagnosed mood disorder, and health-professional diagnosed anxiety disorder.
DISCUSSION
The prevalence of sedative medication use has more than doubled in Canada since 1994, which is concerning given the increased morbidity and mortality associated with these medications.13,14,18–23,39 While sedatives are more frequently used by women and older age groups, surprisingly, notable rises in sedative use are occurring among men, individuals under 60 years of age, and among obese individuals. After adjusting for sociodemographic and psychiatric confounders, greater odds of sedative medication use were found among morbidly obese men and underweight women. Increased sedative use among morbidly obese men may be associated with the presence of underlying obstructive sleep apnea, which may in turn serve in part to explain the known relationship between sedative medications and mortality.
The overall prevalence of sedative medication use in Canada is relatively similar to some countries, like the United States,9 Norway,7 and Italy.6 However, our Canadian prevalence estimates are higher compared to Germany and Britain,6 and lower compared to France,2,6,8 where more than twice the Canadian rate of sedative use has been reported. Our novel finding of increasing sedative medication use over time among Canadians is concerning given the many associated adverse health effects of these medications, including falls and hip fractures,18,19 car accidents,20,21 poisonings,22,23 cognitive impairment,39 and mortality.13,14 While sedative medication use has been previously reported to be declining in Canada,4 this analysis included only Ontarians aged 65+ years and prescription benzodiazepine medication use. In contrast, our analysis included residents from multiple regions of the country, ages 12-80+ years, and captured sedative medication use more broadly. We calculated the change in prevalence during the same period for several other classes of medications, including inhalers, antihypertensives, antibiotics, oral hypoglycemic agents, and antidepressants (data not shown). With the exception of antidepressants, no other medication classes had similar increases over time as sedatives, indicating that the rise in sedative agents is not occurring in the context of rising medication use across the board.
The observed rise in sedative use may be explained by a rising awareness in society of insomnia and its potential consequences, or by a rise in insomnia symptoms in the population. Increasing use of sedative medications for general psychiatric purposes may also potentially explain the temporal rise. The emergence of the potentially safer non-benzodiazepine receptor agonists may also explain the rise in sedative use. Zopiclone entered the Canadian market in 1994,40 which is the first year of our data analysis, and zaleplon became available in Canada in 200040 but was discontinued in 2007.41 Although zolpidem was approved in Canada in 1996 it was never marketed for use,40 and ramelteon is as of yet unavailable. While trends in medication use can also be a function of government drug formulary changes, there were no formulary changes with respect to sedative agents in the largest of Canada’s provinces by population (Ontario) during the study period,42 although other provinces may differ.
Similar to previous studies,2,7,11,12 we have found that women and older age groups use sedatives more frequently. However, our findings that sedative use is also rising among men and the non-elderly is novel. In contrast to previous studies that found no increased or lower sedative use among individuals who smoked cigarettes and consumed alcohol in the general43 and elderly44 populations, we found significantly increased use of sedatives among current and former smokers, but not among consumers of alcohol. While the association between sedative use and smoking may be explained by the higher rates of psychiatric disease among smokers,45 our findings were independent of comorbid mood and anxiety disorders.
Significantly greater odds of sedative medication use were found among morbidly obese men, independent of psychiatric comorbidity, and the prevalence of sedative use is increasing more so among obese versus non-obese individuals in the population. The finding of increased sedative medication use among morbidly obese men might be explained by underlying obstructive sleep apnea. Obesity is a well-accepted risk factor for sleep apnea, with the prevalence of obstructive sleep apnea among obese individuals in population studies ranging from 7% to 36%,30,31 and 25% to 71% in clinical studies.28,29,32 Obesity is even a stronger risk factor for obstructive sleep apnea in men than women,28,30,31 with the prevalence in obese men being as high as 88%.29 A high prevalence of insomnia complaints exists among individuals with objectively diagnosed obstructive sleep apnea, ranging from about 40% to 60%,46,47 the reason for which is not entirely clear. Previous studies have reported high use of sedatives among individuals with obstructive sleep apnea, ranging from 50% to 74%,24,25 but these data were based on small, selected populations, and were univariate associations that did not control for any potential confounders. Given that obstructive sleep apnea is both associated with obesity and increased mortality,26,27 underlying obstructive sleep apnea may therefore serve in part to explain the relationship between sedative medications and mortality. Alternatively, the increased use of sedative medications among obese individuals may be explained by the presence of insomnia due to nocturia48,49 or musculo-articular pains,50 which obese individuals are at increased risk for. The increased use of sedatives among obese individuals is also potentially concerning given that sedatives, and specifically benzodiazepines, may worsen untreated sleep apnea,51–54 although this has not been a consistent finding.55–58
Sedative medications were also found to be used with increased frequency among underweight women, and this relationship was independent of self-reported diagnosed mood and anxiety disorders. While undiagnosed or unreported psychiatric disorder may potentially explain this relationship, the presence of other comorbid medical conditions that are associated with underweight women and associated with sleep disturbance may also serve as possible explanations, including eating disorders,59 hyperthyroidism,60 connective tissue disease,61 and gynecological malignancy.62
Strengths of this study are that it is based on a very large sample of Canadians including data on a wide range of sociodemographic and health status variables. Sedative medication use was broadly asked, including both prescription and non-prescription agents. Although self-reported BMI was used, this had excellent correlation with objectively measured BMI in the CCHS. Upwards of 12 sociodemographic and health status variables were included in our logistic regression models of BMI to control for potential confounding. Our study also has several limitations. Sedative medication use was based on self-report and may have been underestimated because of recall and social desirability biases. Our prevalence estimates of sedative drug use are indeed likely underestimated, given previous research showing that questions on self-reported sleeping pill use underestimate actual sedative medication use by as much as 27%.63 Sedative medication use was asked only with respect to the preceding month, and thus chronic sedative use was not captured, although most sedative medication use has been shown to be consumed by chronic users.64 Breakdown of the data by sedative medication type (e.g., benzodiazepine, non-benzodiazepine) was not possible. Although participants’ response rate to the question on sedative medication use was very high, because these questions were included in the survey for only selected health regions of the country as determined by provincial health representatives in consultation with Statistics Canada (17% of the total health regions in Canada, limited to the provinces of Ontario and British Columbia), results may not be generalizable to all of Canada.
In conclusion, the increasing use of sedatives among Canadians is potentially concerning given the increased morbidity and mortality associated with these medications. Sedative use is rising among certain groups, like men and the non-elderly, who were previously thought to be at lower risk of using sedatives. Greater odds of sedative medication use were found at both extremes of body weight, morbid obesity and underweight, with notable gender discrepancies. The finding of increased sedative use among morbidly obese men may reflect the presence of underlying obstructive sleep apnea, which may in turn serve in part to explain the known relationship between sedative medications and mortality. The increased odds of sedative medication use among morbidly obese men may also be a cause for concern, given that there is some evidence showing that sedatives may worsen sleep apnea. The substantial rise in the use of sedative agents in the general population, in the context of their known multiple adverse health associations, raises potential concerns regarding public health safety.
ACKNOWLEDGMENT
This research was conducted solely at the University of Toronto.
Appendix
Canadian national health survey cycle total samples sizes and combined household-person level response rates
| National health survey cycle | Total sample size | Household-person level response rate |
|---|---|---|
| NPHS 1994 | 17,626 | 88.7% |
| NPHS 1996 | 81,804 | 82.6% |
| NPHS 1998 | 17,244 | 87.6% |
| CCHS 2000/01 | 130,880 | 84.7% |
| CCHS 2003 | 134,072 | 80.7% |
Footnotes
A commentary on this article appears in this issue on page 827.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hogan DB, Maxwell CJ, Fung TS. Prevalence and potential consequences of benzodiazepine use in senior citizens: results from the Canadian Study of Aging and Health. Can J Clin Pharmacol. 2003;10:72–7. [PubMed] [Google Scholar]
- 2.Ohayon MM, Caulet M. Psychotropic medication and insomnia complaints in two epidemiologic studies. Can J Psychiatry. 1996;41:457–64. doi: 10.1177/070674379604100711. [DOI] [PubMed] [Google Scholar]
- 3.Joint Committee on Drug Utilization. Benzodiazepine use in Saskatchewan. Regina: Saskatechewan; 1998. [Google Scholar]
- 4.Tu K, Mamdani MM, Hux JE, Tu J-B. Progressive trends in the prevalence of benzodiazepine in older people in Ontario, Canada. J Am Geriatr Soc. 2001;49:1341–5. doi: 10.1046/j.1532-5415.2001.49262.x. [DOI] [PubMed] [Google Scholar]
- 5.Rojas-Fernandez CH, Carver D, Tonks R. Population trends in the prevalence of benzodiazepine use in older populations of Nova Scotia – a cause for concern? Can J Clin Pharmacol. 1999;6:149–56. [PubMed] [Google Scholar]
- 6.Ohayon MM, Lader MH. Use of psychotropic medication in the general population of France, Germany, Italy, and the United Kingdom. J Clin Psychiatry. 2002;63:817–25. doi: 10.4088/jcp.v63n0912. [DOI] [PubMed] [Google Scholar]
- 7.Omvik S, Pallesen S, Bjorvatn B, Sivertsen B, Havik OE, Nordhus IH. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25:91–100. doi: 10.1097/YIC.0b013e328334e5e6. [DOI] [PubMed] [Google Scholar]
- 8.Quera-Salva MA, Orluc A, Goldenberg F, Guilleminault C. Insomnia and use of hypnotics: study of a French population. Sleep. 1991;14:386–91. doi: 10.1093/sleep/14.5.386. [DOI] [PubMed] [Google Scholar]
- 9.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–32. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 10.US Dept of Health, Education, and Welfare publication (PHS) 80-1559. Use habits among adults of cigarettes, coffee, aspirin, and sleeping pills, United States, 1976. Washington, DC: National Centre for Health Statistics; 1979. [PubMed] [Google Scholar]
- 11.Wysowski DK, Baum C. Outpatient use of prescription sedative-hypnotic drugs in the United States, 1970 through 1989. Arch Int Med. 1991;151:1779–83. [PubMed] [Google Scholar]
- 12.Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997-2002. Clin Ther. 2006;28:1044–53. doi: 10.1016/j.clinthera.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 14.Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle-aged population. Pharmacoepidemiol Drug Safety. 2007;16:913–8. doi: 10.1002/pds.1417. [DOI] [PubMed] [Google Scholar]
- 15.Vinkers DJ, Gussekloo J, van der Mast RC, Westendorp RGJ. Benzodiazepine use and risk of mortality in individuals aged 85 years and older. JAMA. 2003;290:2942–3. doi: 10.1001/jama.290.22.2942. [DOI] [PubMed] [Google Scholar]
- 16.Rumble R, Morgan K. Hypnotics, sleep, and mortality in elderly people. J Am Geriatr Soc. 1992;40:787–91. doi: 10.1111/j.1532-5415.1992.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 17.Drummer OH, Gerostamoulos J, Batziris H, et al. The involvement of drugs in drivers of motor vehicle accidents killed in Australia road traffic crashes. Accid Anal Prev. 2004;36:239–48. doi: 10.1016/s0001-4575(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 18.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Int Med. 2009;169:1952–60. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 19.Moden B, Merlo J, Ohlsson H, Rosvall M. Psychotropic drugs and falling accidents among the elderly: a nested case control study in the whole population of Scania, Sweden. J Epidemiol Community Health. 2010;64:440–6. doi: 10.1136/jech.2009.098947. [DOI] [PubMed] [Google Scholar]
- 20.Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet. 1998;352:1331–6. doi: 10.1016/s0140-6736(98)04087-2. [DOI] [PubMed] [Google Scholar]
- 21.Engeland A, Skurtveit S, Mrland J. Risk of road traffic accidents associated with the prescription of drugs: a registry-based cohort study. Ann Epidemiol. 2007;17:597–602. doi: 10.1016/j.annepidem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Rogers JJ, Heard K. Does age matter? Comparing case fatality rates for selected poisonings reported to US poison centres. Clin Toxicol. 2007;45:705–8. doi: 10.1080/15563650701517491. [DOI] [PubMed] [Google Scholar]
- 23.Gossop M, Stewart D, Treacy S, Marsden J. A prospective study of mortality among drug misusers during a 4-year period after seeking treatment. Addiction. 2002;97:39–47. doi: 10.1046/j.1360-0443.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–71. [PubMed] [Google Scholar]
- 25.Sivertsen B, Omvik S, Pallesen S, Nordhus IH, Bjorvatn B. Sleep disorders in elderly patients who take hypnotics on a regular basis. Tidsskr Nor Laegeforen. 2004;124:2600–2. [PubMed] [Google Scholar]
- 26.Young T, Finn L Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Rajala R, Partinen M, Sane T, Pelkonen R, Huikuri K, Seppalainen AM. Obstructive sleep apnea in morbidly obese patients. J Intern Med. 1991;230:125–9. doi: 10.1111/j.1365-2796.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 29.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring, and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 30.Young T, Peppard P, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Ford ES, Zhao G, Croft JB, Balluz LS, Mokdad AH. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005-2006. Prev Med. 2010 doi: 10.1016/j.ypmed.2010.03.016. In press. [DOI] [PubMed] [Google Scholar]
- 32.Frey W, Pilcher J. Obstructive sleep apnea breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 33.Tambay JL, Catlin G. Sample design of the National Population Health Survey. Health Rep. 1995;7:29–38. [PubMed] [Google Scholar]
- 34.Beland Y. Canadian Community Health Survey – methodological overview. Health Rep. 2002;13:9–14. [PubMed] [Google Scholar]
- 35.World Health Organization. Global Database on Body Mass Index. BMI Classification. Available at: http://apps.who.int/bmi/index.jsp?introPage = intro_3.html.
- 36.Nyholm M, Gullberg B, Merlo J, Lundqvist-Persson C, Rstam L, Lindblad U. The validity of obesity based on self-reported weight and height: Implications for population studies. Obesity (Silver Springs) 2007;15:197–208. doi: 10.1038/oby.2007.536. [DOI] [PubMed] [Google Scholar]
- 37.Ahluwalia IB, Tessaro I, Rye S, Parker L. Self-reported and clinical measurement of three chronic disease risks among low-income women in West Virginia. J Womens Health. 2009;18:1857–62. doi: 10.1089/jwh.2009.1360. [DOI] [PubMed] [Google Scholar]
- 38.Statistics Canada, Health Statistics Division. Canadian Community Health Survey 2003, User Guide for the Public Use Microdata File. Ottawa (Canada): Ministry of Industry; 2005. Cat. No. 82M0013GPE. [Google Scholar]
- 39.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of isks and benefits. BMJ. 2005;331:1169–76. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drug and Health Products, Notice of Compliance Online Query. Health Canada. http://webprod3.hc-sc.gc.ca/noc-ac/index-eng.jsp.
- 41.Drug and Health Products, Drug Product Database Online Query. Health Canada. http://webprod.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp.
- 42.Drugs Funded by Ontario Drugs Benefit (ODB) Program. Ministry of Health and Long-Term Care, Province of Ontario. www.health.gov.on.ca/english/providers/program/drugs/edition_41.html.
- 43.John U, Baumeister SE, Volzke H, Meyer C, Ulbricht S, Alte D. Sedative, hypnotic, anxiolytic and opioid medicament use and its co-occurrence with tobacco smoking and alcohol risk drinking in a community sample. BMC Public Health. 2007;7:337–449. doi: 10.1186/1471-2458-7-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagnaoui R, Moore N, Dartigues JF, Fourrier A, Begaud B. Benzodiazepine use and wine consumption in the French elderly. Br J Clin Pharmacol. 2001;52:455–60. doi: 10.1046/j.0306-5251.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalman D, Moriseette SB, George ST. Comorbidity of smoking in patients with psychiatric and substance abuse disorders. Am J Addic. 2005;14:106–23. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13:287–93. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204. [PMC free article] [PubMed] [Google Scholar]
- 48.Tikkinen KA, Auvinen A, Johnson TM, 2nd, et al. A systematic evaluation of factors associated with nocturia - the population-based FINNO study. Am J Epidemiol. 2009;170:361–8. doi: 10.1093/aje/kwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiri R, Hakama M, Hakkinen J, et al. The effects of lifestyle factors on the incidence of nocturia. J Urol. 2008;180:2059–62. doi: 10.1016/j.juro.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 50.Holliday KL, McWilliams DF, Maciewicz RA, Muir KR, Zhang W, Doherty M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthritis Cartilage. 2011;19:37–43. doi: 10.1016/j.joca.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 51.Berry RB, Kouchi K, Bower J, Prosise G, Light RW. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–4. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 52.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–60. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 53.Dolly FR, Block AJ. Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med. 1982;73:239–43. doi: 10.1016/0002-9343(82)90185-1. [DOI] [PubMed] [Google Scholar]
- 54.Guilleminault C, Silvestri R, Mondini S, Coburn S. Aging and sleep apnea: action of benzodiazepine, alcohol, and sleep deprivation in a healthy elderly group. J Gerontol. 1984;39:655–61. doi: 10.1093/geronj/39.6.655. [DOI] [PubMed] [Google Scholar]
- 55.Berry RB, Patel RB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep. 2006;29:1052–6. doi: 10.1093/sleep/29.8.1052. [DOI] [PubMed] [Google Scholar]
- 56.Cirignotta F, Mondini S, Zucconi M, Gerardi R, Farolfi A, Lugaresi E. Zolpidem: polysomnographic study of the effect of a new hypnotic drug in sleep apnea syndrome. Pharmacol Biochem Behav. 1988;29:807–9. doi: 10.1016/0091-3057(88)90212-2. [DOI] [PubMed] [Google Scholar]
- 57.Hoijer U, Hedner J, Ejnell H, Grunstein R, Odelberg E, Elam M. Nitrazepam in patients with sleep apnea: a double-blind placebo-controlled study. Eur Respir J. 1994;7:2011–5. [PubMed] [Google Scholar]
- 58.Nunes JPL, Faria M, Winck JC. Apnea/hypopnea index and benzodiazepine use in patients with arterial hypertension and excessive weight. Int J Cardiol. 2007;114:416–8. doi: 10.1016/j.ijcard.2005.11.111. [DOI] [PubMed] [Google Scholar]
- 59.Lauer CJ, Krieg J-C. Sleep in eating disorders. Sleep Med Rev. 2004;8:109–16. doi: 10.1016/S1087-0792(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 60.Vita R, Lapa D, Vita G, Trimarchi F, Benvenga S. A patient with stress-related onset and exacerbations of Graves disease. Nat Clin Pract Endocrinol Metab. 2009;5:55–61. doi: 10.1038/ncpendmet1006. [DOI] [PubMed] [Google Scholar]
- 61.Valencia-Flores M, Resendiz M, Castano VA, et al. Objective and subjective sleep disturbances in patients with systemic lupus erythematosus. Arthritis Rheum. 1999;42:2189–93. doi: 10.1002/1529-0131(199910)42:10<2189::AID-ANR21>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 62.Price MA, Zachariae R, Butow PN, et al. Prevalence and predictors of insomnia in women with invasive ovarian cancer: anxiety a major factor. Eur J Cancer. 2009;45:3262–70. doi: 10.1016/j.ejca.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Neutal CI, Walop W. Comparing two different approaches to measuring drug use within the same survey. Chron Dis Can. 2000;21:150–6. [PubMed] [Google Scholar]
- 64.Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biol Psychiatry. 1998;43:687–93. doi: 10.1016/s0006-3223(97)00292-8. [DOI] [PubMed] [Google Scholar]