Abstract
Study Objectives:
To describe the rate, distribution and correlates of periodic limb movements in sleep (PLMS) in children with sickle cell disease (SCD).
Design:
Prospective, cross-sectional.
Setting:
Hospital-based sleep laboratory.
Participants:
Sixty-four children aged 2–18 years with SCD, hemoglobin SS-type who had an overnight polysomnogram and a parent-completed Pediatric Sleep Questionnaire. Mean age was 8.4 years (SD 4.8); 50% were male.
Interventions:
N/A
Measurements and Results:
The mean PLMS index was 3.7 (6.6) and ranged from 0 to 31.8, with 23.4% of the sample having PLMS ≥ 5/h. Sleep efficiency was decreased (P = 0.03), and the total arousal index (P = 0.003) and PLMS arousal index (P < 0.001) were increased in children with PLMS ≥ 5/h compared to those with PLMS < 5/h. PLMS were most frequent in NREM stage 2 sleep and during the fourth hour of sleep. Inter-movement interval duration peaked at 25–30 s. “Growing pains worst in bed” or “restlessness of the legs”, suggesting restless legs syndrome (RLS), were reported in 12.5% of the total sample and were more common in children with elevated PLMS. A PLMS score for identifying elevated PLMS in children, based on items from the Pediatric Sleep Questionnaire, did not significantly predict PLMS ≥ 5/h.
Conclusions:
Elevated PLMS are common in children with SCD and are associated with sleep disruption and symptoms of RLS. Future research into the time structure of PLMS, their causes and consequences, and development of a disease-specific sleep disorders screening questionnaire, is needed in children with SCD.
Citation:
Rogers VE; Marcus CL; Jawad AF; Smith-Whitley K; Ohene-Frempong K; Bowdre C; Allen J; Arens R; Mason TBA. Periodic limb movements and disrupted sleep in children with sickle cell disease. SLEEP 2011;34(7):899-908.
Keywords: Periodic limb movements in sleep, anemia, sickle cell, child, adolescent, sleep disorders, inter-movement interval, sleep stages
INTRODUCTION
Sickle cell disease (SCD) is a group of inherited, chronic hemolytic anemias in which a point mutation on the hemoglobin molecule causes polymerization under certain conditions including hypoxemia. As a result of disease-related pathology, children with SCD experience neurocognitive deficits,1,2 frequent hospitalization and excessive health care costs,3,4 and an elevated death rate in nearly every age group compared to healthy African Americans.5
Sleep-related disorders are emerging as a potential contributor to SCD severity. Nearly half of children with SCD experience nocturnal oxyhemoglobin desaturation,6,7 and low oxygen saturation has been implicated in the development of vaso-occlusive pain crises8 and pulmonary hypertension.9 Obstructive sleep apnea (OSA) is more prevalent and more severe in children with SCD than unaffected children.10–12 Little is known, however, about other sleep disorders in children with SCD, including the occurrence of periodic limb movements in sleep (PLMS).
Periodic limb movements in sleep are repetitive, highly stereotyped movements of the arms or legs occurring during sleep. Evidence increasingly suggests that dysfunction of the dopaminergic neurotransmitter system plays a part in the pathogenesis of PLMS.13 A PLMS index exceeding 5/h in children indicates periodic limb movement disorder, provided additional diagnostic criteria are met including clinical sleep disturbance and absence of another primary disorder that would explain the PLMS.14 Periodic limb movements are relatively unusual in healthy children,15 but have been associated with several comorbid conditions including OSA,16 attention deficit hyperactivity disorder (ADHD),17,18 restless legs syndrome (RLS),19 and iron deficiency.20 Few studies have explored the occurrence of PLMS in children with SCD.
This study was part of a prospective, longitudinal investigation of the prevalence, contributory mechanisms, and pulmonary and vascular consequences of oxyhemoglobin desaturation in children and adolescents with SCD, homozygous hemoglobin SS-type (SCD-SS). The aims of this study were to describe objective measures of PLMS including their rate, sleep stage and time-of-night distribution, and time structure; the relationship of PLMS to subjectively reported symptoms; and the utility of a PLMS score, based on items from the Pediatric Sleep Questionnaire (PSQ),21 for identifying elevated PLMS in a prospective, unreferred, homogenous sample of children with SCD-SS.
METHODS
Participants
This was a prospective, cross-sectional study. Children were recruited if they received care at the Sickle Cell Center of The Children's Hospital of Philadelphia. Inclusion criteria at the point of study entry were age 2 to 18 years with documented SCD-SS and not currently receiving chronic transfusion or hydroxyurea therapies, or having received either during the previous 3 months. Subjects were excluded if they were currently pregnant or had congenital heart disease or a chronic lung condition unrelated to SCD, with the exception of asthma. Studies were performed if subjects were at their baseline state of health without having experienced acute chest syndrome or other exacerbations within the last 2 months. In addition, for these analyses subjects required a completed polysomnogram and PSQ.21 The study was approved by the Institutional Review Board at The Children's Hospital of Philadelphia. All parents/guardians signed informed consent, and children 7 years of age or older signed their assent prior to participation.
Procedure
Polysomnogram
Participants underwent overnight polysomnography at baseline, and some participants completed a polysomnogram at one-year follow-up or post-adenotonsillectomy. The first polysomnogram was included in this study. Four participants did not complete a PSQ at the time of their baseline sleep study, and in these cases, the second polysomnogram was included.
During the study, a Rembrandt polysomnography system (Embla, Broomfield, CO) recorded the following parameters: electroencephalogram (C3/A2, C4/A1, O1/A2, and O2/A1, with later studies also including frontal electroencephalography leads), left and right electroculograms, submental and tibial electromyogram, electrocardiogram, oro-nasal airflow with a 3-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA), nasal pressure with a pressure transducer (Pro-Tech Services, Inc.), rib cage and abdominal wall motion using respiratory inductance plethysmography (Viasys Healthcare, Yorba Linda, CA), end-tidal carbon dioxide level (Novametrix Medical Systems, Inc., Wallingford, CT), and arterial oxygen saturation (SpO2) with pulse waveform (Masimo, Irvine, CA). Subjects were also recorded using infrared videography. Sleep architecture, arousals and respiratory events were scored according to American Academy of Sleep Medicine criteria, using pediatric respiratory scoring rules for all subjects.22 The obstructive apnea-hypopnea index (OAHI) was the number of obstructive apneas, obstructive hypopneas, and mixed apneas per hour of sleep. Subjects had OSA if their OAHI was ≥ 1.5.23–25
Periodic limb movements were measured using 2 surface electromyography electrodes placed over the anterior tibialis muscle, using separate channels for each leg. They were defined as a series of ≥ 4 consecutive leg movements, each lasting between 0.5 and 10 sec, having an amplitude increase in electromyography voltage ≥ 8 μV above resting baseline and a period length between leg movements of 5 to 90 sec. Limb movements were not scored if they occurred within 0.5 sec preceding or following an apnea or hypopnea.22 A PLMS index ≥ 5/h of sleep was considered abnormal.14 Polysomnograms were interpreted by physicians who were board certified in sleep medicine (CM, TM).
For analysis of PLMS distribution, hour of sleep was defined as the hour post sleep onset regardless of intervening wake time. Only periodic limb movements occurring during sleep were analyzed. Fourteen children did not sleep through the entire eighth hour (mean sleep for short sleepers 7.5 h, SD 0.4 h), and no correction factor was applied to account for incomplete data collection during that hour. Inter-movement interval (IMI) was defined as the time between the onset of 2 subsequent PLMS in a single PLMS sequence. All IMI were binned into time interval classes accounting for 5-sec spans of time, covering the range from 5 to 90 sec (e.g., 5s ≤ interval < 10s, 10s ≤ interval < 15s, 15s ≤ interval < 20s,…85s ≤ interval ≤ 90s).
Pediatric Sleep Questionnaire
The PSQ21 is a widely used parent-report questionnaire with established reliability and validity developed to identify children aged 2 to 18 years with sleep disorders. Items from the PSQ have been used in the development of a PLMS score,26 a weighted composite score of 6 PSQ items associated with PLMS in children. The PLMS score was developed to assist in clinical discrimination of PLMS ≥ 5/h of sleep. Items in the PLMS score include (A13) “Does your child describe restlessness of the legs when in bed?”; (A13b) “Does your child have ‘growing pains’ that are worst in bed?”; (A16) “At night does your child usually get out of bed (for any reason)?”; (A44) “Does your child wake more than twice a night, on average?”; (B1) “Does your child wake up feeling unrefreshed in the morning?”; and (B7) “Does your child wake up with headaches in the morning”? Item responses include no = 0, yes = 1, and don't know = missing. These scores are summed, with items A13 and A13b double-scored to improve specificity of the score for PLMS, and the sum is divided by the number of items, yielding a score ranging from 0 to 1. Using a receiver operator characteristic (ROC) curve, authors identified a PLMS score > 0.33 as suggestive of PLMS ≥ 5/h in a validation sample of 113 children aged 2 to 18 years of age referred for polysomnography.26 The PLMS score had a positive predictive value of 0.38, negative predictive value of 0.89, sensitivity of 0.79, and specificity of 0.56 to detect PLMS ≥ 5/h. The PLMS score demonstrated reasonable initial validity, internal consistency and test-retest reliability, although predictive values, sensitivity, and specificity demonstrated limited effectiveness in screening children for PLMS ≥ 5/h in the clinical setting.26
The PSQ was completed on the day of the polysomnogram. Because of the number of tests involved in the parent study, however, some subjects were unable to complete the PSQ at the time of the sleep study, and completed it at the next clinical or study contact. Seventy-six percent (49/64) of subjects completed the PSQ within a day of the sleep study (immediate completers). Completion of the PSQ for the remaining subjects ranged from 12 to 301 days after the sleep study (delayed completers). Immediate completers were compared to delayed completers on the dichotomized PLMS index, age, gender, and OAHI, and no significant differences were found. Consequently, all data were analyzed together.
Statistical Analysis
Descriptive statistics, including mean (standard deviation [SD]), range and percent were used to describe the data. Spearman correlation (ρ) was employed to estimate the association of demographic and clinical variables with the PLMS index. The PLMS index was dichotomized into < 5/h (normal) and ≥ 5/h (elevated). The independent t-test or Mann-Whitney test was used to test differences between groups on variables measured on a continuous scale. Fisher exact tests or χ2 tests were used for comparing frequencies and proportions.
Age was arbitrarily dichotomized into < 10 years and ≥ 10 years of age for comparison of frequency distributions of PLMS by sleep stages, hour of sleep, and duration of IMI. For these analyses, individual PLMS were binned for each polysomnogram, across categories (sleep stage, hour of sleep) and interval classes (IMI duration). A mean and standard error of the mean was calculated across all polysomnograms, and for children < 10 and ≥ 10 years of age, for each category and interval class.
Prediction of the dichotomized PLMS index from individual PSQ items and the PLMS score was accomplished using logistic regression methods. Items on the PSQ having > 2 response categories were dichotomized (0 = “does not apply” or “applies just a little” versus 1 = “applies quite a bit” or “definitely applies most of the time”). An ROC curve modeled the ability of the PLMS score to discriminate a PLMS index < 5 versus ≥ 5/h. Post hoc exploration was performed using univariate logistic regression, with α set at P < 0.20. Analysis of PLMS distributions was performed using Excel 2007 (Microsoft Corp., Redmond, WA). All other analyses were performed using the Statistical Package for the Social Sciences, version 17.0 (SPSS, Inc., Chicago, IL). Alpha was set at 0.05. No correction was made for multiple comparisons as this was an exploratory, observational study, and clinically meaningful directions for future research might otherwise be missed.27
RESULTS
Sixty-four subjects completed both a polysomnogram and a PSQ. All subjects were African American. Characteristics of the sample are shown in Table 1. Medications taken on a daily or as needed basis among subjects during the study included penicillin (n = 57), folic acid (n = 61), albuterol (n = 8), inhaled corticosteroid (n = 10), antihistamine (n = 4), hydroxyurea (n = 1), deferasirox (n = 1), methylphenidate (n = 1), lansoprazole (n = 1), levetiracetam (n = 1), ibuprofen (n = 5), and acetaminophen with codeine (n = 3). No parent reported the use of selective serotonin reuptake inhibitors or any antidepressant medications by their child.
Table 1.
Characteristics of children with sickle cell disease for all subjects and by periodic limb movements in sleep index < 5 and ≥ 5
| Variable | All subjects N = 64 | PLMS < 5/h n = 49 | PLMS ≥ 5/h n = 15 |
|---|---|---|---|
| Age (years) | 8.4 (4.8) | 8.5 (4.7) | 8.2 (5.3) |
| Sex, male (n (%)) | 32 (50) | 24 (49.0) | 8 (53.3) |
| BMI (kg/m2) | 16.9 (2.7) | 17.0 (2.7) | 16.6 (2.8) |
| BMI-z score | −0.20 (1.2) | −0.16 (1.3) | −0.36 (1.0) |
| White blood cell count (109/L) | 13.3 (4.6) | 13.8 (4.6) | 11.9 (4.5) |
| Hemoglobin (g/dL) | 8.2 (1.2) | 8.1 (1.2) | 8.5 (1.3) |
| Reticulocyte count (%) | 14.1 (5.1) | 14.9 (4.9)* | 11.4 (5.3)* |
| Mean red blood cell volume (fl) | 83.9 (7.1) | 83.8 (7.0) | 84.3 (7.5) |
P < 0.05 for comparison between subjects with periodic limb movements in sleep (PLMS) < 5/h vs. PLMS ≥ 5/h. Mean (standard deviation) are reported unless otherwise noted. BMI refers to body mass index.
Polysomnography Findings
The mean PLMS index was 3.7 (SD 6.6), and ranged from 0 to 31.8. Fifteen (23.4%) subjects had a PLMS index ≥ 5. Comparison of polysomnographic parameters between subjects having a PLMS index < 5 versus ≥ 5 is shown in Table 2. As expected, the PLMS index (P < 0.001) and PLMS arousal index (P < 0.001) were significantly higher in subjects with elevated PLMS. The total arousal index was also significantly increased in subjects with elevated PLMS (P = 0.004). Sleep efficiency was decreased in subjects with elevated PLMS compared to those with PLMS < 5/h (P = 0.03). There was a trend toward longer sleep onset latency in subjects with elevated PLMS (P = 0.08). Neither total sleep time nor sleep stages differed in children with normal versus elevated PLMS. Obstructive sleep apnea was identified in 26.6% (17/64) of the sample. Of the children with OSA, 17.6% (3/17) had a PLMS index ≥ 5, while 25.5% (12/47) of children had an elevated PLMS index but did not have OSA, differences that were not significant.
Table 2.
Polysomnography findings in children with sickle cell disease for all subjects and by periodic limb movements in sleep index < 5 and ≥ 5
| Variable | All subjects N = 64 | PLMS < 5/h n = 49 | PLMS ≥ 5/h n = 15 | P |
|---|---|---|---|---|
| Obstructive apnea-hypopnea index (n/h) | 1.7 (3.7) | 1.9 (4.2) | 0.95 (1.6) | 0.21 |
| Central apnea index (n/h) | 0.42 (0.56) | 0.48 (0.60) | 0.20 (0.31) | 0.051 |
| Total sleep time (min) | 432.6 (64.7) | 439.0 (67.5) | 411.7 (50.9) | 0.16 |
| Sleep efficiency (%) | 83.6 (11.5) | 84.8 (11.7) | 79.8 (10.2) | 0.03 |
| Sleep latency (min) | 29.6 (31.6) | 26.7 (31.4) | 39.0 (31.5) | 0.08 |
| Wake after sleep onset (min) | 55.4 (54.3) | 52.2 (54.8) | 65.9 (53.2) | 0.14 |
| N1 (%) | 6.4 (3.8) | 5.8 (3.1) | 8.3 (5.4) | 0.11 |
| N2 (%) | 47.5 (7.6) | 47.8 (8.2) | 46.7 (4.9) | 0.55 |
| N3 (%) | 27.6 (7.7) | 28.0 (7.9) | 26.2 (6.7) | 0.44 |
| REM (%) | 18.5 (5.3) | 18.5 (5.3) | 18.8 (5.4) | 0.85 |
| Total arousal index (n/h) | 14.0 (5.6) | 12.8 (4.7) | 18.0 (6.4) | 0.001 |
| PLMS index (n/h) | 3.7 (6.6) | 0.93 (1.2) | 12.6 (9.1) | < 0.001 |
| PLMS arousal index (n/h) | 0.84 (1.6) | 0.18 (0.33) | 2.9 (2.3) | < 0.001 |
Mean (standard deviation) are reported.
N refers to NREM sleep stage; PLMS, periodic limb movements in sleep.
Twelve children had a second polysomnogram during the course of this study. Time between sleep studies averaged 10.6 months (4.2 months) and ranged from 1.0 to 15.0 months. The change in PLMS index from the first to the second sleep study averaged 0.4 (3.8), ranging from 3.9 to 11.9, with no child changing diagnostic classification (PLMS index above or below 5/h) from the first to the second study. There was no correlation of the PLMS index with age.
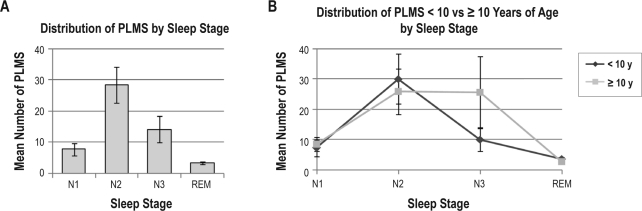
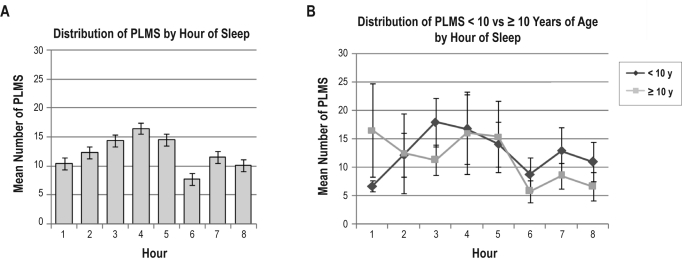
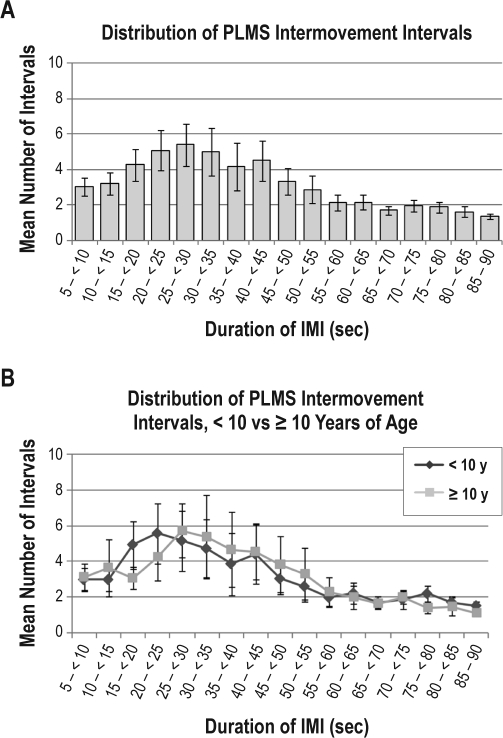
Forty-four polysomnograms containing 1626 individual PLMS were available for analysis of the frequency distribution of PLMS across sleep stages, time of night, and duration of IMI. Considering all PLMS across the sample prior to binning by individual subject, the majority (68.9%) of PLMS occurred during NREM sleep stage (N) 2, with 14.7% occurring during N1, 13.1% during N3 or slow wave sleep, and 3.3% during REM sleep. Binned PLMS across sleep stages demonstrated a similar distribution (Figure 1, Table 3), with the greatest mean number of PLMS for the total sample and in children < 10 years of age occurring during N2, while PLMS in children ≥ 10 years were approximately equally distributed between N2 and N3. The mean number of binned PLMS across hours of sleep (Figure 2) for the total sample peaked during hour 4. However, when examined between younger versus older children, the peak was mainly seen in children < 10 years of age. In the older children, PLMS were more frequent during the first hour of sleep than in the younger children, dipped during hours 2 and 3, then peaked again during hours 4 and 5. Both age groups demonstrated a dip in PLMS frequency during hour 6, with a small peak again during the 7th hour of sleep. The mean number of binned PLMS across IMI 5-sec time interval classes (Figure 3) for the total sample peaked at 25- < 30 s, with the peak extending from 15- < 20 s through 55- < 60 s. In children < 10 years of age, IMIs were of slightly shorter duration than in the older children, but followed the same course of decreasing frequency as IMI duration lengthened.
Figure 1.
Distribution of the mean of binned periodic limb movements in sleep (PLMS) occurring in NREM (N) and REM sleep stages during polysomnography (A) across all children with sickle cell disease and PLMS, and (B) comparing children < 10 versus ≥ 10 years of age. Error bars represent standard error of the mean.
Table 3.
Distribution of mean periodic limb movements in sleep (PLMS) across hour of sleep and sleep stages in children with sickle cell disease and PLMS
| Mean (SD) |
|||
|---|---|---|---|
| Total sample N = 44 | < 10 Years n = 28 | ≥ 10 Years n = 16 | |
| Hour of Sleep | |||
| 1 | 10.3 (12.8) | 6.6 (3.1) | 16.5 (20.0) |
| 2 | 12.3(14.5) | 12.1 (11.7) | 12.4 (18.6) |
| 3 | 14.4 (10.3) | 17.9 (11.9) | 11.2 (8.0) |
| 4 | 16.6 (18.5) | 16.7 (19.4) | 16.4 (18.8) |
| 5 | 14.5 (13.4) | 14.0 (13.0) | 15.3 (15.3) |
| 6 | 7.7 (9.3) | 8.7 (11.0) | 5.7 (5.1) |
| 7 | 11.5 (12.8) | 12.8 (15.1) | 8.5 (5.5) |
| 8* | 10.0 (8.9) | 10.9 (9.8) | 6.5 (3.5) |
| Sleep Stage | |||
| N1 | 8.0 (10.6) | 7.2 (12.0) | 9.1 (8.5) |
| N2 | 28.7 (36.3) | 30.1 (40.7) | 26.4 (29.0) |
| N3 | 14.2 (16.7) | 10.0 (12.3) | 25.8 (23.6) |
| REM | 3.4 (1.8) | 3.6 (1.9) | 2.8 (1.5) |
14 subjects did not sleep a full hour during the eighth hour of sleep. Calculations are not adjusted for short sleep. Mann-Whitney test of differences between age groups were nonsignificant for all categories. N# refers to NREM sleep stage.
Figure 2.
Distribution of the mean number of binned periodic limb movements in sleep (PLMS) occurring in each hour of sleep during polysomnography (A) across all children with sickle cell disease and PLMS, and (B) comparing children < 10 versus ≥ 10 years of age. Error bars represent standard error of the mean.
Figure 3.
Distribution of the mean number of binned periodic limb movements in sleep (PLMS) inter-movement intervals (IMI) in each 5-sec interval class (A) across all children with sickle cell disease and PLMS, and (B) comparing children < 10 versus ≥ 10 years of age. Error bars represent standard error of the mean.
Subjective Symptoms and PLMS Score
Only 4 subjects met American Academy of Sleep Medicine criteria for periodic limb movement disorder.14 Conversely, 60% (9/15) of children with a PLMS index ≥ 5 reported no clinical symptoms, and many children who did not have elevated PLMS reported one or more symptoms, including 65.6% (21/32) of children with a PLMS index < 1 and 52.9% (9/17) of children with a PLMS index between 1 and < 5.
Select responses to the PSQ for the full sample and by PLMS < 5/h versus ≥ 5/h are shown in Table 4. Either growing pains worst in bed or restlessness of the legs was reported by 12.5% of the sample, including 6.1% (3/49) of children with PLMS < 5/h and 33.3% (5/15) of children with PLMS ≥ 5/h (P = 0.014). Items from the PSQ were used to calculate a Sleep-Related Breathing Disorder score, an Inattention/Hyperactivity score and a Daytime Sleepiness score21 (Table 4). Overall, there was no correlation between the PLMS index and any of the scores. However, when only children with PLMS ≥ 5/h were included in the analysis, the correlation between the PLMS index and the Inattention/Hyperactivity score (but not the Sleep-Related Breathing Disorder or the Daytime Sleepiness scores) became significant (ρ = 0.592, P = 0.02). Children with clinical symptoms of sleep disturbance and elevated PLMS had a higher Inattention/Hyperactivity score than did children with elevated PLMS without clinical symptoms (mean 0.39 [0.39] vs. 0.17 [0.28], respectively), but this difference was not significant.
Table 4.
Positive responses to select Pediatric Sleep Questionnaire items for all children with sickle cell disease and by periodic limb movements in sleep index < 5 versus ≥ 5
| Item | All subjects N = 64 | PLMS < 5/h, n = 49 | PLMS ≥ 5/h, n = 15 |
|---|---|---|---|
| A1 Ever snore | 49 (76.6) | 37 (75.5) | 12 (80.0) |
| A3 Always snores | 20 (31.3) | 15 (30.6) | 5 (33.3) |
| A4 Snore loudly | 23 (35.9) | 18 (36.7) | 5 (33.3) |
| A5 Heavy breathing | 10 (15.6) | 10 (20.4) | 0 |
| A6 Trouble breathing | 6 (9.4) | 5 (10.4) | 1 (6.7) |
| A7 Observed apneas | 6 (9.4) | 5 (10.2) | 1 (6.7) |
| A13 Restlessness of legs | 7 (10.9) | 3 (6.1) | 4 (26.7) |
| A13b Growing pains worst in bed | 2 (3.1) | 0 | 2 (13.3) |
| A16 Out of bed | 48 (75.0) | 39 (79.6) | 9 (60.0) |
| A24 Mouth open during day | 12 (18.8) | 9 (18.4) | 3 (20.0) |
| A25 Dry mouth on awakening | 27 (42.2) | 20 (40.8) | 7 (46.7) |
| A32 Nocturnal enuresis | 26 (40.6) | 20 (40.8) | 6 (40.0) |
| A40 Difficult sleep onset | 15 (23.8) | 13 (26.5) | 2 (13.3) |
| A44 Wakes more than twice/night | 17 (26.6) | 13 (26.5) | 4 (26.7) |
| A45 Difficult return to sleep at night | 12 (18.8) | 9 (18.4) | 3 (20.0) |
| A46 Wakes early morning | 9 (14.1) | 9 (16.3) | 1 (6.7) |
| B1 Wakes unrefreshed | 14 (21.9) | 11 (22.4) | 3 (20.0) |
| B2 Daytime sleepiness | 11 (17.2) | 10 (20.4) | 1 (6.7) |
| B4 Sleepy per teacher | 12 (18.8) | 11 (22.4) | 1 (6.7) |
| B6 Hard to wake up | 20 (31.3) | 15 (30.6) | 5 (33.3) |
| B7 Morning headache | 8 (12.5) | 8 (16.3) | 0 |
| B9 Delayed growth | 7 (10.9) | 4 (8.2) | 3 (20.0) |
| B22 Obesity | 3 (4.7) | 3 (6.1) | 0 |
| C3 Does not listen | 16 (25.0) | 12 (24.5) | 4 (26.7) |
| C5 Difficulty organizing | 5 (7.8) | 5 (10.2) | 0 |
| C8 Easily distracted | 13 (20.3) | 8 (16.3) | 5 (33.3) |
| C10 Fidgets | 9 (14.1) | 5 (10.2) | 4 (26.7) |
| C14 On the go | 18 (28.1) | 12 (24.5) | 6 (40.0) |
| C18 Interrupts | 14 (21.9) | 10 (20.4) | 4 (26.7) |
| Composite Scores | Mean (SD) | Mean (SD) | Mean (SD) |
|---|---|---|---|
| PLMS score (n = 57) | 0.20 (0.14) | 0.19 (0.13) | 0.23 (0.16) |
| Sleep-Related Breathing Disorder score (n = 58) | 0.21 (0.15) | 0.21 (0.15) | 0.21 (0.16) |
| Inattention/Hyperactivity score (n = 58) | 0.20 (0.28) | 0.18 (0.26) | 0.26 (0.33) |
| Daytime Sleepiness score (n = 61) | 0.23 (0.26) | 0.25 (0.24) | 0.17 (0.29) |
Number (percent) are reported unless otherwise noted. There were no significant differences between subjects with PLMS index < 5 vs. ≥ 5 on any composite score.26 PLMS refers to periodic limb movements in sleep. PLMS score includes A13, A13b, A16, A44, B1 and B7. Sleep-Related Breathing Disorder score includes A1, A3, A4, A5, A6, A7, A24, A25, A32, B1, B2, B4, B6, B7, B9, B22, C3, C5, C8, C10, C14, and C18. Inattentive/Hyperactive score includes C3, C5, C8, C10, C14, and C18. Daytime Sleepiness score includes B1, B2, B4, and B6.
The mean PLMS score was 0.20 (0.14), and ranged from 0 to 0.63. The dichotomized PLMS index was regressed on the PLMS score in an unadjusted logistic regression model (Table 5). The PLMS score was not a significant predictor of PLMS ≥ 5/h. The odds ratio was substantial (OR 12.1), yet the 95% confidence interval was excessively large and classification of PLMS ≥ 5/h, the prediction of interest, was poor at 6.7%. The area under the ROC curve (0.608) was not significantly better than that predicted by chance. The model was then adjusted for age, sex, nightly snoring, OAHI, and nadir SpO2.26 In the adjusted model, prediction of PLMS ≥ 5/h was 13.3%, an improvement over the unadjusted model; however, this model was likewise not significant.
Table 5.
Unadjusted and adjusted logistic regression models of periodic limb movements in sleep (PLMS) ≥ 5/h predicted by the PLMS score in children with sickle cell disease, n = 57
| Variable | B (SE) | P value | Odds ratio (95% CI) | Classification (% correct) |
AUC | |
|---|---|---|---|---|---|---|
| PLMS < 5/h | PLMS ≥ 5/h | |||||
| PLMS scorea | 2.50 (2.17) | 0.251 | 12.10 (0.2, 858.0) | 100.0 | 6.7 | 0.608 |
| Adjusted PLMS scoreb,c | 2.67 (2.38) | 0.261 | 14.43 (0.1, 1521.4) | 92.9 | 13.3 | N/A |
AUC refers to area under the receiver operator characteristic curve.
Cox & Snell R2 = 0.023, Nagelkerke R2 = 0.033.
Adjusted for age, sex, nightly snoring, obstructive apnea-hypopnea index, and nadir oxyhemoglobin saturation.
Cox & Snell R2 = 0.116, Nagelkerke R2 = 0.170.
To explore reasons for the lack of predictive value of the PLMS score in children with SCD, we performed post hoc testing of variables included in the PLMS score, and additionally tested other PSQ items that might be associated with PLMS in children with SCD. Additional items included those related to restless sleep, leg kicks during sleep, sleep disruption, daytime sleepiness, sleep disordered breathing symptoms, and inattentive/hyperactive behaviors. Items achieving P < 0.20 in univariate logistic regression models of the dichotomized PLMS index are shown in Table 6. Only one item in the PLMS score, (A13) restless legs, met this criterion in the expected direction (e.g., problematic sleep behavior reported more often in children with elevated PLMS). Additional significant items in the expected direction included (A14) “While your child sleeps, have you seen repeated kicks or jerks of the legs at regular intervals?”; (B5) “Does your child usually take a nap during the day?”; and (C10) “This child often fidgets with hands or feet or squirms in seat.” Unexpectedly, children with PLMS ≥ 5/h had fewer reported problematic sleep behaviors than children with normal PLMS for the items (A16) “At night, does your child usually get out of bed (for any reason)”?; (A17) “At night, does your child usually get out of bed to urinate?”; (B4) “Has a teacher or other supervisor commented that your child appears sleepy during the day?”; and (B16) “Has your child felt an irresistible urge to take a nap at times, forcing him or her to stop what he or she is doing in order to sleep?”
Table 6.
Univariate logistic regression of periodic limb movements in sleep (< 5/h versus ≥ 5/h) on Pediatric Sleep Questionnaire items achieving P < 0.20 in children with sickle cell disease
| Variable | Beta (SE) | P value | Odds ratio | 95% CI of Odds Ratio | AUC |
|---|---|---|---|---|---|
| A13 Restlessness of legs | 1.65 (0.84) | 0.048 | 5.21 | 1.01, 26.79 | 0.601 |
| A14a Repeated kicks | 1.68 (0.97) | 0.083 | 5.38 | 0.80, 35.94 | 0.578 |
| A16 Out of bed | −1.06 (0.64) | 0.099 | 0.35 | 0.098, 1.22 | 0.394 |
| A17 Out of bed to urinate | −1.31 (0.64) | 0.040 | 0.27 | 0.08, 0.94 | 0.362 |
| B4 Sleepy per teacher | −1.45 (1.09) | 0.183 | 0.23 | 0.03, 1.98 | 0.416 |
| B5 Takes naps | 0.90 (0.62) | 0.147 | 2.46 | 0.73, 8.25 | 0.609 |
| B16 Urge to nap | −1.54 (1.09) | 0.157 | 0.21 | 0.03, 1.81 | 0.408 |
| C10 Fidgets | 1.04 (0.75) | 0.166 | 2.84 | 0.65, 12.40 | 0.577 |
AUC refers to area under the receiver operator characteristic curve.
DISCUSSION
This study described PLMS in a sample of children with SCD-SS, including their rate, sleep stage and time-of-night distribution, time structure, and association with clinical characteristics and subjective symptoms. It further explored the usefulness of a screening algorithm for identifying elevated PLMS in clinical practice, developed from a parent-report questionnaire of sleep related symptoms. In children, PLMS have been associated with RLS,19 ADHD,17,18 low iron stores,20 and the use of antidepressant medications.28 Factors associated with PLMS in children with SCD are unknown. This study demonstrated a high rate of PLMS in these children, which was associated with sleep fragmentation and an elevated symptom score for RLS.
Polysomnography Findings
This was the first identified study to report elevated PLMS in a prospective, unreferred sample of children with SCD. Nearly one-quarter of our sample had a PLMS index ≥ 5. This is a remarkable finding, considering that PLMS are unusual in children. In comparison to our findings, prevalence estimates for PLMS ≥ 5/h in otherwise healthy children range from 1.2% to 8%.15,23 In the one recently published study reporting PLMS in children with SCD, a rate of PLMS > 5/h of 26% was found in a retrospective sample of children with SCD referred to a sleep center for evaluation of sleep disordered breathing,12 similar to the findings of our study.
Measures of sleep disruption were evident on polysomnography in children with elevated PLMS. Sleep efficiency was decreased and arousals were increased in children with PLMS ≥ 5/h over those with normal PLMS. There is now extensive research showing that microarousals, in the absence of frank awakenings, cause significant morbidity, including adverse neurocognitive outcomes and cardiovascular disease.29,30 PLMS have also been associated with transient but pronounced cardiac acceleration, changes in cardiovascular variability,31 and nocturnal hypertension in children,32 and fluctuations in cerebral hemodynamics in adults.33 These findings, considered together with evidence from one study demonstrating that RLS (and associated PLMS) in the presence of stroke risk factors posed an increased risk of silent stroke,34 and another demonstrating an increase in the relative odds of ischemic stroke in middle-aged men reporting RLS symptoms on questionnaire,35 are particularly concerning in children with SCD. Sickle cell disease is the most common cause of childhood stroke, with a stroke risk 333 times that of a healthy child without SCD or heart disease.36 Additionally, their cardiovascular system is already stressed due to the increased cardiac workload caused by severe anemia. Thus, the possibility exists that PLMS may contribute to adverse cardiovascular and cerebrovascular outcomes including stroke in children with SCD. However, these theoretical connections are far from supported, and further investigation is needed before a causal connection can be established. Neither subjectively reported frequent night awakenings nor awakenings on polysomnography differed between children with normal versus elevated PLMS, suggesting that some components of sleep disruption may be influenced by SCD-related pathology, such as pain or kidney dysfunction, rather than sleep disorders.
Obstructive sleep apnea was common and was diagnosed by polysomnography in over one-quarter of our sample. Periodic limb movements are often associated with OSA, and obstructive respiratory events can confound the scoring of PLMS.16,37 Nevertheless, in our sample the PLMS index was not significantly associated with the OAHI, and OSA was more common in children with normal PLMS than in those with a PLMS index ≥ 5/h, suggesting that the presence of OSA did not influence the occurrence of PLMS in these children.
Periodic limb movements show a high degree of variability from night to night,38 and in adults they increase in frequency with age.39 While no consecutive night polysomnograms were performed in our study, repeat polysomnography demonstrated surprising stability over time, with no child crossing the diagnostic threshold for periodic limb movement disorder of 5/h from the first to the second polysomnogram, despite the lapse of one year between most studies. Moreover, PLMS were not associated with age. Unfortunately, only one child undergoing repeat polysomnography had a PLMS index > 5. These findings suggest that children with few PLMS are unlikely to develop elevated PLMS over time. Further, the lack of correlation between PLMS and age preliminarily suggests that change over time in children with elevated PLMS may also be unlikely, although further investigation is required to better establish variability over time in children with elevated PLMS.
Our finding of a predominance of PLMS during N2 and slow wave sleep (N3) differ somewhat from other studies in which the highest frequency of PLMS occurred in N1 sleep and showed a decrease across N2 and slow wave sleep, with the lowest occurrence in REM sleep.16,40 In our sample, PLMS occurred with low but approximately equal frequency in N1 and N3 sleep. However, similar to other studies, few occurred during REM sleep. In the younger children, the frequency of PLMS peaked during N2 followed by a decrease to less than half that mean value in N3, while in the older children, PLMS occurred with about equal frequency during N2 and N3 sleep. The distribution of PLMS across hours of sleep in our sample peaked about halfway through the night, during hour 4, with a smaller peak during hour 7. This is in contrast to studies in adults with RLS showing a decline in the number of PLMS from the beginning to the end of the sleep period, but in agreement with the findings of Ferri et al.,41 in which this circadian variation was not apparent in children with RLS under the age of 15 years. The findings of our study could relate to the young age of our subjects, or it could be that the occurrence of PLMS in children with SCD is more closely related to their disease processes than to circadian influences or RLS.
Inter-movement intervals of PLMS in our sample spanned the range of time interval classes from 5- < 10 s through 85-90 s, with a peak in the frequency distribution in the 25- < 30 s interval class. Compared to older children, the peak IMI in the younger children was of slightly shorter duration but followed essentially the same distribution. While this peak in the distribution of IMI duration was small, it is still unusual in that the few other published studies of pediatric data found no peak in the IMI either in children with RLS41 or in healthy children,39 with the exception of a number of short IMI (e.g., < 10 s), which it has been suggested may represent more frequent movement in children during sleep rather than PLMS.39 Preliminary work in pediatric ADHD also reportedly demonstrated a PLMS peak at less than 10 seconds,38 similar to other pediatric studies. Much more work needs to be done to further elucidate time-of-night, sleep stage pattern, and time structure of PLMS in healthy children and in children with disorders that may disrupt sleep or may be affected by sleep disorders, such as SCD. The significance of our findings is unknown, yet the frequency distribution of PLMS during sleep and across sleep stages, and their time structure, may be markers of SCD phenotypes or may suggest differences in the pathophysiological mechanisms of PLMS42 in children with SCD that differ from other children.
Subjective Symptoms and PLMS Score
Nearly all individuals with RLS have elevated PLMS, and there is evidence that the two disorders may be associated through common dopaminergic pathways.43,44 Occurrence of RLS symptoms in children with SCD has not been previously examined. In our sample, over 12% of children had complaints of restlessness of the legs or growing pains worst in bed, and these symptoms were more common in children with PLMS ≥ 5/h. In comparison, a large population-based study found a prevalence of RLS of about 2% in children 8 to 17 years of age,45 while a rate of 5.9% was found in a sample of children referred to a pediatric sleep disorders clinic.46 These findings suggest that RLS may be more common in children with SCD than in the general population of children. Further investigation of the prevalence of RLS in children with SCD is needed, using RLS diagnostic criteria,47 to further elucidate a possible link between RLS symptoms and PLMS in this population and to identify children who might benefit from treatment.
Children with ADHD also frequently have elevated PLMS.16,18 We did not have an ADHD diagnosis for children in our sample, and did not measure inattention/hyperactivity using an instrument validated to screen for ADHD. We did, however, screen for inattentive/hyperactive behaviors using the subscale score reported by Chervin, et al.21 We found that among children with PLMS ≥ 5/h, there was a significant association between the PLMS index and the Inattention/Hyperactivity score. Our sample was too small to test for differences in inattention/hyperactivity in children meeting the American Academy of Sleep Medicine International Classification of Sleep Disorders-2 criteria14 for periodic limb movement disorder. Nevertheless, an association of sleep disturbance and short sleep with behavior dysregulation is well established.48,49 In a larger sample, this relationship may have held true for children with SCD as well.
The PLMS Score, as designed by Chervin and Hedger,26 did not perform well in our sample. Results of both logistic regression and ROC analysis of the PLMS score were nonsignificant. At least part of the lack of fit in our sample can be attributed to our finding that items on the PSQ are not necessarily appropriate for children with chronic illnesses such as SCD. For example, obesity is relatively unusual in children with SCD due to their high metabolic demands. Likewise, the physical demands of chronic illness, including pain and fatigue, may contribute to differences in responses to other items including more restlessness, increased nighttime arousals, sleep initiation and maintenance difficulties, unrefreshing sleep, and daytime sleepiness over those of healthy children. Indeed, the finding that certain items normally interpreted as problematic sleep behaviors were more common among children with normal levels of PLMS in our sample demonstrated some of the shortcomings of using the PSQ in children with SCD. These findings point out the need to develop disease-specific measures of sleep disorders for children with SCD that better account for their unique sleep- and disease-related symptoms. Moreover, taken together with our finding that PLMS occurred with equal frequency in children with and without OSA, they further suggest that distinctions between PLMS ≥ 5/hour and periodic limb movement disorder based on additional clinical criteria and absence of a comorbid sleep disorder may be less meaningful in children with SCD than in other pediatric populations.
Possible Mechanisms of PLMS in Sickle Cell Disease
We can only speculate as to the possible pathophysiology of PLMS in children with SCD, given the lack of research in this area. Iron dysregulation is implicated in the pathogenesis of PLMS. Yet, deficient iron storage as a cause of PLMS in SCD presents a paradox. Ferritin levels are often elevated, rather than decreased, in SCD due to prior blood transfusions and recurrent acute phase response to ongoing vaso-occlusive tissue damage.50 However, there is evidence in individuals with RLS, a disorder that is genetically linked to PLMS,51,52 that despite normal serum levels, iron stores in the central nervous system may be deficient.53,54 In a study of two adult patients with hemochromatosis and severe RLS, these patients were found to have decreased brain iron levels by magnetic resonance imaging, despite systemic iron overload, compared to healthy controls,55 underscoring the possibility that RLS symptoms are linked to low regional brain iron rather than peripheral iron availability. Brain iron stores have not been studied in children with SCD, but this presents a possible mechanism for elevated PLMS. An alternative mechanism is suggested by recent evidence demonstrating differences in the control of iron metabolism in SCD compared to unaffected individuals. Specifically, inflammation in SCD may prevent the release of iron from storage sites, particularly macrophages, thereby decreasing iron transport to tissues with high iron requirements.56 This finding is suggestive in light of evidence of iron metabolism abnormalities in peripheral lymphocytes in women with early-onset RLS versus control subjects, despite comparable ferritin subtypes and transferrin levels.57 Iron regulation in SCD as a cause of PLMS remain unexplored but presents intriguing direction for future research.
We cannot reconcile the difference in reticulocyte count between children with normal versus elevated PLMS based on what is known or hypothesized about their sequelae. We anticipated that children with more severe SCD would be the ones with higher PLMS indices and would, as a result of increased hemolysis, have greater reticulocytosis. What we found instead was the reverse, that the children with normal PLMS had higher reticulocyte counts. As hemoglobin levels were comparable between groups, and the difference in reticulocytes was small (3%), we suspect that this finding is merely of statistical rather than of clinical significance. Nevertheless, the possibility that PLMS might be associated with hematological changes warrants further study.
Limitations
This study had some important limitations. The PSQ for a small number of children was completed substantially later than their sleep study, and changes in children's sleep and sleep-related symptoms might have occurred during the time lapse between the two measures. Certain measures, including serum iron and ferritin levels were not collected during this research, so that associations of PLMS with correlates previously identified in children with other disorders, such as low body iron stores, could not be tested in our sample. Night-to-night variability of PLMS could not be evaluated due to the lack of consecutive polysomnograms, thus we were unable to account for children in our study with normal PLMS who may have exhibited elevated PLMS on repeat polysomnogram and whose change in diagnostic classification may have significantly altered our results. Finally, there is a difference between having elevated PLMS and having periodic limb movement disorder, a diagnosis which requires, in addition to a PLMS index ≥ 5/h on polysomnography, evidence of clinical sleep disturbance and lack of confounding sleep disorders. We had only 4 subjects who met these criteria, a sample size too small to carry out meaningful comparisons. A larger sample is required to more fully explore the impact of PLMS ≥ 5/h versus periodic limb movement disorder on daytime functioning and health outcomes in children with SCD.
CONCLUSION
We identified a high rate of abnormally elevated PLMS in a prospective, unreferred sample of children with SCD. Furthermore, PLMS were associated with sleep disturbances and RLS symptoms. While the clinical significance of our findings are unknown, deficient sleep in children has been associated with behavioral and academic deficits,58 and children with SCD are already at risk for neurocognitive deficits.59 Moreover, PLMS are associated with sympathetic bursts31 and changes in cerebral hemodynamics33 which may, theoretically, increase the risk of stroke in an at-risk population. Therefore, given the possible impact on the health of children with SCD and PLMS over a lifetime, there is a need for expanded research in this area, in order to better identify the causes and long-term outcomes of PLMS in children with SCD.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Marcus has received research support and equipment from Phillips Respironics unrelated to this study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Joel Traylor, BS, RPsgT, for his assistance in the collection of data from the Rembrandt polysomnography system pertaining to the distribution and time structure of periodic limb movements, and James R. Rogers, PhD for his assistance in the analysis of periodic limb movement distribution and inter-movement interval data.
This study was performed at The Children's Hospital of Philadelphia, PA. Research was sponsored by National Institutes of Health/NHLBI grant #5RO1HL079911-04.
ABBREVIATIONS
- ADHD
Attention deficit hyperactivity disorder
- AUC
Area under the receiver operator characteristic curve
- IMI
Inter-movement interval
- N
NREM sleep stage
- OAHI
Obstructive apnea-hypopnea index
- OSA
Obstructive sleep apnea
- PLMS
Periodic limb movements in sleep
- PSQ
Pediatric Sleep Questionnaire (Chervin, et al., 2000)
- RLS
Restless legs syndrome
- ROC
Receiver operator characteristic (curve)
- SCD
Sickle cell disease
- SCD-SS
Sickle cell disease, homozygous hemoglobin SS genotype
- SD
Standard deviation
- SpO2
Arterial oxygen saturation
REFERENCES
- 1.Hogan AM, Pit-ten Cate IM, Vargha-Khadem F, Prengler M, Kirkham FJ. Physiological correlates of intellectual function in children with sickle cell disease: hypoxaemia, hyperaemia, and brain infarction. Devel Sci. 2006;9:379–87. doi: 10.1111/j.1467-7687.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 2.Steen RG, Xiong X, Mulhern RK, Langston JW, Wang WC. Subtle brain abnormalities in children with sickle cell disease: relationship to blood hematocrit. Ann Neurol. 1999;45:279–86. doi: 10.1002/1531-8249(199903)45:3<279::aid-ana2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Lanzkron S, Haywood C, Jr, Segal JB, Dover GJ. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81:927–32. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 4.Ballas SK. The cost of health care for patients with sickle cell disease. Am J Hematol. 2009;84:320–2. doi: 10.1002/ajh.21443. [DOI] [PubMed] [Google Scholar]
- 5.Shankar SM, Arbogast PD, Mitchel E, Cooper WO, Wang WC, Griffin MR. Medical care utilization and mortality in sickle cell disease: a population-based study. Am J Hematol. 2005;80:262–70. doi: 10.1002/ajh.20485. [DOI] [PubMed] [Google Scholar]
- 6.Needleman JP, Franco ME, Varlotta L, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–22. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–34. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–8. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 9.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. Br J Haematol. 2009;144:736–41. doi: 10.1111/j.1365-2141.2008.07501.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaleyias J, Mostofi N, Grant M, et al. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30:659–65. doi: 10.1097/MPH.0b013e31817eb7ef. [DOI] [PubMed] [Google Scholar]
- 11.Spivey JF, Uong EC, Strunk R, Boslaugh SE, DeBaun MR. Low daytime pulse oximetry reading is associated with nocturnal desaturation and obstructive sleep apnea in children with sickle cell anemia. Pediatr Blood Cancer. 2008;50:359–62. doi: 10.1002/pbc.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6:374–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Vetrugno R, Provini F, Montagna P. Restless legs syndrome and periodic limb movements. Rev Neurol Dis. 2006;3:61–70. [PubMed] [Google Scholar]
- 14.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 15.Kirk VG, Bohn S. Periodic limb movements in children: prevalence in a referred population. Sleep. 2004;27:313–5. doi: 10.1093/sleep/27.2.313. [DOI] [PubMed] [Google Scholar]
- 16.Bokkala S, Napalinga K, Pinninti N, et al. Correlates of periodic limb movements of sleep in the pediatric population. Pediatr Neurol. 2008;39:33–9. doi: 10.1016/j.pediatrneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Picchietti DL, England SJ, Walters AS, Willis K, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:588–94. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- 18.Crabtree VM, Ivanenko A, Gozal D. Clinical and parental assessment of sleep in children with attention-deficit/hyperactivity disorder referred to a pediatric sleep medicine center. Clin Pediatr. 2003;42:807–13. doi: 10.1177/000992280304200906. [DOI] [PubMed] [Google Scholar]
- 19.Picchietti DL, Stevens HE. Early manifestations of restless legs syndrome in childhood and adolescence. Sleep Med. 2008;9:770–81. doi: 10.1016/j.sleep.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 21.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson A, Quan SF American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated event: rules, terminology and technical specifications. [Google Scholar]
- 23.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 24.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 25.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 26.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2:501–10. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 28.Hoque R, Chesson AL., Jr Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6:79–83. [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien LM, Ivanenko A, Crabtree VM, et al. Sleep disturbances in children with attention deficit hyperactivity disorder. Pediatr Res. 2003;54:237–43. doi: 10.1203/01.PDR.0000072333.11711.9A. [DOI] [PubMed] [Google Scholar]
- 30.Javaheri S. Sleep and cardiovascular disease: present and future. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 1157–60. [Google Scholar]
- 31.Walter LM, Foster AM, Patterson RR, et al. Cardiovascular variability during periodic leg movements in sleep in children. Sleep. 2009;32:1093–9. doi: 10.1093/sleep/32.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing YK, Zhang J, Ho CK, Au CT, Li AM. Periodic limb movement during sleep is associated with nocturnal hypertension in children. Sleep. 2010;33:759–65. doi: 10.1093/sleep/33.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizza F, Biallas M, Wolf M, Valko PO, Bassetti CL. Periodic leg movements during sleep and cerebral hemodynamic changes detected by NIRS. Clin Neurophysiol. 2009;120:1329–34. doi: 10.1016/j.clinph.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elwood P, Hack M, Pickering J, Hughs J, Gallacher J. Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Community Health. 2006;60:69–73. doi: 10.1136/jech.2005.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. 2009;114:5117–25. doi: 10.1182/blood-2009-05-220921. [DOI] [PubMed] [Google Scholar]
- 37.Exar EN, Collop NA. The association of upper airway resistance with periodic limb movements. Sleep. 2001;24:188–92. doi: 10.1093/sleep/24.2.188. [DOI] [PubMed] [Google Scholar]
- 38.Picchietti MA, Picchietti DL, England SJ, et al. Children show individual night-to-night variability of periodic limb movements in sleep. Sleep. 2009;32:530–5. doi: 10.1093/sleep/32.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pennestri MH, Whittom S, Adam B, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: prevalence and interval distribution. Sleep. 2006;29:1183–7. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 40.Pollmacher T, Schulz H. Periodic leg movements (PLM): their relationship to sleep stages. Sleep. 1993;16:572–7. [PubMed] [Google Scholar]
- 41.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9:790–8. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 43.Trenkwalder C, Hening WA, Walters AS, Campbell SS, Rahman K, Chokroverty S. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14:102–10. doi: 10.1002/1531-8257(199901)14:1<102::aid-mds1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Parker KP, Rye DB. Restless legs syndrome and periodic limb movement disorder. Nurs Clin North Am. 2002;37:655–73. doi: 10.1016/s0029-6465(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 45.Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents--The Peds REST Study. Pediatrics. 2007;120:253–66. doi: 10.1542/peds.2006-2767. [DOI] [PubMed] [Google Scholar]
- 46.Kotagal S, Silber MH. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56:803–7. doi: 10.1002/ana.20292. [DOI] [PubMed] [Google Scholar]
- 47.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 48.Wiater AH, Mitschke AR, von Widdern S, Fricke L, Breuer U, Lehmkuhl G. Sleep disorders and behavioural problems among 8- to 11-year-old children. Somnologie. 2005;9:210–4. [Google Scholar]
- 49.Smedje H, Broman JE, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged six to eight years: a study based on parents' perceptions. Eur Child Adolesc Psychiatry. 2001;10:1–9. doi: 10.1007/s007870170041. [DOI] [PubMed] [Google Scholar]
- 50.Hedo CC, Aken'ova AY, Okpala IE, Durojaiye AO, Salimonu LS. Acute phase reactants and the severity of homozygous sickle cell disease. J Intern Med. 1993;233:467–70. doi: 10.1111/j.1365-2796.1993.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 51.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 52.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–6. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 53.Mizuno S, Mihara T, Miyaoka T, Inagaki T, Horiguchi J. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14:43–7. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 54.Earley CJ, Barker PB, Horska A, Allen RP. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006;7:458–61. doi: 10.1016/j.sleep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Haba-Rubio J, Staner L, Petiau C, Schunck T, Macher JP. Restless legs syndrome and low brain iron levels in patients with haemochromatosis. J Neurol Neurosurg Psychiatry. 2005;76:1009–10. doi: 10.1136/jnnp.2003.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter PB, Harmatz P, Vichinsky E. Iron metabolism and iron chelation in sickle cell disease. Acta Haematol. 2009;122:174–83. doi: 10.1159/000243802. [DOI] [PubMed] [Google Scholar]
- 57.Earley CJ, Ponnuru P, Wang X, et al. Altered iron metabolism in lymphocytes from subjects with restless legs syndrome. Sleep. 2008;31:847–52. doi: 10.1093/sleep/31.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 59.Schatz J, Finke RL, Kellett JM, Kramer JH. Cognitive functioning in children with sickle cell disease: a meta-analysis. J Pediatr Psychol. 2002;27:739–48. doi: 10.1093/jpepsy/27.8.739. [DOI] [PubMed] [Google Scholar]