Summary
Endocytic events are critical for neuronal survival in response to target-derived neurotrophic cues, but whether local axon growth is mediated by endocytosis-dependent signaling mechanisms remains unclear. Here, we report that Nerve Growth Factor (NGF) promotes endocytosis of its TrkA receptors and axon growth by calcineurin-mediated dephosphorylation of the endocytic GTPase, dynamin1. Conditional deletion of calcineurin in sympathetic neurons disrupts NGF-dependent innervation of peripheral target tissues. Calcineurin signaling is required locally in sympathetic axons to support NGF-mediated growth in a manner independent of transcription, during the initial phase of axonal outgrowth. We show that calcineurin associates with dynamin1 via a PxIxIT interaction motif found only in specific dynamin1 splice variants. PxIxIT-containing dynamin1 isoforms co-localize with surface TrkA receptors, and their phosphoregulation is selectively required for NGF-dependent TrkA internalization and axon growth in sympathetic neurons. Thus, NGF-dependent phosphoregulation of dynamin1 is a critical event coordinating neurotrophin receptor endocytosis and initial axonal growth.
Introduction
Neurotrophins are trophic factors secreted by target tissues that coordinate multiple aspects of neuronal development including cell survival, axonal and dendritic growth, and synapse formation (Huang and Reichardt, 2001). In polarized neurons, neurotrophins elicit their effects by activating signaling pathways characterized by their sub-cellular site of action (Heerssen and Segal, 2002). Local signaling in distal axons and growth cones mediates acute responses including rapid axon growth, branching and guidance. In contrast, retrograde signaling to the cell body and nucleus elicits long-term changes in gene expression necessary for neuronal survival and differentiation. The neurotrophin, NGF, secreted by peripheral target tissues, supports survival of sympathetic and sensory neurons by regulating endocytosis and retrograde vesicular trafficking of NGF:TrkA complexes (Zweifel et al., 2005). Although much is known about the mechanisms regulating retrograde survival signaling to the nucleus, how target-derived NGF activates TrkA receptors in nerve terminals to induce axonal outgrowth remains unclear.
In the developing sympathetic nervous system, the neurotrophins, NT-3 and NGF, act through the same TrkA receptor to orchestrate sequential stages of axon growth (Glebova and Ginty, 2005; Kuruvilla et al., 2004). NT-3, highly expressed in intermediate targets such as the vasculature, promotes early stages of axon growth. NGF, which is highly expressed in final peripheral targets, supports final target innervation (Glebova and Ginty, 2004; Kuruvilla et al., 2004). Unlike NGF, NT-3 cannot promote endocytosis and retrograde transport of TrkA (Kuruvilla et al., 2004). Although both NGF and NT-3 promote robust axon growth in sympathetic neurons, only NGF supports neuronal survival. Thus, differential trafficking of TrkA seems to be responsible only for differences in the ability of NGF and NT-3 to promote neuronal survival. Consistent with the idea that activation of cell-surface TrkA receptors is sufficient to support local axonal growth, NGF immobilized on beads elicits acute axonal responses including growth cone extension, branching and guidance (Gallo et al., 1997; Gallo and Letourneau, 1998). However, axon growth along intermediate targets is characteristically distinct from final stages of target innervation (Rubin, 1985). Furthermore, NGF- and NT-3-treated neurons display distinct morphological responses (Orike et al., 2001). Currently, it remains unclear whether NGF and NT-3 employ distinct signaling mechanisms downstream of a common TrkA receptor to promote axonal growth. In particular, the contribution of endocytic trafficking of TrkA receptors to neurotrophin-mediated axonal growth remains poorly defined.
In sensory neurons, a calcineurin/NFAT-dependent transcriptional program has been reported to control axonal growth in response to NGF and NT-3 (Graef et al., 2003). Calcineurin is a calcium-responsive serine/threonine phosphatase, consisting of a catalytic subunit (calcineurin A) and a regulatory subunit (calcineurinB). Ca2+-dependent activation of calcineurin results in dephosphorylation and nuclear import of NFAT transcription factors (NFAT1-4) (Flanagan et al., 1991). Mice deficient in calcineurin/NFAT signaling show defects in neurotrophin-dependent sensory axon growth, without any disruption of neuronal differentiation or survival (Graef et al., 2003). Although NFAT has received the most attention among calcineurin substrates, calcineurin has many other downstream targets that may play important roles in neuronal development (Li et al. 2011). Here, we identify a new endocytic mechanism by which calcineurin regulates neurotrophin-dependent axonal growth. We found that calcineurin activity is specifically required for NGF, but not NT-3-mediated axon growth in sympathetic neurons. We identified dynamin1 as a local target of calcineurin signaling in axons that is critical for NGF-mediated growth, in a manner independent of transcription. A PxIxIT box present within specific dynamin1 splicing isoforms promotes interactions with calcineurin. Phosphoregulation of these PxIxIT-containing dynamin1 isoforms by NGF is required for TrkA internalization and axon growth. Together, our results point to a novel regulatory pathway by which NGF promotes axonal growth via calcineurin-mediated dephosphorylation of PxIxIT motif-containing dynamin1 isoforms and endocytosis of TrkA receptors.
Results
Calcineurin is required for NGF-, but not NT-3-mediated sympathetic axon growth
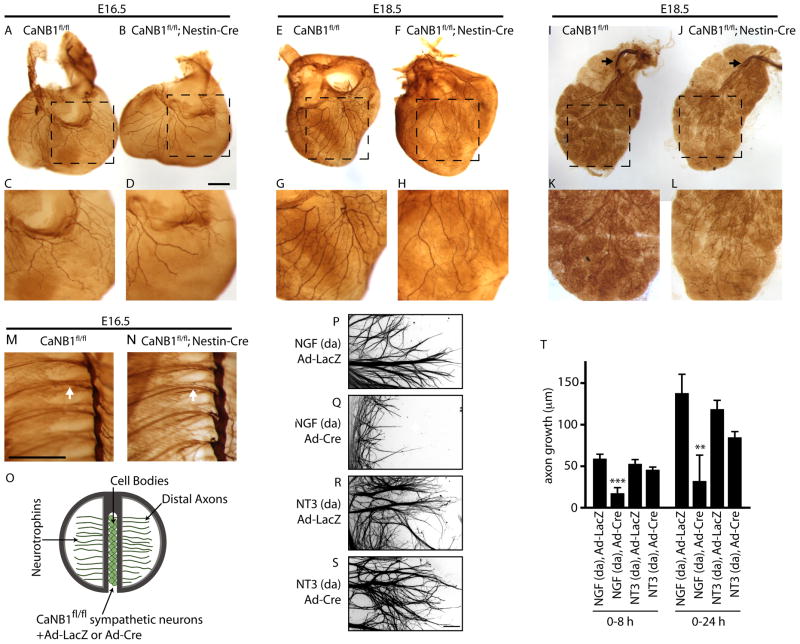
To assess the role of calcineurin in neurotrophin-dependent sympathetic axon growth in vivo, we examined innervation of target tissues in mice with conditional ablation of calcineurin in neurons. Selective disruption of calcineurin in neurons was accomplished by crossing mice harboring a LoxP-based conditional calcineurin allele (CaNB1fl/fl mice) (Zeng et al., 2001) to Nestin-Cre transgenic mice (Tronche et al., 1999). There are two mammalian isoforms of the calcineurin regulatory subunit, CalcineurinB; CaNB1 is ubiquitously expressed while CaNB2 is only expressed in testes. Immunoblotting analyses of sympathetic ganglia from CaNB1fl/fl;Nestin-Cre mice showed reductions in the levels of CaNB1 and the calcineurinA catalytic subunit (CaNA) (Supplemental Figure S1A). A whole-mount tyrosine hydroxylase (TH) immunohistochemical assay was employed to visualize axonal growth out of sympathetic ganglia and innervation of several peripheral targets at late embryonic stages (E16.5–E18.5). TH immunostaining of E16.5 embryos revealed sympathetic fibers beginning to innervate the heart in both CaNB1fl/fl;Nestin-Cre and wild-type litter-mates (Figures 1A–D). However, in CaNB1fl/fl;Nestin-Cre mutants, sympathetic axons were shorter and less branched (Figures 1B and 1D) as compared to that in wild-type embryos (Figures 1A and 1C). Deficits in sympathetic innervation were also observed in the dorsal face of the heart (Supplemental Figures S1B–E), and in the kidneys (Supplemental Figures S1F–I). At E18.5, while the main axonal fibers continued to elaborate into finer branches in the heart in wild-type mice (Figures 1E and 1G, and Supplemental Figures S1J and S1L), there were marked reductions in terminal extension and arborization of sympathetic fibers in CaNB1fl/fl;Nestin-Cre mice (Figures 1F and 1H, and Supplemental Figures S1K and S1M). Similar deficits were observed in E18.5 salivary glands (Figures 1I–L) and kidneys (Supplemental Figures S1N–Q). In contrast to innervation deficits observed in final targets, axonal outgrowth from sympathetic ganglia (Figures 1M, N) and projections along the vasculature (Supplemental Figures S1R and S1S) appeared normal in CaNB1fl/fl;Nestin-Cre embryos. In addition, there were no differences in overall morphology of the sympathetic chain between mutant and wild-type embryos (Figures 1M, N). These results suggest that calcineurin is required for sympathetic innervation of final target tissues, an NGF-mediated process, but that, axon growth along the vasculature, an NT-3-mediated process, occurs via calcineurin-independent mechanisms.
Figure 1. Calcineurin is required for NGF, but not NT-3-mediated axon growth in sympathetic neurons.
(A–L) Whole mount TH immunostaining shows reduced sympathetic fibers in target tissues in CaNB1fl/fl;Nestin-Cre mice as compared to CaNB1fl/fl controls, at E16.5 (heart: A–D), and E18.5 (heart: E–H and salivary glands: I–L). Higher magnification images are shown in the lower panels. Black arrows in I, J indicate sympathetic fibers approaching the salivary glands. Scale bar: 500 μm. (M–N) There are no differences in sympathetic chain organization between E16.5 CaNB1fl/fl (M) and CaNB1fl/fl;Nestin-Cre (N) mice. White arrow indicates TH-positive sympathetic fibers extending from sympathetic ganglia in both wild-type and mutant mice. Scale bar: 500 μm. (n=2 embryos for each genotype at E16.5, and at E18.5) (O) CaNB1fl/fl sympathetic neurons were infected with adenoviral vectors expressing Cre (Ad-Cre) or LacZ (Ad-LacZ). Neurotrophins were added only to distal axons (da). (P–S) Cre-mediated calcineurin deletion specifically decreases NGF, but not NT-3-mediated axon growth. Axons were stained with β-III-tubulin for visualization after quantification of axon growth. Scale bar, 80μm. (T) Quantification of axon growth in compartmentalized cultures over 0–8 hr and 0–24 hr, ** p<0.01, ***p<0.001. Results are mean ± SEM from n=5 experiments.
To directly test the requirement for calcineurin in promoting growth downstream of NGF and NT-3, we examined neurotrophin-dependent growth in compartmentalized cultures. In this culture system, neuronal cell bodies and axon terminals are segregated into distinct fluid compartments by a teflon-grease barrier (Figure 1O). Target-derived neurotrophins can be applied exclusively to axon terminals, recapitulating the in vivo situation. To genetically disrupt calcineurin activity in vitro, compartmentalized sympathetic cultures established from P0.5 CaNB1fl/fl mice were infected with adenoviral vectors expressing either Cre recombinase or LacZ as control. Immunoblotting analyses showed significant reductions in the levels of CaNB and CaNA, 48 hr after infecting CaNB1fl/fl sympathetic neurons with Cre adenovirus (Supplemental Figure S1T). CaNB1fl/fl axons were then exposed to either NGF or NT-3, and growth was measured over 0–8 hr and 0–24 hr. NGF (100 ng/ml) supports approximately 60 μm of axon growth over 8 hr, and 130 μm of axon growth over 24 hr (Figure 1T). Similar rates of axon growth were observed with NT-3 (100 ng/ml; 52 ± 6.4 μm and 117 ± 11.8 μm over 8 hr and 24 hr, respectively). Cre-mediated calcineurin depletion significantly decreased NGF-dependent axonal growth (Figures 1P, 1Q and 1T). In contrast, NT-3-mediated axonal growth was not affected by the absence of calcineurin at 8 hr, and largely unaffected at 24 hr (Figures 1R, 1S and 1T). Together with our in vivo results, these findings provide evidence that calcineurin activity in sympathetic neurons is required for axon growth in response to NGF, but not NT-3.
Calcineurin signaling in distal axons is required for NGF-mediated axon growth
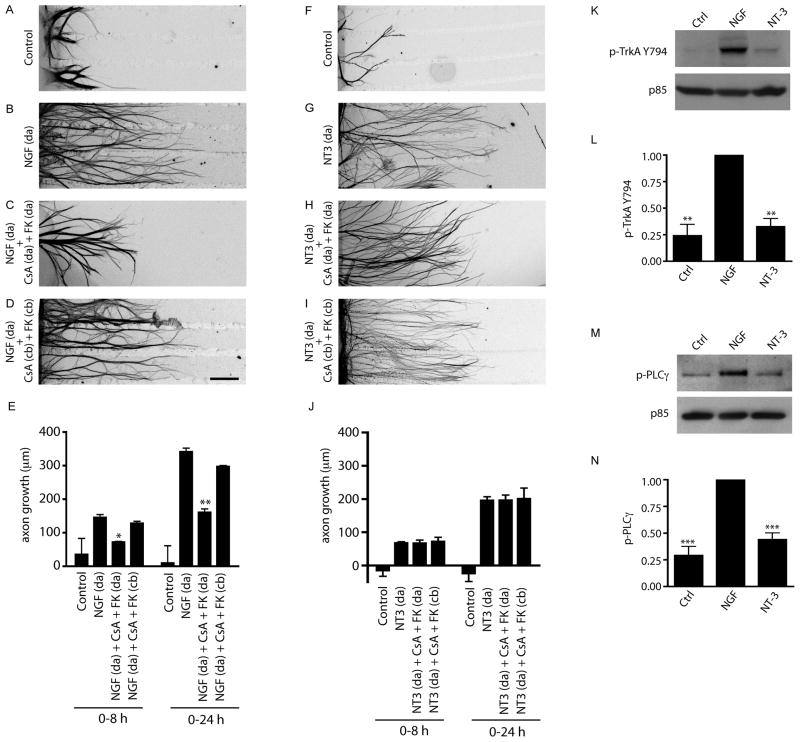
Since target-derived NGF can activate calcineurin signaling either locally in axons or retrogradely in cell bodies, we asked whether calcineurin activity was required in cell bodies or in axons to promote axonal growth. Cell bodies or axons of rat sympathetic neurons grown in compartmentalized cultures were exposed to the calcineurin inhibitors Cyclosporin A (CsA) (2 μg/ml) and FK506 (0.2 μg/ml), and growth in response to axon-applied NGF (100 ng/ml) was assessed. As reported previously (Graef et al., 2003), pharmacological inhibition of calcineurin activity in neurons required the use of CsA and FK506 together since only partial inhibition was observed with either alone. NGF-dependent axon growth (Figures 2A and 2B) was markedly reduced when calcineurin inhibitors were added to distal axons (Figure 2C), but not cell bodies (Figures 2D). Decrease in NGF-dependent growth of axons exposed to calcineurin inhibitors was observed within 8 hr (Figure 2E), suggesting that calcineurin activity in axons is required for rapid axonal extension in response to NGF. Quantification revealed that calcineurin inhibition in distal axons significantly reduced NGF-dependent axonal growth by 51% over 8 hr, and by 54% over 24 hr, (Figure 2E). Consistent with our previous results, NT-3-dependent axon growth was not affected by the addition of CsA and FK506 to distal axons or cell bodies (Figures 2F–J).
Figure 2. Calcineurin signaling is required in axons for NGF-mediated growth.
(A–D) NGF-mediated axon growth is reduced by addition of calcineurin inhibitors (CsA+FK506) to distal axons (da) (C), but not cell bodies (cb) (D). NGF (100 ng/ml) was added only to distal axons. Axons were stained with β-III-tubulin for visualization. Scale bar, 320μm. (E) Quantification of NGF-mediated axon growth in compartmentalized cultures over 0–8 hr or 0–24 hr. * p<0.05, **p<0.01, n=4 experiments. (F–I) Calcineurin signaling is not required for NT-3-mediated axon growth. (J) Quantification of NT-3-mediated axon growth, n=4. (K) NGF but not NT-3 induces phosphorylation of TrkA on Tyr-794. Neuronal lysates were probed for phospho-TrkA (Y794). Immunoblots were reprobed for p85. (L) Densitometric quantification of phospho-TrkA (Y794). **p<0.01, n=3. (M) NGF treatment selectively promotes tyrosine phosphorylation of PLC-γ. Lysates were immunoprecipitated with anti-phospho-tyrosine and probed for PLC-γ. Supernatants were probed for p85. (N) Densitometric quantification of PLC-γ phosphorylation, ***p<0.001, n=4.
Given that NGF-, but not NT-3-dependent axon growth requires calcineurin, we considered whether these two neurotrophins differ in their ability to activate calcineurin in sympathetic neurons. It is likely that neurotrophin signaling promotes activation of calcineurin through recruitment of PLC-γ to TrkA receptors (Graef et al., 2003) and the subsequent ability of PLC-γ to release Ca2+ from intracellular stores (Huang and Reichardt, 2003). To assess activation of the PLC-γ pathway in sympathetic neurons treated with either NGF (100 ng/ml) or NT-3 (100 ng/ml), we examined phosphorylation of TrkA at Tyr794, previously identified as the PLC-γ binding site on rat TrkA (Loeb et al., 1994). Immunoblotting analyses with a phospho-specific antibody (Rajagopal et al., 2004) revealed that NGF increased TrkA phosphorylation at the PLC-γ interaction site (Y794), as compared to untreated control cultures, or cultures treated with NT-3 (Figures 2K and 2L). Recruitment of PLC-γ to TrkA upon neurotrophin stimulation leads to tyrosine phosphorylation of PLC-γ (Loeb et al., 1994), which is a pre-requisite step for activation of its enzymatic activity. We assessed PLC-γ tyrosine phosphorylation in untreated, NGF- and NT-3-treated sympathetic neuronal lysates by immunoprecipitating with an antibody directed against phosphotyrosine and probing immunoblots with a PLC-γ antibody. Only NGF treatment of sympathetic neurons resulted in enhanced tyrosine phosphorylation of PLC-γ (Figures 2M and 2N). These results suggest that selective activation of calcineurin by NGF in sympathetic neurons occurs via engagement of the PLC-γ signaling pathway.
NGF-mediated axonal responses require calcineurin signaling independent of transcriptional activity
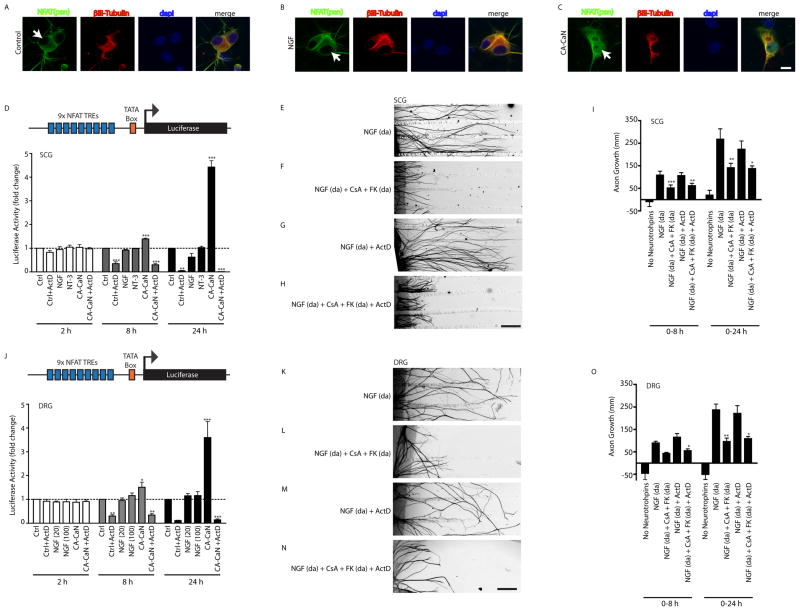
Previously, NFAT transcription factors were reported to be the major calcineurin substrate relevant for neurotrophin-mediated axon growth in developing sensory neurons (Graef et al., 2003). To test whether NGF stimulation promotes nuclear translocation of NFAT transcription factors in sympathetic neurons, we performed confocal microscopic analyses of neurons immunostained with a pan-NFAT antibody. Sympathetic neurons express all four Ca2+-sensitive NFAT1-4 isoforms (Supplemental Figure S2A). In unstimulated neurons, NFAT immunoreactivity was observed to be predominantly cytoplasmic with staining observed both in cell bodies and axons including growth cones (Figure 3A and Supplemental Figures S2B and S2C). Importantly, exposure to NGF for 30 min failed to elicit any changes in NFAT sub-cellular localization (Figure 3B). However, expression of a constitutively active form of calcineurin (CA-CaN) that lacks the regulatory domain (De Windt et al., 2000) resulted in nuclear accumulation of NFAT (Figure 3C). As a more sensitive and quantitative assay, sympathetic neurons were infected with an adenoviral construct expressing a NFAT luciferase reporter containing nine multimerized NFAT binding sites upstream of a minimal TATA-containing promoter fused to luciferase (Figure 3D) (Wilkins et al., 2004). Exposure of neurons to NGF (100 ng/ml) for 2 hr, 8 hr or 24 hr did not induce NFAT-dependent transcriptional activity (Figure 3D). Similarly, NT-3 had no effect on NFAT transcriptional activity (Figure 3D). However, adenovirus-mediated expression of constitutively active calcineurin increased luciferase reporter activity, at 8 hr and 24 hr post-infection (1.4-fold and 4.4-fold at 8 hr and 24 hr, respectively), as compared to control untreated cultures (Figure 3D). Together, these results suggest that, at least over 24 hr, NGF does not induce activation of NFAT-dependent transcriptional activity in sympathetic neurons.
Figure 3. Calcineurin supports NGF-mediated axon growth in a transcription-independent manner.
(A–C) NGF does not promote nuclear import of NFAT transcription factors in sympathetic neurons. NFAT immunostaining shows that NGF treatment (100 ng/ml, 30 min) does not induce nuclear localization of NFAT (B, arrow), while NFAT nuclear labeling is evident in neurons expressing CA-CaN (C, arrow). Neurons were also immunostained with β-III Tubulin and DAPI. Scale bar, 10μm. (D) NFAT-luciferase reporter assay shows that NGF and NT-3 do not activate NFAT-dependent transcription in sympathetic neurons. Neurons expressing CA-CaN show activation of NFAT-dependent transcription at 8 and 24 hr post-infection with NFAT-adenovirus, which is blocked by ActD (0.1 μg/ml). **p<0.01 and ***p<0.001. Results are means± SEM from 3 experiments. (E–I) Calcineurin signaling is required for NGF-mediated axon growth in the absence of transcription. NGF and calcineurin inhibitors (CsA+FK506) were added only to distal axons. ActD (0.1 μg/ml) was bath applied. Scale bar, 320μm. (I) Quantification of sympathetic axon growth in compartmentalized cultures over 0–8 hr and 0–24 hr after treatments described in (E–H). *p<0.05, **p<0.01, and ***p<0.001, n=3 experiments. (J) NFAT-luciferase assay shows that NGF does not activate NFAT-dependent transcription in DRG sensory neurons. DRG neurons expressing CA-CaN show robust activation of NFAT-dependent transcription. *p<0.05, **p<0.01, and ***p<0.001, n=5 experiments. (K–N) Calcineurin signaling is required for NGF-mediated axon growth in DRG neurons, in the absence of transcription. Scale bar, 320μm. (O) Quantification of DRG axon growth in compartmentalized cultures. *p<0.05, ** p<0.01, n=4.
To determine whether calcineurin signaling mediates NGF-dependent axon growth via transcriptional responses, we directly tested the role of transcription in short-term axonal growth in response to NGF. Sympathetic neurons were grown in compartmentalized culture chambers, and transcriptional activity was blocked by adding ActinomycinD (ActD, 0.1 μg/ml). We confirmed that this concentration of ActD effectively blocked the ability of CA-CaN to induce NFAT-dependent transcription in sympathetic neurons (Figure 3D). ActD did not significantly influence NGF-dependent growth in sympathetic neurons over 8 hr or 24 hr (Figures 3E, 3G and 3I). Similarly, treatment of sympathetic neurons with a different transcriptional inhibitor, α-amanitin (0.2 μg/ml), also had no effect on NGF-dependent axonal growth over 24 hr (Supplemental Figure S2D). However, by 48 hr, we observed a complete cessation of NGF-mediated axonal growth with transcriptional inhibitors (Supplemental Figure S2D), indicating that continued axonal growth after 24 hr requires new transcription. Importantly, application of the calcineurin inhibitors, CsA and FK506 to axon terminals significantly reduced NGF-mediated axon growth over 24 hr, to 40%–60% of control values, in the presence (Figures 3H and 3I) or absence of ActD (Figures 3F and 3I). Together, these results provide evidence that calcineurin has a role in NGF-mediated axonal growth that is independent of transcription. Consistent with its role in transcriptionally-independent NGF responses, calcineurin activity is required for rapid changes in growth cone morphology in response to NGF (Supplemental Figures S2E–I). NGF stimulation (15 min) leads to rapid increases in growth cone area (Supplemental Figures S2F and S2H) and filopodia number (Supplemental Figures S2F and S2I). These short-term effects of NGF are attenuated by calcineurin inhibition (Supplemental Figures S2G–I).
To determine whether our findings extend to other NGF-responsive neuronal populations, we asked whether calcineurin has a transcription-independent growth-promoting effect in dorsal root ganglia (DRG) sensory neurons. Exposure of DRG neurons to NGF (20 ng/ml or 100 ng/ml) for 2 hr, 8 hr or 24 hr did not induce NFAT-dependent transcriptional activity (Figure 3J), as reported by the NFAT-luciferase assay. However, expression of constitutively active calcineurin increased luciferase reporter activity in DRG neurons (Figure 3J). Transcriptional inhibition did not significantly influence NGF-dependent growth in compartmentalized DRG cultures over 8 hr or 24 hr, but stopped axon growth by 48 hr (Figure 3K, 3M, 3O, and Supplemental Figure S2J). Similar to our results with sympathetic neurons, application of the calcineurin inhibitors, CsA and FK506, to DRG axon terminals reduced NGF-mediated axon growth (40%–50% of control values) in the presence (Figures 3N and 3O) or absence of ActD (Figures 3L and 3O). Together, these results uncover a transcription-independent role for calcineurin in NGF-mediated axon growth in both sympathetic and DRG neurons.
PLC-γ/calcineurin signaling promotes TrkA endocytosis
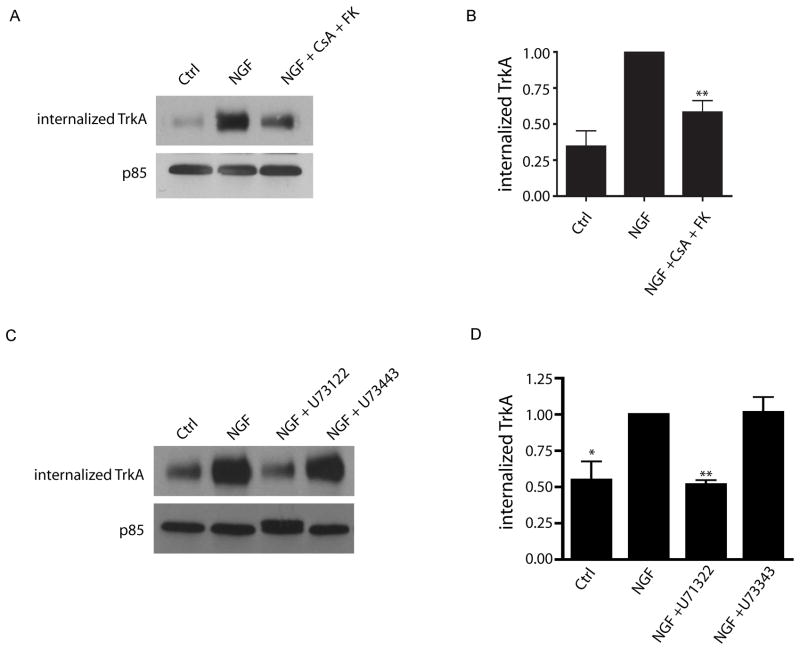
The primary difference between NGF and NT-3 signaling in sympathetic neurons is that NGF is able to induce endocytosis of TrkA receptors while NT-3 cannot (Kuruvilla et al., 2004). Given that calcineurin signaling is required for NGF-, but not NT-3-dependent axonal responses, we hypothesized that calcineurin signaling might be required for NGF-mediated endocytosis of TrkA receptors. To test this hypothesis, a cell surface biotinylation assay was performed to measure NGF-dependent internalization of TrkA receptors in sympathetic neurons, in the presence or absence of calcineurin signaling. As previously reported (Kuruvilla et al., 2004), NGF treatment leads to robust internalization of TrkA receptors (Figures 4A and 4B). Levels of biotinylated TrkA receptors internalized in response to NGF were markedly reduced (42% decrease) when calcineurin activity was blocked with CsA and FK506 (Figures 4A and 4B). Calcineurin inhibitors had no effect on surface levels of TrkA in the absence of NGF (Supplemental Figures S3A and S3B), indicating calcineurin signaling is not required for maintenance of TrkA receptors on the plasma membrane. Since our results suggest that NGF-mediated activation of calcineurin occurs via recruitment of the TrkA effector, PLC-γ, we tested whether PLC-γ activity is required for TrkA endocytosis. Inhibition of PLC-γ activity with a selective inhibitor, U73122 (10 μM) markedly reduced NGF-dependent endocytosis of TrkA receptors (Figures 4C and 4D). However, treatment of neurons with an inactive analog, U73443, had no effect. These results suggest that NGF promotes endocytosis of TrkA receptors by activation of a PLC-γ/calcineurin signaling pathway.
Figure 4. PLC-γ and calcineurin mediate TrkA endocytosis.
(A) Calcineurin activity is required for NGF-dependent internalization of TrkA receptors. Cell surface biotinylation and TrkA immunoblotting shows that NGF-dependent TrkA endocytosis is reduced by calcineurin inhibiton. (B) Densitometric quantification of internalized TrkA, **p<0.01, n=6. (C) TrkA endocytosis is dependent on PLC-γ activity. PLC-γ inhibitor (U73122) decreases NGF-dependent TrkA endocytosis. (D) Densitometric quantification of internalized TrkA, *p<0.05 and **p<0.01, n=4. In (A) and (C), supernatants were probed for p85 for normalization.
Calcineurin mediates NGF-dependent growth via dephosphorylation of dynamin
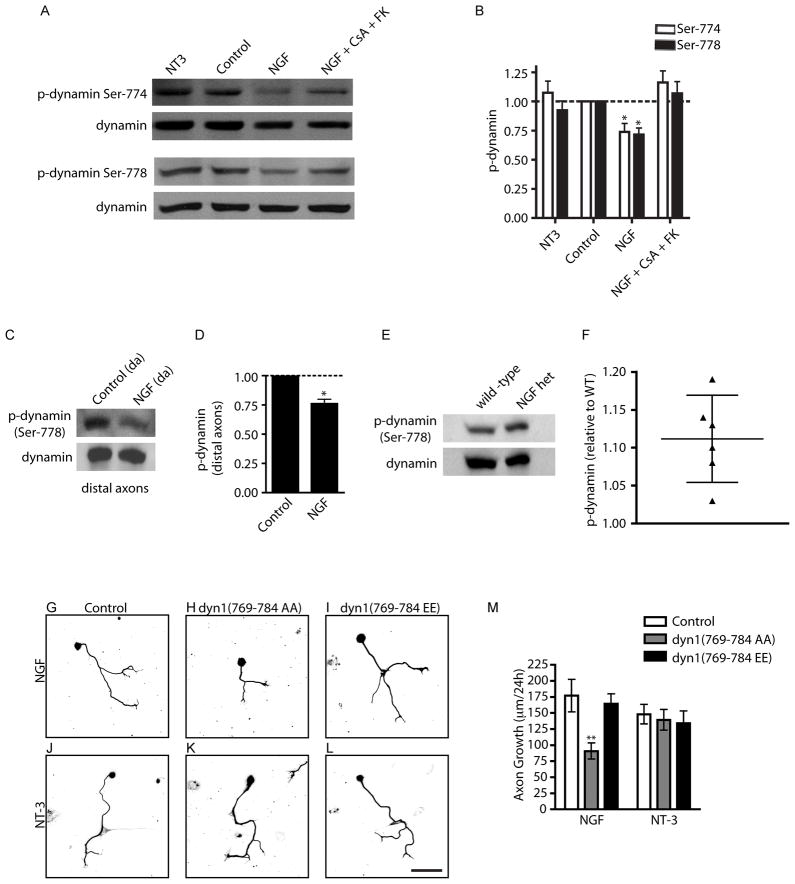
Given that calcineurin signaling is required for TrkA endocytosis, we asked what calcineurin substrate/s mediates this response? Our clue came from previous studies in synaptic vesicle endocytosis (SVE) where calcineurin-dependent dephosphorylation of the endocytic GTPase dynamin1 is essential for the retrieval of synaptic vesicle membranes (Liu et al., 1994). Nerve terminal depolarization leads to calcineurin-dependent dephosphorylation of dynamin1 on at least two serine residues, Ser-774 and Ser-778, located within a phospho-box region in the proline-rich C-terminus (Clayton et al., 2009). Site-directed mutagenesis indicated that phosphoregulation of these residues on dynamin1 is required for calcineurin-dependent endocytosis of synaptic vesicles (Clayton et al., 2009). To ask whether NGF stimulation leads to dynamin dephosphorylation in a calcineurin-dependent manner, sympathetic neurons were exposed to NGF for 20 min, and levels of phosphorylated dynamin1 assessed using phospho-specific antibodies that specifically recognize dynamin1 phosphorylated on Ser-774 and Ser-778. NGF induced a significant decrease in dynamin1 phosphorylation on Ser-774 and Ser-778 (26.2% and 28.5%, respectively), which was blocked by CsA and FK506 treatment (Figures 5A and 5B). As predicted, the phosphorylation status of dynamin1 was unaffected by NT-3 treatment (Figures 5A and 5B). Thus, NGF, but not NT-3, leads to calcineurin-dependent dephosphorylation of dynamin, providing further support for this mechanism underlying the differential trafficking of TrkA receptors downstream of NGF and NT-3 (Kuruvilla et al., 2004).
Figure 5. NGF promotes axon growth through dynamin dephosphorylation.
(A) NGF stimulation results in dephosphorylation of dynamin1 in a calcineurin-dependent manner. Neuronal lysates were immunoblotted using phospho-Ser774 and phospho-Ser778 dynamin antibodies. Immunoblots were stripped and reprobed for total dynamin1. (B) Densitometric quantification of phospho-dynamin1 levels *p<0.05, n=6. (C) NGF stimulation results in dephosphorylation of dynamin1 (Ser 778) in distal axons. Immunoblots were reprobed for total dynamin1. (D) Densitometric quantification of phospho-dynamin1 (Ser778) in axons, *p<0.05, n=3. (E–F) NGF+/− mice have increased levels of phospho-dynamin1 in sympathetic axons in vivo. Salivary gland lysates from P0.5 wildtype and NGF+/− mice were immunoblotted using phospho-dynamin1 (Ser778) antibody. Immunoblots were reprobed for total dynamin1. (F) Densitometric quantification of phospho-dynamin1 (Ser778) after treatments as described in (E), represented as a scatter plot with 95% confidence intervals. n=6 pups for each genotype. (G) Dephosphorylation-dependent dynamin1 function is required for NGF-mediated axon growth. Introduction of dyn1(769-784 AA) (H) but not the dyn1(769-784 EE) (I) peptide decreased NGF-dependent axon growth over 24 hr. NT-3-mediated growth was unaffected by introduction of dyn1 phosphopeptides (J–L). Scale bar, 100μm. (M) Quantification of axon growth. **p<0.01, n=3.
Given that target-derived NGF acts directly on projecting axons to promote growth, we tested whether axon-applied NGF locally modulates dynamin1 phosphorylation in nerve terminals, in vitro and in vivo. Sympathetic axons in compartmentalized cultures were stimulated with NGF (100 ng/ml, 20 min) and axonal lysates immunoblotted with phospho-dynamin1 (Ser 778) antibody. Similar to results in mass cultures, NGF treatment of distal axons leads to a reduction (24% decrease) in phosphorylated dynamin1, in comparison to control treatment (Figure 5C and 5D). To test whether NGF regulates phosphorylation of dynamin1 in axons in vivo, we analyzed the levels of phospho-dynamin1 in a sympathetic target tissue, the salivary glands, in wild-type and heterozygous NGF (NGF+/−) mice. Given that dynamin1 is neuron-specific (Urrutia et al., 1997), immunoblotting of salivary gland lysates with the phospho-dynamin1 antibody should reveal the status of dynamin1 phosphorylation locally in sympathetic nerve terminals that innervate the target tissue. If target-derived NGF regulates dynamin1 phosphorylation in vivo, then we would expect to see increased dynamin1 phosphorylation levels under conditions of reduced NGF signaling. We employed NGF+/− mice for this analysis because these mice display haploinsufficiency with reduced levels of NGF and sympathetic target innervation (Brennan et al., 1999; Ghasemlou et al., 2004), in contrast to homozygous NGF null mice, that completely lack sympathetic innervation (Glebova and Ginty, 2004). We found that NGF+/− mice have higher levels of phosphorylated dynamin1 on Ser-778 in sympathetic axons innervating the salivary glands, as compared to wild-type animals (11.2 ± 2% increase, Figure 5E and 5F). These findings provide in vivo evidence for NGF-dependent phosphoregulation of dynamin1 locally in sympathetic axons.
To assess the role of dynamin1 dephosphorylation in supporting neurotrophin-dependent axon growth, sympathetic neurons were exposed for 24 hr to a cell permeable peptide spanning the dynamin1 phospho-box (amino acids 769-784, incorporating Ser-774 and Ser-778) in which the two serines 774/778 were replaced with alanine (Ser774/778-Ala, dyn1769-784AA). The dyn1769-784AA peptide blocks dephosphosphorylation-dependent dynamin1 functions by binding and sequestering downstream effector molecules, such as syndapin1 (Anggono et al., 2006). Delivery of dyn1769-784AA (300 μM) into sympathetic neurons reduced NGF-mediated axon growth from an average of 177 ± 14 μm/day to 90.6 ± 7.2 μm/day (Figures 5G, 5H and 5M). In contrast, introduction of the phospho-mimetic peptide dyn1769-784EE (in which the serines 774/778 were substituted with glutamate) had no effect on NGF-mediated axon growth (Figures 5I and 5M). NT-3-mediated axon growth was not affected by delivery of either dyn1769-784AA or dyn1769-784EE (Figures 5J, 5K, 5L and 5M). Together, these results provide evidence that calcineurin-mediated dephosphorylation of dynamin1 is a key signaling mechanism necessary for NGF- but not NT-3-mediated axon growth.
Isoform-specific interaction of calcineurin with dynamin1 via a PxIxIT domain
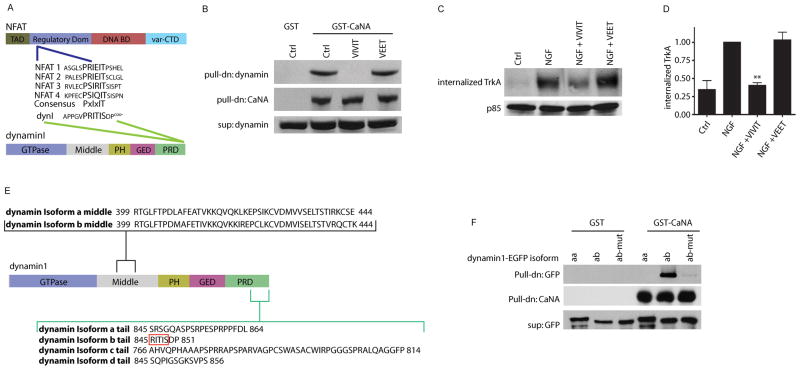
There are three dynamin genes expressed in mammals with dynamin1 reported to being neuron-specific, dynamin2 being ubiquitously expressed and dynamin3 expressed in brain, lungs and testes (Urrutia et al., 1997). We asked whether calcineurin interacted with all 3 dynamins by performing calcineurinA-GST pulldowns of rat brain lysates, and probing for calcineurin interaction using antibodies specific to dynamin1, 2 and 3. As previously demonstrated (Lai et al., 1999), dynamin1 binds calcineurinA-GST (Supplemental Figure S4A); in contrast, dynamin2 and dynamin3 do not detectably bind calcineurinA-GST (Supplemental Figure S4A).
While exploring the mechanism of calcineurin-dynamin1 association, we observed that the dynamin1 C-terminal proline rich domain (PRD) harbors a putative calcineurin interaction sequence, PRITIS, within the amino acids 844-849 (Figure 6A). This motif has high sequence identity to the “PxIxIT box”, a consensus sequence present in NFAT transcription factors and that mediates the docking of calcineurin to the NFAT regulatory domains (Aramburu et al., 1998). Deletion studies using a yeast-two hybrid assay had restricted the calcineurin-interaction region of dynamin1 to the last 135 amino acids at the C-terminus (Lai et al., 1999), encompassing this putative PxIxIT box. To test whether calcineurin-dynamin1 interaction is mediated by the PxIxIT motif present in dynamin1, we took advantage of the VIVIT peptide, a high affinity molecular mimic of the PxIxIT box domain that acts as a competitive inhibitor of calcineurin-PxIxIT box interactions (Aramburu et al., 1999). CalcineurinA-GST pulldowns of rat brain lysates were performed either in the presence or absence of VIVIT, and immunoblotting was performed to detect dynamin1 interaction. The VIVIT peptide completely blocked calcineurin-dynamin1 interaction while a control peptide, VEET, had no effect (Figure 6B).
Figure 6. Calcineurin-dynamin1 interaction is mediated by a PxIxIT motif found in specific dynamin1 isoforms.
(A) Schematic of PxIxIT box consensus sequence found in the regulatory domain of NFAT (1–4) transcription factors and the PRITIS sequence in the proline-rich domain (PRD) of dynamin1. TAD is the transactivation domain, DNA BD is the DNA binding domain, var-CTD is the variable C-terminal domain for NFAT. PH is the pleckstrin homology domain and GED is the GTPase effector domain for dynamin. (B) Calcineurin-dynamin1 interaction is dependent on the PxIxIT motif. VIVIT peptide (a PxIxIT box mimic), but not a control VEET peptide, blocks association of CaNA with dynamin1. Pulldown with GST alone is shown as control. (C) Calcineurin-dynamin1 interaction via the PxIxIT motif is required for NGF-dependent TrkA internalization. Cell surface biotinylation assay shows that VIVIT, but not VEET treatment decreases NGF-dependent internalization of TrkA receptors. Supernatants were probed for p85. (D) Densitometric quantification of internalized TrkA, **p<0.01, n=4 (E, F) Calcineurin interaction is specific to dynamin1 variants with a PxIxIT box. (E) Schematic of dynamin1 splicing variants. Red box indicates xIxIS portion of the PRITIS box sequence, only present in b tail isoforms. (F) GST pull-down assays with HEK293 lysates show that calcineurin interacts with dynamin1ab via the PxIxIT box, but not dynamin1aa isoforms. HEK293 cells were transfected with dynamin1aa-EGFP, dynamin1ab-EGFP, or dynamin1ab-EGFP with PRITIS sequence mutated to ARATAA.
To investigate whether calcineurin signaling regulates TrkA endocytosis via its interaction with dynamin1, calcineurin-dynamin1 interaction was blocked by exposing cultured sympathetic neurons to a cell permeable VIVIT peptide (1 μM) and a cell-surface biotinylation assay performed to assess internalization of TrkA receptors in response to NGF. We observed that internalized TrkA levels following NGF treatment were significantly reduced (60% decrease) in the presence of VIVIT peptide, while application of the control peptide had no effect on TrkA internalization (Figures 6C and 6D). Treatment of sympathetic neurons with VIVIT or VEET did not significantly change the basal levels of surface TrkA receptors (Supplemental Figures S4B and S4C). Thus, calcineurin association with dynamin1 via the PxIxIT box is required for NGF-dependent internalization of TrkA receptors.
Given that calcineurin-dynamin1 interaction is required for TrkA internalization, we asked whether this association is required for NGF-mediated axonal growth. Sympathetic neurons were grown in compartmentalized cultures, and axon growth in response to NGF was assessed over 24 hr. To disrupt calcineurin-dynamin1 interactions exclusively in cell bodies or distal axons, the VIVIT peptide was added either to cell body or axonal compartments. VIVIT application reduced axon growth only when added to distal axons, indicating that association of calcineurin with PxIxIT-containing proteins in axons is required for NGF-dependent growth (Supplemental Figure S4D–G). VIVIT peptide did not disrupt NT-3-dependent axon growth (data not shown).
Although eight alternative spliced isoforms of dynamin1 are expressed in neurons (Cao et al., 1998), only two isoforms contain the PxIxIT motif. Dynamin1 contains two splicing regions; the usage of the first splicing region results in two isoforms of equal size but different nucleotide sequences (a and b forms). Additionally, there are at least four splicing variants of the C-terminal region resulting in four distinct tail regions: a, b, c, and d (Figure 6E). Thus, dynamin1 has at least eight spliced variants, aa, ba, ab, bb, ac, bc, ad and bd (Cao et al., 1998). Interestingly, only splice variants with the b tail region (ab and bb) contain the PxIxIT box motif (Figure 6E). To test whether dynamin1 splice variants bearing the b tail specifically interact with calcineurin, dynamin1aa (without PxIxIT) and dynamin1ab (with PxIxIT) were tagged with EGFP and expressed in HEK293 cells. Cell lysates were tested for interaction of the specific dynamin1 isoforms with calcineurinA-GST in pull-down assays. As predicted, dynamin1ab isoform that contains the PxIxIT box interacted with calcineurinA-GST. Neither the dynamin1aa isoform nor a dynamin1ab isoform with a mutated PxIxIT box (PRITIS->ARATAA) were able to bind calcineurinA-GST in pull-down assays (Figure 6F).
Phosphoregulation of PxIxIT-containing dynamin1 isoforms is required for TrkA endocytosis and axon growth
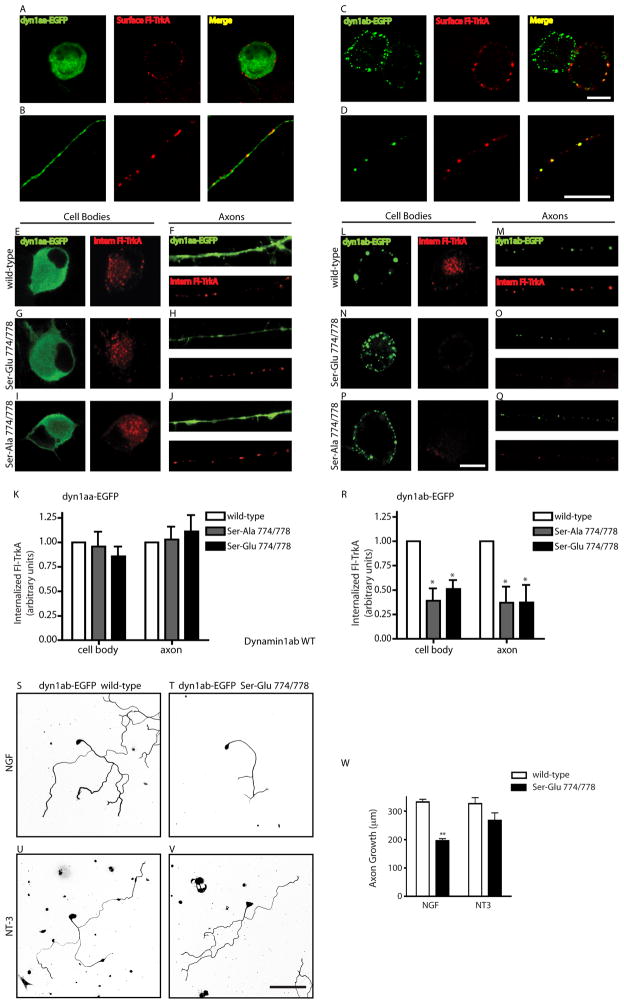
Different dynamin1 splicing isoforms display different subcellular localization in heterologous expression systems (Cao et al., 1998). To examine the subcellular localization of dynamin1aa and dynamin1ab isoforms, sympathetic neurons were electroporated with vectors expressing EGFP-tagged dynamin1aa or dynamin1ab. The two dynamin isoforms showed striking differences in subcellular localization in sympathetic neurons. Dynamin1aa-EGFP showed diffuse cytoplasmic localization throughout the cell bodies (Figure 7A) and axons (Figure 7B). In stark contrast, dynamin1ab-EGFP expression resulted in a punctate staining along the plasma membrane and throughout the cytoplasm in cell bodies (Figure 7C) and axons (Figure 7D). As the amino acid sequence of these two isoforms differ only in the C-terminal region containing the PxIxIT motif (Figure 6E), these results indicate that relatively small differences in primary sequence can result in striking changes in cellular localization. We next examined whether the punctate distribution of dynamin1ab-EGFP co-localizes with surface TrkA receptors. To visualize surface TrkA receptors, a live-cell antibody feeding assay was performed in sympathetic neurons (Ascano et al., 2009) expressing both N-terminal FLAG-tagged TrkA receptors and dynamin1-EGFP isoforms. Using an antibody directed against the extracellular FLAG epitope of the TrkA receptors, we found that the punctate distribution of dynamin1ab-EGFP fluorescence at the plasma membrane was in close apposition to surface TrkA immunoreactivity in neuronal soma (Figure 7C) and axons (Figure 7D), under non-permeabilizing conditions. In contrast, little co-localization between surface TrkA labeling and dynamin1aa-EGFP was observed (Figures 7A and 7B). Together, these results suggest that dynamin1ab isoforms might mediate TrkA endocytosis in sympathetic neurons.
Figure 7. Phosphoregulation of dynamin1ab is required for TrkA endocytosis and axon growth.
(A–D) Dynamin1aa isoform (A,B) shows diffuse cytoplasmic localization while dynamin1ab isoform (C,D) shows punctate localization in cell bodies and axons. FLAG immunostaining shows surface FLAG-TrkA receptors in cell bodies (A,C) and in axons (B,D). Scale bar, 10 μm. (E–J) Phosphoregulation of dynamin1aa is not required for NGF-dependent TrkA internalization. Neurons were transfected with FLAG-TrkA and either wild-type dynamin1aa-EGFP (E, F), Ser-Glu 774/778 dynamin1aa-EGFP (G,H), or Ser-Ala 774/778 dynamin1aa-EGFP (I,J). Cell bodies are shown in E, G, I. Axons are shown in F, H, J. Scale bar, 10μm. (K) Quantification of NGF-dependent TrkA internalization in cell bodies and axons. n=3. (L–Q) Phosphomutants of dynamin1ab disrupt NGF-dependent internalization of TrkA. Neurons were transfected with FLAG-TrkA and either wild-type dynamin1ab-EGFP (L,M), Ser-Glu 774/778 dynamin1ab-EGFP (N,O), or Ser-Ala 774/778 dynamin1ab-EGFP (P,Q). Cell bodies are shown in L, N and P. Axons are shown in M, O and Q. Scale bar, 10μm. (R) Quantification of internalized TrkA. *p<0.01, n=3. (S–V) Phosphoregulation of dynamin1ab is required for NGF-, but not NT-3-, dependent axon growth. NGF-mediated growth is blocked in sympathetic neurons expressing dynamin1ab-EGFP Ser-Glu 774/778 (T) as compared to wild-type dynamin1ab-EGFP (S). NT-3-mediated growth was unaffected (U,V). (W) Quantification of neurite length. **p<0.01, n=3. Scale bar: 50 μm.
To test whether phosphoregulation of dynamin1 is critical for NGF-dependent endocytosis of TrkA receptors, we generated phosphomutants of the dynamin1aa and dynamin1ab isoforms. As NGF stimulation results in dephosphorylation of dynamin1 on Ser 774 and 778, we generated dynamin1aa and dynamin1ab mutants bearing mutations of both serine residues to either alanine (Ser774/778-Ala; non-phosphorylatable forms) or glutamate (Ser774/778-Glu; phosphomimetic forms). Previous studies had shown that both the non-phosphorylatable and phosphomimetic forms of dynamin1 act as dominant negative inhibitors of activity-dependent synaptic vesicle endocytosis (Anggono et al., 2006; Clayton et al., 2009).
To label and follow endocytic trafficking of surface TrkA receptors, sympathetic neurons co-expressing FLAG-TrkA and the dynamin1 constructs were live-labeled with a calcium-sensitive FLAG antibody. After exposure to NGF for 30 min to allow internalization of labeled receptors, surface-bound antibodies were stripped, leaving antibodies bound only to the internalized pool of receptors. FLAG antibodies bound to internalized receptors were then visualized with Alexa-546 labeled secondary antibodies. We observed robust internalization of TrkA receptors in cell bodies and axons in response to NGF stimulation in cells expressing wild-type (Figures 7E and 7F), phosphomimic (Ser774/778 to Glu) (Figures 7G and 7H) or phosphomutant (Ser774/778 to Ala) (Figures 7I and 7J) dynamin1aa-EGFP. In contrast, expression of either dynamin1ab-EGFP phosphomimetic mutant (Ser774/778-Glu) (Figure 7N) or the non-phosphorylatable dynamin1ab-EGFP mutant (Ser774/778-Ala) (Figure 7P) significantly reduced NGF-mediated TrkA internalization in cell bodies to 39% and 50%, respectively, when compared to neurons expressing wild-type dynamin1ab-EGFP (Figures 7L and 7R). Expression of both phosphomutant forms of dynamin1ab-EGFP similarly reduced NGF-dependent internalization in axons (63% decrease) (Figures 7M, 7O, 7Q and 7R). Expression of mutant dynamin1ab-EGFP forms did not affect surface expression of FLAG-TrkA receptors in the absence of NGF treatment, nor influence the ability of FLAG antibodies to bind surface receptors (Supplemental Figures S5A–C), indicating that decreased intracellular accumulation of FLAG-TrkA in mutant dynamin1ab-expressing cells indeed reflects a block in endocytosis. The finding that both the non-phosphorylatable (Ser-Ala) and phosphomimetic (Ser-Glu) mutations inhibited receptor internalization suggests that similar to synaptic vesicle endocytosis (Anggono et al., 2006), the cycle between dynamin phosphorylation and dephosphorylation is critical for NGF-dependent endocytosis of TrkA receptors. Together these results indicate that NGF promotes internalization of its receptors through calcineurin-mediated dephosphorylation of specific spliced variants of dynamin1 harboring a PxIxIT interaction motif.
To determine whether phosphoregulation of dynamin1ab isoforms is also important for NGF-dependent axon growth, we infected sympathetic neurons with adenoviruses expressing either wild-type dynamin1ab-EGFP or the phosphomimetic dynamin1ab-EGFP mutant (Ser774/778-Glu), and measured axon growth in response to NGF over 36 hr. Expression of the mutant dynamin1ab-EGFP significantly reduced NGF-dependent neurite outgrowth, as compared to that in neurons expressing wild-type dynamin1ab-EGFP (Figures 7S and 7T). When quantified, the longest neurite in dynamin1ab-EGFP mutant (Ser774/778-Glu)-expressing cells was on average 136 μm shorter than axons of control cells (195.8 ± 7 μm in dynamin1ab-EGFP Ser774/778-Glu-expressing neurons versus 332.2 ± 9.2 μm in wild-type dynamin1ab-EGFP neurons) (Figure 7W). Consistent with our previous results, NT-3 mediated axon growth was not significantly affected by the dynamin1ab phosphomutant (Figures 7U, 7V and 7W). Together, these results suggest that phosphoregulation of PxIxIT box-containing dynamin1 isoforms is necessary to mediate NGF-dependent endocytosis of TrkA receptors and axonal growth.
Discussion
Endocytosis of NGF and its receptor, TrkA, in developing neurons provide one of the best examples of the significance of growth factor receptor trafficking in neurobiology. However, surprisingly little is known of the mechanisms by which NGF signaling modulates the core endocytic machinery to promote its own trafficking; and even less is known about the role of endocytosis in local NGF-promoted growth events in axon terminals. Here, we identify calcineurin-mediated dephosphorylation of dynamin1, as a mechanism by which target-derived NGF promotes internalization of its TrkA receptors and axon growth (Figure 8). We show that specific spliced variants of dynamin1 interact with calcineurin, and phosphoregulation of only these isoforms mediate neurotrophin receptor endocytosis and axon growth. Importantly, our results point to critical differences in the mechanisms by which two neurotrophins, NT-3 and NGF, act on a common TrkA receptor to promote proximal and distal stages of axonal growth during sympathetic nervous system development, with NGF-dependent growth showing a selective need for calcineurin-mediated endocytic events locally in nerve terminals.
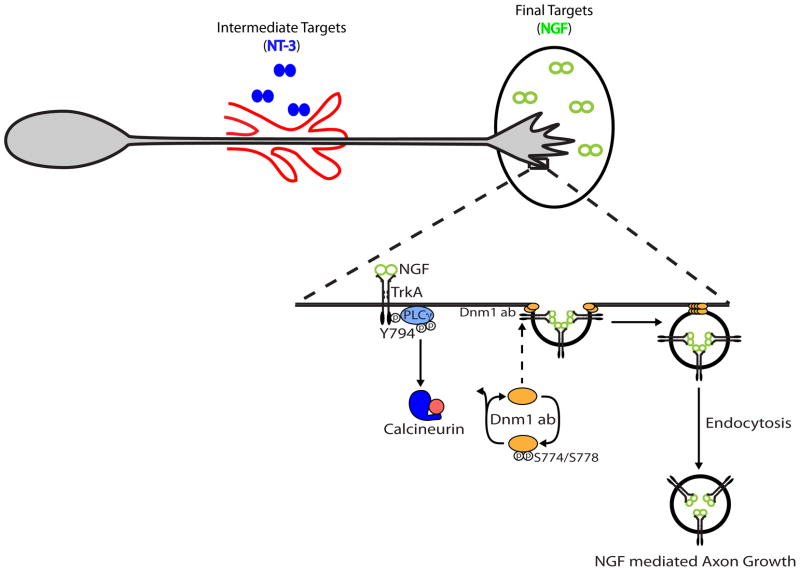
Figure 8. NGF and NT-3 differentially employ calcineurin signaling to promote axonal growth in sympathetic neurons.
NT-3 and NGF act upon a common TrkA receptor in sympathetic neurons to regulate distinct stages of axonal growth. NT-3 promotes proximal axon extension along the vasculature, whereas NGF is required for innervation of final peripheral targets. NGF-specific engagement of the PLC-γ effector pathway activates the calcium-responsive phosphatase, calcineurin. Calcineurin dephosphorylates PxIxIT motif-containing dynamin1 isoforms to promote TrkA endocytosis. Calcineurin-mediated endocytic trafficking of TrkA receptors signals locally in sympathetic nerve terminals to promote NGF-dependent axonal extension and branching within final target tissues.
Previously, transcriptional programs controlled by calcineurin have been demonstrated to be critical for axonal growth, dendritic structure and synapse formation, (Flavell et al., 2006; Graef et al., 2003; Schwartz et al., 2009). Our results uncover a new transcription-independent mechanism by which calcineurin mediates neuronal responses to extrinsic neurotrophic cues. We found that over 24 hr, axon growth in response to NGF acting locally at axon terminals in sympathetic and DRG sensory neurons was significantly attenuated by calcineurin inhibition, but not transcriptional blockade. Thus, we favor the hypothesis that calcineurin-mediated TrkA trafficking influences early growth events through local axonal mechanisms. Currently, it remains unclear as to why TrkA endocytosis might be selectively required for NGF-, but not NT-3-mediated axonal growth in sympathetic neurons. One possible explanation might be that since NGF uniquely promotes TrkA endocytosis in nerve terminals for carrying retrograde survival signals back to neuronal soma, this process has been co-opted for local control of NGF-mediated axonal growth, via mechanisms that remain to be identified. It is possible that TrkA localization to endocytic vesicles might enhance downstream signaling perhaps by prolonging association with downstream signaling effectors, spatially concentrating activated receptors, or by recycling receptors back to the membrane for repeated interaction with ligand.
Our findings that NGF does not induce NFAT activation within 24 hr in sympathetic and DRG sensory neurons do not preclude a requirement for calcineurin/NFAT-mediated transcriptional activity in supporting long-term axonal growth. Although we found that transcriptional activity is not required for NGF-mediated axonal growth over the first 24 hr, continued axonal growth after 24 hr requires new gene expression. This may reflect a specific role for NGF-mediated transcriptional responses, acting either via the calcineurin/NFAT, MAPK/SRF (Wickramasinghe et al., 2008) or CREB pathways (Lonze et al., 2002). Alternatively, this may reflect a general loss of proteins important for axonal growth during the extended treatments with transcriptional inhibitors. Together with the previously published study by Graef et. al (Graef et al., 2003), our findings might reflect a biphasic mechanism of action for calcineurin in neurotrophin-mediated axonal growth. Thus, calcineurin might act early via trafficking of TrkA receptors in axons and local activation of growth-promoting pathways, and at later stages via activation of NFAT-mediated transcription. NFATc2/c3/c4 triple null mice die early at embryonic day E11.5 (Graef et al., 2001), prior to the formation of sympathetic axons and innervation of target tissues. Further studies using mice with conditional deletion of NFAT isoforms will be needed to elucidate the contribution of NFAT-mediated transcription to the developing sympathetic nervous system. Nevertheless, our results indicate that NFAT transcription factors are not the sole targets of calcineurin relevant for neurotrophin-mediated axon growth. Our identification of a novel endocytic mechanism by which calcineurin signaling promotes neurotrophin-dependent axonal growth provides insight into the versatility of calcineurin signaling in nervous system development, and in particular, neurotrophin-mediated functions.
Our study suggests parallels between neurotrophin receptor endocytosis in developing neurons and local synaptic vesicle recycling in mature nerve terminals. During synaptic vesicle endocytosis, dynamin1 is among a group of structurally distinct proteins collectively called dephosphins that undergo a cycle of dephosphorylation and rephosphorylation in nerve terminals to mediate synaptic vesicle recycling and synaptic transmission (Cousin and Robinson, 2001). We show that calcineurin-dependent dephosphorylation of dynamin1 is a common mechanism underlying TrkA and synaptic vesicle endocytosis. Neurotrophins modulate synaptic transmission in mature neurons (Lu, 2004) and our results suggest that a potential target for neurotrophin actions at presynaptic terminals might be the regulation of calcineurin-dynamin1-dependent retrieval of synaptic vesicles after exocytosis.
Alternative splicing of the 3 dynamin genes generates over 25 different variants (Cao et al., 1998) that could greatly increase the diversity of dynamin functions in the mammalian nervous system. We provide evidence that specific dynamin1 splicing isoforms exhibit distinct sub-cellular localizations in neurons and perform discrete biological functions. In addition to synaptic vesicle retrieval, calcineurin-dynamin1 mediated endocytosis has been shown to be critical for regulation of AMPA receptor densities at postsynaptic spines during paradigms of synaptic plasticity such as long-term depression (LTD) (Beattie et al., 2000; Lin et al., 2000). Our findings raise the possibility that PxIxIT-containing dynamin1 isoforms might mediate all other calcineurin-regulated endocytosis in neurons.
The role of NGF-dependent regulation of calcineurin in endocytosis and axon outgrowth may have implications that extend beyond early neural development to the pathogenesis of some neurodegenerative disorders. Defective NGF trafficking in basal forebrain cholinergic neurons has been implicated in degeneration and atrophy of these neurons in Down’s Syndrome and Alzheimer’s Disease (Cooper et al., 2001; Salehi et al., 2006). Over-expression of Regulator of Calcineurin 1 (RCAN1) encoding for an endogenous calcineurin inhibitor has also been implicated in neuropathology of Down’s syndrome and Alzheimer’s Disease (Ermak et al., 2001; Fuentes et al., 2000). In future experiments, it will be intriguing to investigate the role of regulated calcineurin-dependent endocytosis in the trafficking of TrkA receptors and in maintaining the integrity of basal forebrain cholinergic neurons in normal and diseased states.
Experimental Procedures
Animals
To generate conditional mutants of CaNB1, floxed CaNB1 (CaNB1fl/fl) mice (Jackson Laboratory) were crossed to Nestin-Cre mice (Jackson Laboratory). NGF+/− mice (Crowley et al., 1994) were obtained from the Jackson Laboratory. All procedures relating to animal care and treatment conformed to institutional and NIH guidelines.
Immunostaining
Whole-mount tyrosine hydroxylase immunohistochemistry was performed on E16.5–E18.5 mouse embryos, as previously described (Kuruvilla et al., 2004). For NFAT immunostaining, sympathetic neurons were treated with 100 ng/ml NGF for 30 min, neurons were fixed and immunostained using pan-NFAT antibody, β-III-tubulin, and DAPI (4′,6-diamidino-2-phenylindole). Images representing 1 μm optical slices were acquired using a Zeiss LSM 510 confocal scanning microscope equipped with diode (405 nm), Ar (458–488 nm), and He/Ne (543–633) lasers.
Neuronal cultures
Sympathetic neurons were harvested from P0.5 Sprague-Dawley rats and grown in mass cultures or compartmentalized cultures as described previously (Kuruvilla et al., 2004). Dissociated DRG neurons were isolated from E15–16 rats and grown in mass cultures or compartmentalized cultures, using culture conditions similar to that described for sympathetic neurons. Plasmids, adenoviral vectors, pharmacological reagents and antibodies used in this study are described in detail in Supplemental Experimental Procedures.
Axon growth assays
Axon growth in compartmentalized cultures was quantified by capturing phase contrast images of the distal axon compartments over 8 hr or consecutive 24-hr intervals using a Zeiss Axiovert 200 microscope with a Retiga EXi camera. Rate of axonal growth (μm/day) was measured using Open lab 4.04. For all neurite growth assays in mass cultures, images were taken using an Axio Imager M1 (Zeiss) microscope, and length of the longest neurite was measured using Axiovision software (Zeiss). Measurements from 30–50 neurons were averaged for each condition for a single experiment. Details of analyses of neurotrophin-dependent neurite growth with dynamin1 phosphopeptides and short-term changes in growth cone morphologies are described in Supplemental Experimental Procedures.
Luciferase reporter assays
Sympathetic neurons were infected with NFAT-luciferase reporter adenovirus for 24 hr and then neurons were stimulated with control media, NGF or NT-3 (100 ng/ml) for 2, 8 and 24 hr and reporter gene activity was assessed with Luciferase Reporter Assay System (Promega, E1910). Similar analyses were used to report NFAT transcriptional activity in DRG neurons.
TrkA receptor internalization assays
Cell-surface biotinylation assays were performed in cultured sympathetic neurons as previously described (Kuruvilla et al., 2004). Live cell antibody feeding assays were performed as previously described (Ascano et al., 2009).
Immunoblotting, immunoprecipitation and pulldown assays
For analysis of tyrosine phosphorylation of PLC-γ, sympathetic neurons were treated with NGF or NT-3 (100ng/ml) for 30 min at 37°C. Cells were lysed with RIPA solution, and lysates were subjected to immunoprecipitation with anti-phosphotyrosine (PY-20; Sigma) and incubated with Protein-A agarose beads (Santa Cruz Biotechnology). Immunoprecipitates were then immunoblotted for PLC-γ. To detect phosphorylated dynamin1 in sympathetic axons in vitro, sympathetic neurons grown in compartmentalized cultures were stimulated with NGF applied to distal axons for 20 min or treated with control medium, axonal lysates prepared, and subjected to immunoblotting with the phospho-dynamin1 (Ser 778) antibody. To assess dynamin1 phosphorylation in sympathetic nerve terminals in vivo, salivary glands harvested from P0.5 wild-type and NGF+/− mice were subjected to immunoblotting with the phospho-dynamin1 (Ser 778) antibody. All immunoblots were visualized with ECL Plus Detection Reagent (GE Healthcare) and scanned with a Typhoon 9410 Variable Mode Imager (GE Healthcare). For pull-down assays, CalcineurinA-GST recombinant protein expression was induced with 100 μM IPTG for 4–6 hr at 25°C. Calcineurin-GST protein was immunoprecipitated from bacterial cell lysates with 500 μl of 50% glutathione-agarose. CalcineurinA-GST was resuspended in PBS+ phenylmethanesulphonylfluoride (PMSF, 1 mM)+sodium azide (10 μM). P0.5 rat brain (1g) was homogenized in calcium-containing lysis buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.2% Triton X-100, 0.5 mM B-mercaptoethanol, 5 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF, and 10 μM sodium azide) and centrifuged. Calcineurin-GST pulldowns of rat brain lysates were performed at 4°C for 1 hr. A similar protocol was used for Calcineurin-GST pull-downs from HEK 293 lysates.
Statistical Analyses
InStat software was used for statistical analyses. All Student’s t tests were performed assuming Gaussian distribution, two-tailed, unpaired, and a confidence interval of 95%. One-way or two-way ANOVA analyses were performed when more than two groups were compared.
Supplementary Material
Acknowledgments
We thank Antonella Riccio, Samer Hattar and Haiqing Zhao for insightful comments on this manuscript. We thank Mark McNiven for providing dynamin1 constructs, Moses Chao for the P-TrkA (Y794) antibody, and Lois Greene for the adenovirus-Cre. This work was supported by US National Institutes of Health (R01 MH080738) and Whitehall Foundation award to R.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anggono V, Smillie KJ, Graham ME, Valova VA, Cousin MA, Robinson PJ. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9:752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- Ascano M, Richmond A, Borden P, Kuruvilla R. Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci. 2009;29:11674–11685. doi: 10.1523/JNEUROSCI.1542-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Anggono V, Smillie KJ, Chau N, Robinson PJ, Cousin MA. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J Neurosci. 2009;29:7706–7717. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc Natl Acad Sci U S A. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts MS, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, Kitsis RN, Molkentin JD. Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- Ermak G, Morgan TE, Davies KJ. Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer’s disease. J Biol Chem. 2001;276:38787–38794. doi: 10.1074/jbc.M102829200. [DOI] [PubMed] [Google Scholar]
- Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemlou N, Krol KM, Macdonald DR, Kawaja MD. Comparison of target innervation by sympathetic axons in adult wild type and heterozygous mice for nerve growth factor or its receptor trkA. J Pineal Res. 2004;37:230–240. doi: 10.1111/j.1600-079X.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and Netrins Require Calcineurin/NFAT Signaling to Stimulate Outgrowth of Embryonic Axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25:160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Lai MM, Hong JJ, Ruggiero AM, Burnett PE, Slepnev VI, De Camilli P, Snyder SH. The calcineurin-dynamin 1 complex as a calcium sensor for synaptic vesicle endocytosis. J Biol Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- Loeb DM, Stephens RM, Copeland T, RKD, Greene LA. A Trk nerve growth factor (NGF) receptor point mutation affecting interactin with phospholipase C-gamma 1 abolishes NGF-promoted peripherin induction byt not neurite outgrowth. J Biol Chem. 1994;269:8901–8910. [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–150. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- Orike N, Thrasivoulou C, Wrigley A, Cowen T. Differential regulation of survival and growth in adult sympathetic neurons: an in vitro study of neurotrophin responsiveness. J Neurobiol. 2001;47:295–305. doi: 10.1002/neu.1036. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: ganglion cell maturation. J Neurosci. 1985;5:673–684. doi: 10.1523/JNEUROSCI.05-03-00673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Schwartz N, Schohl A, Ruthazer ES. Neural activity regulates synaptic properties and dendritic structure in vivo through calcineurin/NFAT signaling. Neuron. 2009;62:655–669. doi: 10.1016/j.neuron.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci U S A. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe SR, Alvania RS, Ramanan N, Wood JN, Mandai K, Ginty DD. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.