Abstract
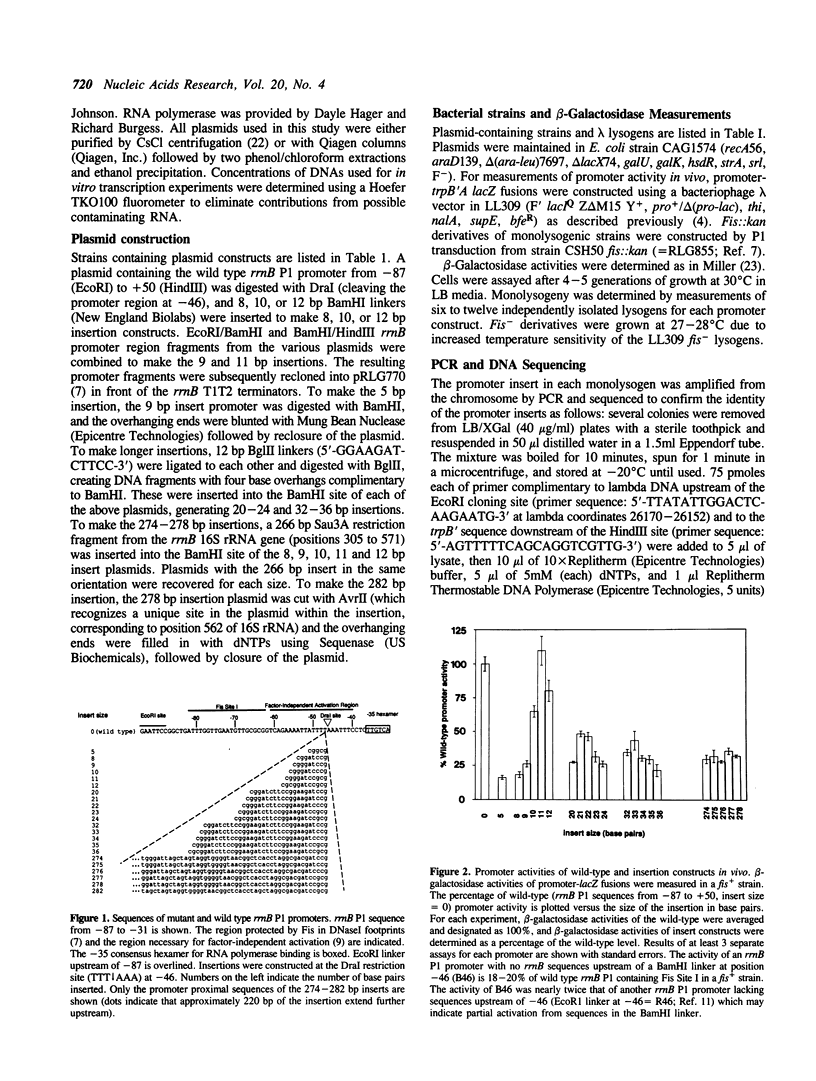
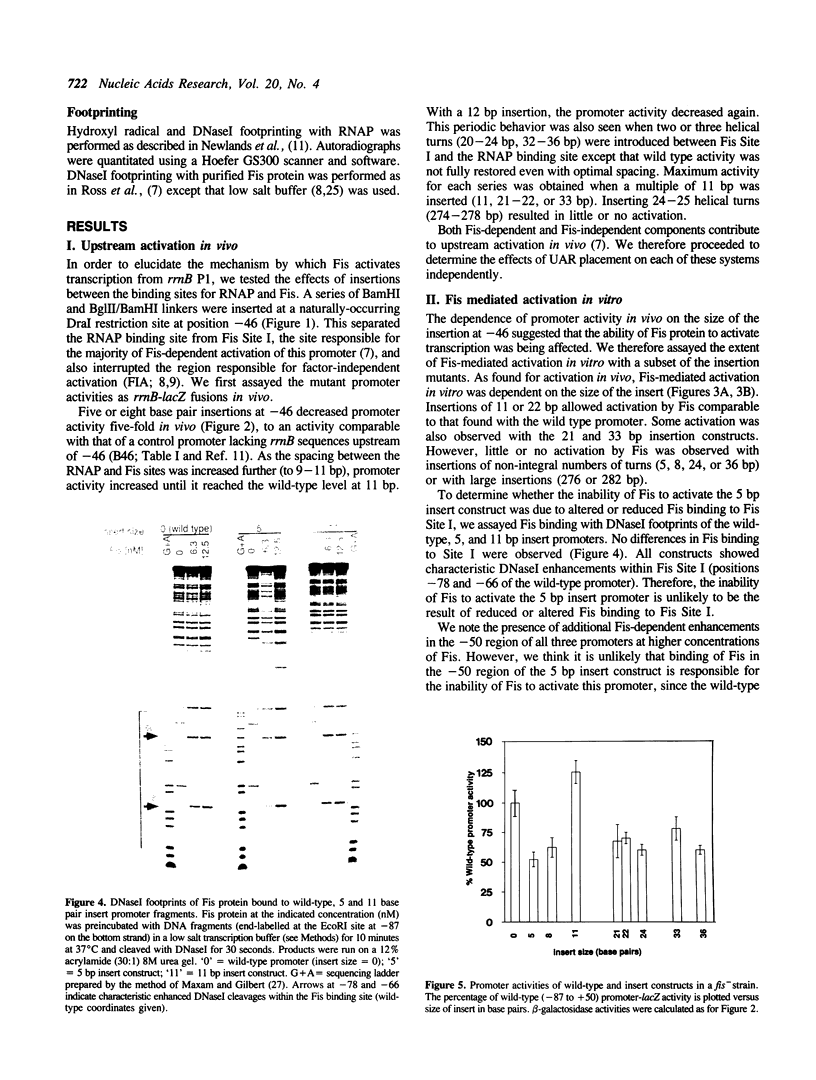
Transcription from the Escherichia coli rrnB P1 promoter is increased by a cis-acting sequence which extends upstream of the -35 hexamer to about -150 with respect to the transcription initiation site, the Upstream Activation Region (UAR). Activation by the UAR involves two components: (1) a trans-acting protein, Fis, which binds to three sites in the UAR between -60 and -150, and (2) the UAR sequences themselves which affect RNA polymerase (RNAP) activity independent of other proteins. We refer to the latter as Factor-Independent Activation (FIA). In addition to its interactions with the -10 and -35 hexamers typical of E. coli promoters, RNAP makes contacts to the -53 region of rrnB P1, which may be related to the FIA effect. We constructed a series of insertion mutants containing integral and non-integral numbers of helical turns at position -46, between the Fis binding sites and the -35 region, and the resulting promoter activities were measured in vitro and in vivo. The data suggest that both Fis-dependent and factor-independent activation are face of the helix dependent: the Fis binding site and the sequences responsible for factor-independent activation must be correctly oriented relative to RNA polymerase in order to activate transcription. These results, in conjunction with other evidence, support a model for the involvement of direct Fis-RNAP interactions in upstream activation. We also demonstrate that RNAP interacts with the -53 region of the rrnB P1 UAR even when these sequences are displaced upstream of the RNAP binding site, and that these interactions correlate with factor-independent activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell A., Gaston K., Williams R., Chapman K., Kolb A., Buc H., Minchin S., Williams J., Busby S. Mutations that alter the ability of the Escherichia coli cyclic AMP receptor protein to activate transcription. Nucleic Acids Res. 1990 Dec 25;18(24):7243–7250. doi: 10.1093/nar/18.24.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenlauer A. C., Reznikoff W. S. Escherichia coli catabolite gene activator protein mutants defective in positive control of lac operon transcription. J Bacteriol. 1991 Aug;173(16):5024–5029. doi: 10.1128/jb.173.16.5024-5029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Barkei J., Dickson R. R., deBoer H. A., deHaseth P. L., Alavi H., Gourse R. L. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J Bacteriol. 1989 Sep;171(9):4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K., Bell A., Kolb A., Buc H., Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990 Aug 24;62(4):733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Gourse R. L. Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988 Oct 25;16(20):9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Gralla J. D. Bacterial gene regulation from distant DNA sites. Cell. 1989 Apr 21;57(2):193–195. doi: 10.1016/0092-8674(89)90955-0. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. The effect of a lambda repressor mutation on the activation of transcription initiation from the lambda PRM promoter. Cell. 1983 Feb;32(2):327–333. doi: 10.1016/0092-8674(83)90452-x. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Irwin N., Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983 Feb;32(2):319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- Irwin N., Ptashne M. Mutants of the catabolite activator protein of Escherichia coli that are specifically deficient in the gene-activation function. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8315–8319. doi: 10.1073/pnas.84.23.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josaitis C. A., Gaal T., Ross W., Gourse R. L. Sequences upstream of the-35 hexamer of rrnB P1 affect promoter strength and upstream activation. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):307–311. doi: 10.1016/0167-4781(90)90186-6. [DOI] [PubMed] [Google Scholar]
- Lee D. H., Schleif R. F. In vivo DNA loops in araCBAD: size limits and helical repeat. Proc Natl Acad Sci U S A. 1989 Jan;86(2):476–480. doi: 10.1073/pnas.86.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirmo S., Gourse R. L. Factor-independent activation of Escherichia coli rRNA transcription. I. Kinetic analysis of the roles of the upstream activator region and supercoiling on transcription of the rrnB P1 promoter in vitro. J Mol Biol. 1991 Aug 5;220(3):555–568. doi: 10.1016/0022-2836(91)90100-k. [DOI] [PubMed] [Google Scholar]
- Li X. M., Krakow J. S. Monoclonal antibodies that inhibit activation of transcription by the Escherichia coli cyclic AMP receptor protein. J Biol Chem. 1988 Mar 5;263(7):3448–3453. [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Initiation of Escherichia coli ribosomal RNA synthesis in vivo. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5480–5484. doi: 10.1073/pnas.76.11.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Ozawa Y., Mizuno T., Mizushima S. Stereospecific positioning of the cis-acting sequence with respect to the canonical promoter is required for activation of the ompC gene by a positive regulator, OmpR, in Escherichia coli. J Mol Biol. 1988 Aug 5;202(3):433–441. doi: 10.1016/0022-2836(88)90276-8. [DOI] [PubMed] [Google Scholar]
- Mandecki W., Caruthers M. H. Mutants of the lac promoter with large insertions and deletions between the CAP binding site and the -35 region. Gene. 1984 Nov;31(1-3):263–267. doi: 10.1016/0378-1119(84)90219-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minchin S. D., Austin S., Dixon R. A. Transcriptional activation of the Klebsiella pneumoniae nifLA promoter by NTRC is face-of-the-helix dependent and the activator stabilizes the interaction of sigma 54-RNA polymerase with the promoter. EMBO J. 1989 Nov;8(11):3491–3499. doi: 10.1002/j.1460-2075.1989.tb08514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M., Oka A. The structure of a transcriptional unit on colicin E1 plasmid. Eur J Biochem. 1979 Jul;97(2):435–443. doi: 10.1111/j.1432-1033.1979.tb13131.x. [DOI] [PubMed] [Google Scholar]
- Newlands J. T., Ross W., Gosink K. K., Gourse R. L. Factor-independent activation of Escherichia coli rRNA transcription. II. characterization of complexes of rrnB P1 promoters containing or lacking the upstream activator region with Escherichia coli RNA polymerase. J Mol Biol. 1991 Aug 5;220(3):569–583. doi: 10.1016/0022-2836(91)90101-b. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Vanet A., Vijgenboom E., Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkney M., Hoggett J. G. Binding of the cyclic AMP receptor protein of Escherichia coli to RNA polymerase. Biochem J. 1988 Mar 15;250(3):897–902. doi: 10.1042/bj2500897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Ren Y. L., Garges S., Adhya S., Krakow J. S. Cooperative DNA binding of heterologous proteins: evidence for contact between the cyclic AMP receptor protein and RNA polymerase. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4138–4142. doi: 10.1073/pnas.85.12.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Thompson J. F., Newlands J. T., Gourse R. L. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990 Nov;9(11):3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Barthelemy I., Salas M. Transcription activation at a distance by phage phi 29 protein p4. Effect of bent and non-bent intervening DNA sequences. J Mol Biol. 1991 Jun 5;219(3):403–414. doi: 10.1016/0022-2836(91)90182-6. [DOI] [PubMed] [Google Scholar]
- Su W., Porter S., Kustu S., Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushida C., Aiba H. Helical phase dependent action of CRP: effect of the distance between the CRP site and the -35 region on promoter activity. Nucleic Acids Res. 1990 Nov 11;18(21):6325–6330. doi: 10.1093/nar/18.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek H., Nilsson L., Bosch L. FIS-induced bending of a region upstream of the promoter activates transcription of the E coli thrU(tufB) operon. Biochimie. 1991 Jun;73(6):713–718. doi: 10.1016/0300-9084(91)90051-2. [DOI] [PubMed] [Google Scholar]
- Wedel A., Weiss D. S., Popham D., Dröge P., Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990 Apr 27;248(4954):486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- de Boer H., Nomura M. In vivo transcription of rRNA operons in Escherichia coli initiates with purine nucleoside triphosphates at the first promoter and with CTP at the second promoter. J Biol Chem. 1979 Jul 10;254(13):5609–5612. [PubMed] [Google Scholar]