Abstract
Polybrominated diphenyl ethers (PBDEs) are flame retardants applied as coatings to many consumer products, including household items. PBDEs are released and produce airborne vapors and dusts. Inhalation of particle-phase and/or gas-phase PBDEs is therefore a major route of exposure. In an attempt to mimic realistic airborne exposures, actual uptake and deposition of particles and vapors, we prepared and characterized particles for future animal exposure studies. To trace the particles in environmental and biological systems, we employed fluoro-tagging. We synthesized, characterized and employed three PBDE congeners 35, 47 and 99, and five fluoro-substituted-PBDEs (F-PBDEs), 17-F5′, 25-F5′, 28-F3′, 35-F5′, 47-F3, 99-F3′ for this study. The PBDE congeners were selected because they are commonly found in house dust. For that reason we coated spherical silica particles of 3 μm and C18 endcapped silica as representative and inert support materials, with 20%, 30% and 40% PBDEs. We determined the particle size distributions by aerodynamic particle size spectrometry and the morphology by scanning electron microscopy. The suitability of the fluoro-tagged tracers to mimic their corresponding parent PBDEs was investigated by extraction studies from spiked blood serum. Our study is of fundamental importance to the development of xenobiotic tracers for monitoring routes of human exposure to PBDEs and understanding uptake of PBDEs from particles and vapors.
Introduction
Polybrominated diphenyl ethers (PBDEs) are incorporated into plastics, foams, and textiles to provide consumer products with flame retardant properties (1). They may be released as fine particles and/or partition into the air because of their semi-volatile nature (2). The concentrations of many persistent organic pollutants, such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo dioxins (PCDDs) and pesticides are decreasing, whereas those of PBDEs are on the rise (3–6). PBDEs are distributed globally and detected in most environmental media including air (7,8), water (9), soil (10), sediment (11,12), and biota (3, 9, 13). They are also found in human tissue, breast milk, and blood (14–18), and have been associated with endocrine disruption (19), and reproductive (20) developmental (21), and neuro-toxicity (22). Recent in vitro studies have shown that hydroxylated metabolites of PBDEs bind with high affinity to thyroid hormone transport proteins, i.e., transthyretin, (23,24) and to thyroid hormone receptors (24,25). Only the fully brominated technical DecaPBDE has been tested for its carcinogenic potential, and these tests have shown some evidence of carcinogenicity in animals (26).
Despite their potential to promote adverse health effects, little is known about the relative importance of different routes by which humans are exposed to PBDEs. The main route of exposure is sometimes ingestion, such as through consumption of fish that have accumulated PBDEs in their fatty tissue (27,28). Alternatively, the predominate route of exposure may be inhalation or dermal for particle-phase and/or gas-phase PBDEs in indoor (29) or occupational settings (e.g., computer/electronics repair, maintenance or dismantling) (30,31). Appropriate toxicology studies are complex to design, and control strategies that minimize exposures are difficult to craft, without a better understanding of the routes by which PBDEs enter the body.
Two key issues hamper our ability to evaluate routes of human exposure to PDBEs. First, toxicity tests are often conducted with a toxicant carried in a vehicle, such as dimethylsulfoxide (DMSO), dimethylformamide (DMF) or perfluoro surfactants like perfluorooctanoic acid (PFOA). Solvents can alter the biological uptake of toxins or PBDEs by functioning as membrane carriers (32,33) and have very little environmental relevance. However, PBDEs are applied often as coatings to a parent backbone material that may range from an inorganic material to an organic polymer (34). Thus, the size distribution, morphology, surface area, surface chemistry, particle reactivity, particle composition and backbone material may influence the behavior of PBDEs and their biological uptake (35). Consistent with the message of the recent Toxicological Highlight (36) entitled How meaningful are the results of nanotoxicology studies in the absence of adequate material characterization, we characterized PBDEs prior their application for in vivo and in vitro toxicological studies.
Second, there are major deficiencies in the methodologies that are used to track particles in exposure studies to follow routes of PBDE uptake and distribution. This is a major challenge with the analytical tools available. Employing tracers is a strategy to address this. In tracer labeling, the compound of interest is tagged facilitating its detection in exposure studies. A basic requirement is that the tagged analogue must resemble the compound of interest in terms of its chemical and physical properties, but not be a normal constituent of the sample. Tags often include unusual isotopes, such as deuterated, 13C and radio-labeled analogues. Although 13C and radioactive labeled analogues are ideal tracers in some cases, they provide poor or no structural information. Mass-selective detection (MS) and/or radioactivity determinations are the only analytical approach to distinguish between isotopic and native compounds. These determinations are often uninformative. In addition, radio-labeled compounds are challenging to handle, their use is therefore restricted, and radioactive contamination of instrumentation is problematic.
Monofluorinated PBDEs (F-PBDEs) can act as tracers in environmental, toxicological and pulmonary exposure studies aimed to monitor absorption, distribution, excretion, bioaccumulation and magnification of PBDEs. F-PBDEs have a number of advantages in analytical identification and quantification (37,38). Specific techniques can be applied to detect F-PBDEs, e.g. MS and atomic emission detection (AED) for trace analysis. The utilization of F-PBDEs by means of MS, electron capture detection (ECD) or AED allows a simultaneous detection without separation by gas chromatography (GC) (37,38). Application of 19F nuclear magnetic resonance (NMR) spectroscopy allows characterizing chemical structures by chemical shift and spin-coupling. In addition, F-PBDEs show very similar physico-chemical behaviors compared to their corresponding parent PBDEs and do not occur in the environment, which makes them ideal internal standards and tracers.
It is our hypothesis that defined dusts composed of PBDEs or dusts coated with PBDEs will best mimic environmentally-relevant exposures. Moreover, we believe that F-tagging of these dusts will provide a useful tool for determining the relative importance of various potential exposure routes. Consequently, we synthesized and employed pairs of PBDEs/F-PBDEs, produced and characterized fine powders of these compounds as well as coatings of PBDEs/F-PBDEs pairs on polar and non-polar C18 endcapped silica particles. F-PBDE/PBDE pairs were chosen on the basis of origin and toxicity. The nomenclature of F-PBDEs used herein follows the Ballschmitter-Zell-Luthe (BZL) numbering system (37,38). 2,2′,4,4′-tetrabromodiphenyl ether (PBDE 47) and 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE 99) are the most detected congeners found in house dust (39). 3,3′,4-tribromodiphenyl ether (PBDE 35) is one of the main tribromoPBDEs detected in breast milk and in human blood serum (14–18). The F-PBDEs, 5′-fluoro-3,3′,4-tribromodiphenyl ether (F-PBDE 35-F5′), 3-fluoro-2,2′,4,4′-tetrabromodiphenyl ether (F-PBDE 47-F3), and 3′-fluoro-2,2′,4,4′,5-pentabromodiphenyl ether (F-PBDE 99-F3′) were utilized as tracers. In addition 5′-fluoro-2,2′,4-tribromodiphenyl ether (F-PBDE 17-F5′), 5′-fluoro-2,3′,4-tribromodiphenyl ether (F-PBDE 25-F5′), and 3′-fluoro-2,4,4′-tribromodiphenyl ether (F-PBDE 28-F3′) were used as surrogate standards. We characterized the particle size distributions using an aerodynamic particle sizer (APS) and the morphology by scanning electron microscopy (SEM).
To evaluate the concept of F-tagging, we investigated the discrimination of F-PBDE relative recoveries versus their corresponding parent PBDEs in blood serum spiked with F-PBDE/PBDE solution and coated silica gels. The employed work-up procedure is based on a procedure for blood analysis of polychlorinated biphenyls (40). The study presented here is of fundamental importance to develop particles of xenobiotic tracers to monitor and understand routes of human exposure to PBDEs.
Experimental Procedures
Materials
2,2′,4,4′-Tetrabromodiphenyl iodonium chloride (1a) (yield, 0.91g, (86 %)), and 3,3′,4,4′-tetrabromodipenyl iodonium chloride (1b) (yield was 1.01g, (95 %)) were synthesized according to published methods (37,38,40).
2,2′,4,4′,5,5′-heaxabromodipenyl iodonium chloride (1c) was synthesized with modifications of the procedure. Fuming sulfuric acid (20%, SO3 free, 1 mL) and fuming nitric acid (100%, 0.15 mL) were added to iodine (0.161 g, 0.64 mmol), into a round bottom flask at room temperature. The reaction mixture was stirred at 70–80 °C for 2 h. The obtained iodyl sulfate was diluted in sulfuric acid (5mL). Under vigorous stirring and cooling to 5°C 1.0 g 1,2,4-tribromobenzene (3.2 mmol) was slowly added to the iodyl sulfate (IO)2SO4. The reaction mixture was stirred at room temperature overnight. After this, the blend was cooled to 0 °C and ice (3.5 g) was added carefully in small portions [CAUTION: exothermic reaction!]. After standing for 15 h, the aqueous solution was filtered under aspirator pressure and the crude product was washed with water and ether (5mL). The crude product was dissolved in methanol (50 mL) and crystallized as the chloride salt by addition of concentrated hydrochloric (0.2 mL) acid dissolved in 1mL methanol. In addition, the solvent volume was reduced to 10 mL. The mixture was maintained for 2 h to precipitate 2,2′,4,4′,5,5′-hexadiphenyliodonium chloride. The product was filtered under aspirator pressure and washed with ether (3×2 mL). Yield was 0.49g 0.62 mmol (49 %).
2,4-dibromo-3-fluorophenol, (2c). 2.55 g (9.55 mmol) aluminum-(III)-bromide was diluted in 3.97 g (14.8 mmol) of bromine under nitrogen atmosphere and stirring. 1.50 g (9.55 mmol) of 5-fluoro-2-nitrophenol were added portion wise (CAUTION: HBr was produced). After all compounds were added, the mixture was kept overnight. The remaining bromine and hydrogen bromide were removed by a gentle stream of nitrogen. Ice was added under stirring. The obtained precipitation was filtered under reduced pressure. The yield was (crude) 3.0g, 9.55 mmol (99%). The obtained crude 6-nitro-3-fluoro-2,4-dibromophenol (2a) was dissolved in 100 mL of ethanol:water (1:1, v:v). 16.6 g (160 mmol) sodium hydrosulfite were added and the reaction mixture was stirred at room temperature for 2 h. Ethanol was evaporated under reduced pressure and the precipitate was filtered under aspirated pressure. The yield was (crude), 1.9 g, 6.69 mmol (70%). 6-Amino-3-fluoro-2,4-dibromophenol (2b) was dissolved in 15 mL dioxane and 5 mL hydrochloric acid (20%) and cooled to 0°C. A solution of sodium nitrite (1.32 g, 20.1 mmol) in 5 ml water was added drop wise over 30 min under stirring and maintained for 30 min at 0°C. 20 mL H2PO3 were added and the reaction mixture was warmed slowly to 45°C while stirring over night. The product (2c) was extracted with methylene chloride, dried over magnesium sulfate, and the solvent evaporated under reduced pressure. The crude product was purified by flash column chromatography with silica gel as the stationary phase and hexanes: ethyl acetate (9:1) as the mobile phase (yield 0.65g 2.41 mol (35%), overall yield 0.65g 2.41 mmol (25.2%)).
General procedure for nucleophilic substitution of the iodyl salts by the phenols, on the example F-PBDE 17-F5′, 5′-fluoro-2,2′,4-tribromodiphenyl ether (3a). 2,2′,4,4′-tetrabromodiphenyl iodonium chloride (0.5 g, 0.79 mmol) and 2-bromo-5-fluorophenol (0.30g, 1.58 mmol) were diluted in 15 mL dimethyl formamide (DMF). Sodium hydroxide (0.03 g, 0.8 mmol) was added, and the mixture was stirred and refluxed overnight. After the reaction was complete, the mixture was filtered employing flash column with silica gel and washed with n-hexane to remove remaining DMF and inorganic impurities. Purification by flash column chromatography with silica gel and n-hexane resulted in 0.29 g, 0.68 mmol (86 %), GC-MS purity 99%).
F-PBDE 25-F5′, 5′-fluoro-2,3′,4-tribromodiphenyl ether (3b) (yield, 0.215g, 0.51 mmol (64 %), purity 99%.), F-PBDE 28-F3′, 3′-fluoro-2,4,4′-tribromodiphenyl ether (3c) (yield, 0.320g 0.75 mmol (95%), purity 99%), F-PBDE 47-F3, 3-fluoro-2,2′,4,4′-tetrabromodiphenyl ether (3g) (yield, 0.24g, 0.48 mmol (39 %), purity 99%), PBDE 99, 2,2′,4,4′,5-pentabromodiphenyl ether (3h) (yield, 0.21g, 0.37 mmol (94 %), purity 99%), F-PBDE 99-F5, 5-fluoro-2,2′,4,4′,5-pentabromodiphenyl ether (3i) (yield, 0.28g, 0.48 mmol (50 %), purity 99%) were performed as described above.
PBDE 35, 3,3′,4-tribromodiphenyl ether (3d) was synthesized under modified conditions. 3,3′,4,4′-Tetrabromodiphenyl iodonium chloride (1.58 g, 2.5 mmol) and 3-bromophenol (0.43 g, 2.5 mmol) were diluted in 20 mL water:dioxane (1:1, v:v). Sodium hydroxide (0.1 g, 2.5 mmol) was added, and the mixture was stirred and refluxed overnight. After the reaction was completed, it was extracted with diethyl ether (3 × 15 mL). The unified organic layers were dried over magnesium sulfate, and the solvent was evaporated under reduced pressure. The crude product was purified by flash column chromatography with silica gel and n-hexane (yield, 0.36g, 0.88 mmol (35 %), purity 99%).
F-PBDE 35-F5′, 5′-fluoro-3,3′,4-tribromodiphenyl ether (3e) (yield, 0.215g, 0.51 mmol (65 %), purity 99%) and PBDE 47, 2,2′,4,4′-tetrabromodiphenyl ether (3f) (yield, 0.640g, 1.31 mmol (83 %), purity 99%) were synthesis was performed as described above in (3d).
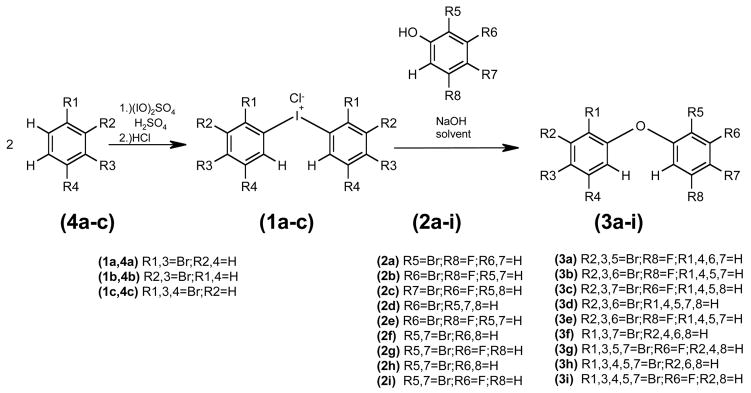
For synthesis see Figure 1, for 1H, 13C and MS characterization see Tables S1-5 (supporting materials).
Figure 1.
Synthesis of the diphenyliodonium salts (1a–c) from bromobenzenes (4a–c) and the synthesis of F-PBDEs and PBDEs (3a–i) by nucleophilic aromatic substitution of the diphenyliodonium salts (1a–c) with bromophenols (2a–i).
Silica gel (60 Å C:C 40–63 μm), silica gel (Nucleosil, 100 Å, 3 μm), and endcapped silica gel C18 (Nucleosil 100 Å, 3 μm) from Fisher Scientific (Pittsburgh, PA, USA).
Methods
Preparation and characterization of dust composed exclusively of PBDE/F-PBDE and coated spherical silica gel particles with PBDE/F-PBDE, preparation of dust composed exclusively of PBDE/F-PBDE
Dusts composed exclusively of PBDEs were prepared by dissolving approximately 50 mg of the PBDE/F-PDBE in 0.5 mL diethyl ether. A small volume (25 μL) of this solution was pipette with a glass capillary onto a glass slide measuring 2.55 cm × 7.65 cm. The ether was evaporated, and the thin layers of PBDEs or F-PBDEs were scraped off with another glass slide to give the intended particles. Dusts of the individual congeners PBDEs 35, 47 and 99, and of the F-PBDEs 35-F5′, 47-F3, and 99-F3′ and a mixture were prepared according to this procedure.
Preparation of dust composed of spherical silica gel particles coated with F-PBDE/PBDE
PBDEs are broadly used to coat materials ranging from inorganic materials to organic polymers. To mimic organic and inorganic backbones while comparing the materials, we choose silica gel as a monodispersed and properly sized support material. Endcapped silica gel particles by C18 chains were applied to mimic a general organic backbone. The material properties of both backbones are identical in particle size range, mesh, porosity and chemical composition. Since the organic matter is chemically bound to the particle, the organic component of the backbone should not function as a carrier for transport across the membrane. This is an additional benefit of this chosen approach. The size of the spherical silica gel particles was on average 3 μm in diameter. This is in the range of household dust particles that can contain PBDEs.
A stock solution was prepared with a total F-PBDE/PBDE concentration of 2 mg/mL, containing equal molar amounts of each congener PBDE 35, 47, 99 and F-PBDE 35-F5′, 47-F3 and 99-F3′. A backbone of silica gel (Nucleosil, average diameter 3 μm, pore size 100 Å) was used as support material, with C18 endcapped and original surface. Four different coating procedures for both support materials were applied to optimize the coating process: (A) support material (40 mg), stock solution (5 mL) and diethyl ether (5 mL) as solvent were mixed in a round bottom flask and the solvent directly evaporated under reduced pressure in a rotary evaporator with tumbling at 30 rpm, without outside warming; (B) processed as above with outside warming to 36 C; (C) incubation with diethylether and F-PBDE/PBDE mixture for 12 h prior evaporation at 36 C; and (D) incubation with only diethylether for 12 h and subsequent addition of the F-PBDE/PBDE mixture and evaporation at 36 C. In all variations of the coating process, the silica gel particles were re-suspended 5 times with 1.5 mL diethyl ether and the solvent evaporated according to the descriptions of the individual procedures above. Following procedure (B) particles of C18 endcapped and original silica gel with 20%, 30%, and 40% F-PBDE/PBDE coating were prepared (40mg (20%), 35 mg (30%) or 30 mg (40%) of silica gel and 5.0 mg (20%), 7.5 mg (30%), or 10.0 mg (40%) of F-PBDE/PBDE mixture).
Characterization of dusts by aerodynamic particle sizer (APS) and scanning electron microscopy (SEM)
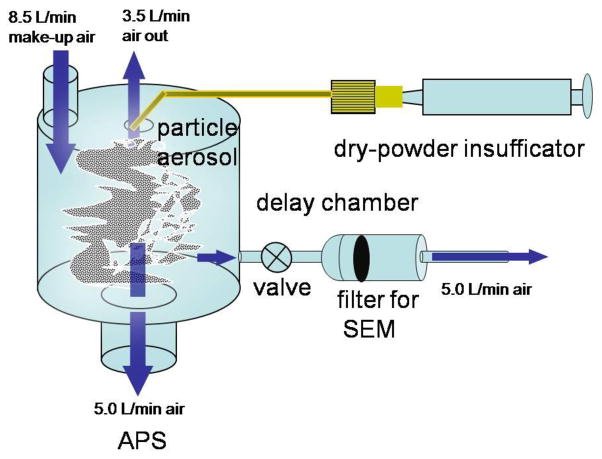
As shown in Figure 2, a dry powder insufflator (model DP-4, Penn-Century, Inc., Philadelphia, PA, USA) was used to aerosolize 0.5 mg of each compound into a cylindrical delay chamber (8 cm diameter x 12 cm height). This type of insufflator has been used to instill particles into rodents during exposure studies. Particle free air at 8.5 L/min was fed into the chamber to provide makeup air. The size distribution of the aerosolized dusts in the chamber was measured in triplicate with a aerodynamic particle sizer (APS, Model 3321, TSI Incorporated, Shoreview, MN, USA). The APS measures particle number concentration by aerodynamic diameter in 32 channel per decade from 0.5 to 20 μm aerodynamic diameter. Prior to conducting these measurements, an aerosol composed of 3 μm polystyrene latex spheres (Polysciences, Inc., Warrinton, PA) was measured to be within +/− 10% of their stated size to verify proper calibration of the APS.
Figure 2.
Set-up of the F-PBDE/PBDE particle measurements by aerodynamic particle sizer (APS) and collections on a filter by aerosolization via a dry powder insuffication into a delay chamber. The blue arrows indicate the air flows in the system.
To observe their morphology by scanning electron microscopy (SEM), a sample of the aerosolized particles in the chamber was collected onto a 0.4-μm polycarbonate filter (E0425-MB, SPI Supplies and Structure Probe, Inc., West Chester, PA) at a flow rate of 5 L/min for 30 sec. after injection. A portion of the filter was mounted on an SEM stub with double sided, graphite tape, and the edge of the filter was painted with silver paint to promote conductivity. The surface of the sample was coated with a gold layer under an inert argon atmosphere by material beam for 2.5 min at 10 Torr and 200 A. Images of the sample were taken with an SEM (Hitachi S-4800, Boston, MA) operated at an accelerating voltage of 1 kV.
Proof of concept of the F-tagging tool for particle tracking
Sprangue-Dawley rats were anesthetized and exsanguinated under an Institutional Animal Care and Use Committee (IACUC)-approved protocol. Pooled rat blood serum was spiked (66.7 mg F-PBDE/PBDE/g blood serum) with (i) F-PBDE/PBDE coated silica gel particles directly and inverted for 120 min prior to extraction, (ii) F-PBDE/PBDE coated C18-endcapped silica gel particles and inverted for 120 min prior to extraction, (iii) F-PBDE/PBDE coated silica gel particles pre-treated with 1.0 mL HCl (6 mol/l) and 6.0 mL n-hexane and inverted for 120 min prior to extraction, and (iv) F-PBDE/PBDE coated silica gel particles pre-treated with 1.0 mL HCl (6 mol/l) and 6.0 mL n-hexane and inverted for 120 min prior to extraction. We normalized these values with previously dissolved stock F-PBDE/PBDEs in n-hexane. Recovery was above 99%. Each sample was separated equally into 3 centrifuge tubes (12 mL, tube 1) into which HClaq (1.0 mL, 6 M), 2-propanol (3.5 mL), and n-hexane:MTBE (3.5 mL, v:v, 1:1) were added. After each addition the samples were mixed by a vortex mixer. The samples were inverted (5 min, 60 rpm) and centrifuged (1.380 G, 5 min). The organic layer was transferred (tube 2) and KClaq (4 mL, 1%) added to it. The aqueous layer was extracted (tube 1) with n-hexane:MTBE (3.0 mL, v;v, 1:1), inverted (5 min) centrifuged (5 min, 1.380 G), the organic layer transferred and unified (tube 2). The aqueous solution was extracted likewise and the organic layers unified (tube 2). The solvent was removed with a gentle stream of nitrogen on a water bath, till 0.5 mL remained. n-Hexane (6 mL) and H2SO4 (2 mL, conc.) were added (tube 2), inverted (2 min, 60 rpm), centrifuged (5 min, 1380 G) and the organic layer transferred (tube 3). The aqueous layer (tube 2) was extracted with n-hexane (3 mL), inverting (2 min, 60 rpm), centrifuged (5min, 1.380 G) and the organic layers again unified (tube 3). The solvent was evaporated as described above and the remaining concentrate transferred to a column (15 cm, 0.1g of acidified silica gel (silica gel:H2SO4 2:1) in the bottom, 1g of silica gel on the top) and eluted with n-hexane (10 mL). The solvent was evaporated and the concentrate transferred into a GC-vial (1.5 mL). Analytical separation and quantification was carried out by GC-MS as described below. Comparisons of mean recoveries across experimental conditions were carried out with mixed linear regression models. Standard errors were calculated. Tests for mean differences were two-sided and carried out with the SAS statistical software (Cary, NC).
Results
Synthesis of the PBDEs, F-PBDEs (3a–i)
The nucleophilic substitution of the diaryl iodonium chlorides by phenols was used for the synthesis of all PBDE and F-PBDE congeners The nucleophilic substitution of the diaryl iodonium chlorides by phenols resulted in satisfactory yields for PBDEs 35 (3d), 47 (3f), and F-PBDEs 35-F5′ (3e). For the synthesis of all other model compounds this procedure was modified by using dimethyl formamide (DMF) as the solvent and changing the molar ratios of the starting materials to iodonium salt:phenol molar ratios of 1:2. This resulted in yields of F-PBDE 17-F5′ (3a), 25-F5′ (3b), 28-F3′ (3c), 47-F3 (3g), 99 F-3′ (3i) and 99 (3h). Synthesis of diaryl iodonium salts (1a–c): The preparation of the diaryl iodonium salts was based on the procedures reported by Luthe et al. (37) and Marsh et al. (41). The synthesis was conducted at lower temperature to prevent decomposition and was reported for the first time. We observed that the purities of the obtained products were higher, easing the work-up procedure and the yields of the purified compounds.
Preparation and characterization of PBDE/F-PBDE dusts, PBDE/F-PBDE dusts
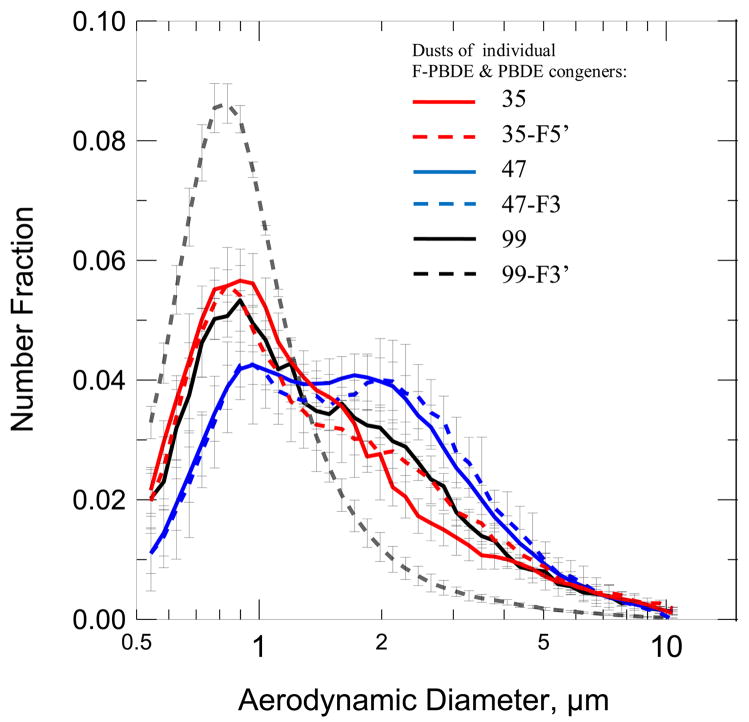
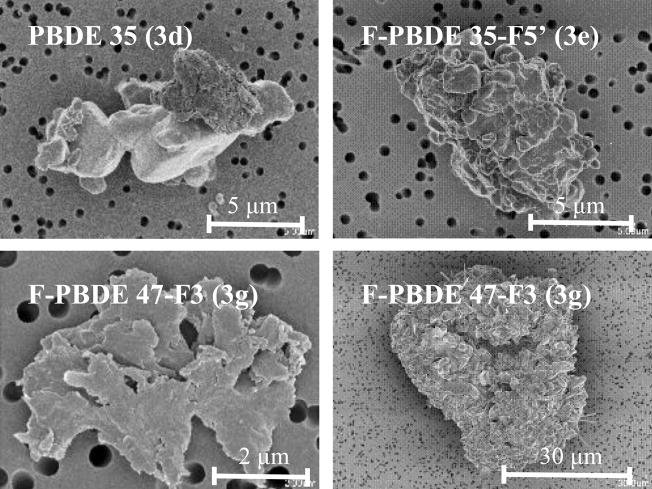
Figure 3 shows the particle size distributions of the individual pure PBDEs and F-PBDEs dusts. The number median diameter (NMD) and geometric standard deviation (GSD, provided in parentheses) for the different powders were: PBDE 35, 1.2 μm (1.89); F-PBDE 35-F5′ 1.2 μm (1.97); PBDE 47, 1.6 μm (1.86); F-PBDE 47-F3, 1.6 μm (1.85); PBDE 99, 1.3 μm (1.90); F-PBDE 99F3′, 0.91 μm (1.57); and mixture, 1.2 μm (1.97). SEM images shows the broad size distributions and also revealed a wide variety in the morphology of the particles (Figure 4). Morphologies included flat particles, crystals, aggregates, and amorphous particles.
Figure 3.
Particle size distribution [μm] of pure dusts composed of congeners F-PBDEs 35-F5′(3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h).
Figure 4.
SEM of individual dust particles of F-PBDEs 35-F5′ (3e), 47-F3 (3g) and PBDE 35 (3d) exhibiting the wide range of morphology.
Dusts of F-PBDE/PBDE coated silica gel particles
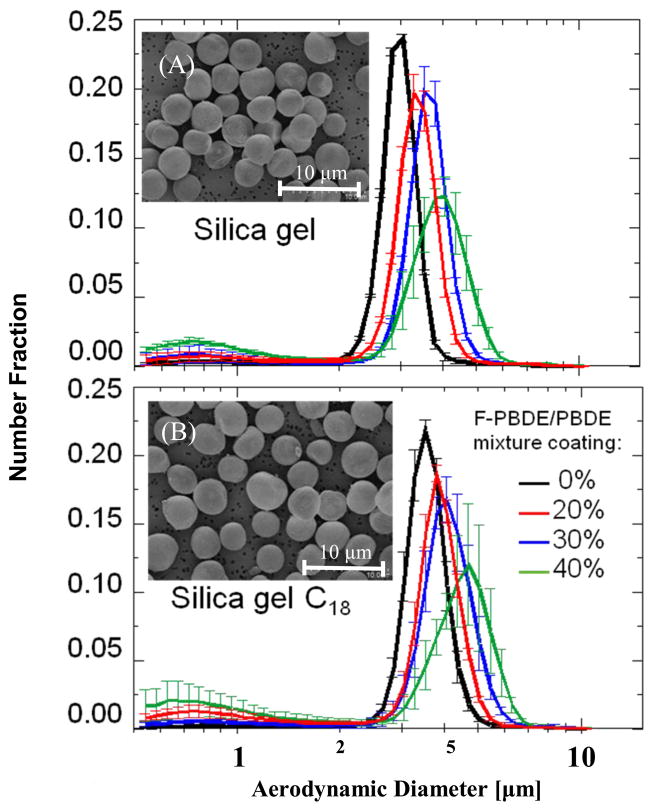
Using procedure (B) we coated both the supporting materials with 20%, 30% and 40% total amount of mixtures of PBDEs/F- PBDEs pairs 35, 47 and 99. Figure 5 shows the particle size distributions of the common and C18 endcapped silica gel particles with the equimolar PBDE/F-PBDE mixture and typical images, observed by SEMs. The NMD and (GSD) were: 0% coating, 3.0 μm; 20%, 3.30μm; 30%, 3.55μm and 40%, 3.68μm for silica gel and 0%, 3.51μm; 20%, 3.72μm, 30%, 3.99μm and 40%, 4.01μm for the C18 chain endcapped silica gel particles. Particle size distributions do not only show an increased median with a higher percentage of coating, but also an increased GSD, especially for 40% coating. Under these optimized conditions, coatings with percentages above 40% resulted in artifacts of crystals from PBDEs.
Figure 5.
Number fraction of dusts composed of silica gel particles coated with 0%, 20%, 30% and 40% PBDE/F-PBDE pairs 35, 47 and 99: (A) non surface modified silica gel, and (B) endcapped silica gel (100 A pore size, 3 μm average particle size). The inset electron micrograph provides a representative image of the coated dust.
Proof of concept of the F-tagging tool for particle tracing
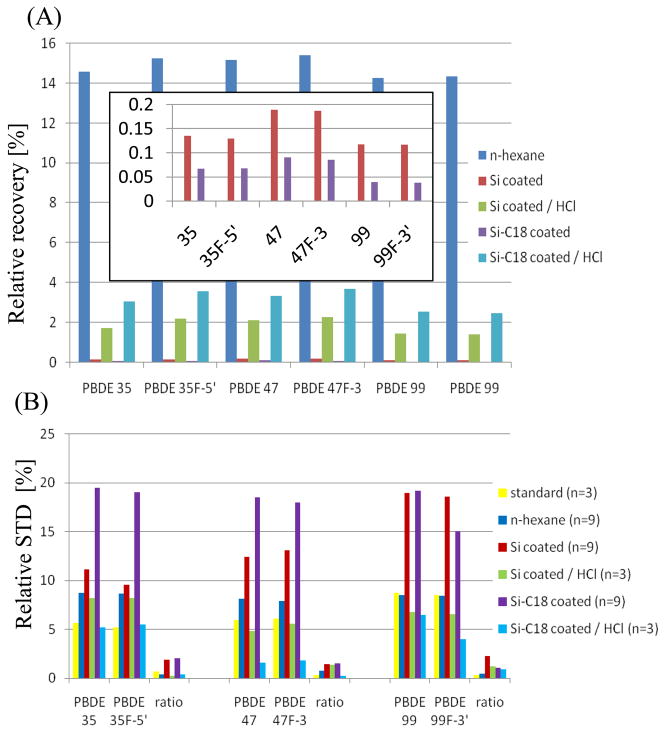
Figure 6 exhibits the results of relative recoveries (5A) and relative standard deviations (STDs) (5B) of PBDE/F-PBDE pairs. The recovery in blood serum was above 99% for the diluted PBDEs/F-PBDEs. Highest recoveries in our experiment were found for the suspension in n-hexane for all PBDE/F/PBDE congeners with values around 15 %. Second highest recoveries were found for the particle solutions in n-hexane and HCl (1.4 – 2.2% for silica coated gel and 2.5 3.7% for coated endcapped silica gel). Lowest recoveries were obtained by spiking with the coated particles directly (0.12–0.19% for silica coated gel and 0.04–0.07% for coated endcapped silica gel). While major differences in the relative recoveries occurred between the exposure procedures, the fluoro- tagged analogues closely resembled the corresponding parent PBDEs. While the recovery from the C18 endcapped backbone was lower compared with the silica gel, the finding becomes different after HCl treatment. The C18 chains functioned as a bonded liquid phase. The PBDEs/F-PBDEs were less stronger bound to the lipophilic C18 backbone. Figure 6B demonstrates the applicability of F-tagged analogues to serve as recovery standards for blood serum quantifications. The relative STD decreases for the investigated congeners from 5.2 8.7 % down to 0.3–0.7 % for injection triplets of the standard solutions. Additional investigations of the repeatability of the blood spiking extractions demonstrated that the STD decreases from 7.9–8.7% to 0.4–0.8% for a solution in n-hexane, 11.1–19.0% to 1.5–2.3 for coated silica particles and 8.2-6.8% to 0.3–1.3% for HCl pre-treated silica particles, 18.5–19.5% to 2.1-1.1% for coated C18 endcapped silica particles and 6.4–5.2% to 0.9-0.3% for HCl pre-treated particles. Irrespective of the high, medium or low recoveries experimentally determined, the PBDE/F-PBDE recovery ratios are consistent within 0.3–2.3% STD.
Figure 6.
Relative recoveries (A) with insertation of the magnification and standard deviations (STDs) (B) of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) from pooled blood serum (Sprague Dawley rat) carried out by liquid-liquid extraction. Spiking was carried out with coated particles and a solution in n-hexane, and pre-treatment with HCl.
Discussion
Synthesis of the PBDEs, F-PBDEs (3a–i)
The advantages of nucleophilic substitution of the diaryl iodonium chlorides by phenols are the regio-selectivity and the suppression of side reactions, resulting in high congener purity. Alternative pathways are the direct bromination of the diphenyl ether (37) and the Ullmann-reaction (37,38,40). Both alternative pathways give product mixtures, which are difficult to separate. For the synthesis of all other model compounds this procedure was modified by using dimethyl formamide (DMF) as the solvent and changing the molar ratios of the starting materials to iodonium salt:phenol molar ratios of 1:2. DMF exhibits a higher solubility for the diaryl iodonium salts and diminishes their decomposition. In addition, the excess of phenol suppresses the phenol substitution, originating from the decomposition of the iodonium salt. The synthesis and characterization of F-PBDE 47-F3 (3g), 99-F3′ (3i), 17-F5′ (3a), 25-F5′ (3b) and 35-F3′ (3e) are presented here for the first time.
Synthesis of diaryl iodonium salts (1a–c)
An excess of solvent was used to increase the solubility of the starting material at the lower reaction temperature, resulting in higher purities. Synthesis of 2,4-dibromo-3-fluorophenol (2g): The synthesis of 2,4-dibromo-3-fluorophenol (2g) is reported for the first time. The preparation via di-bromination of 5-fluoro-2-nitrophenol (4d), followed by the reduction of the nitrogroup to the amino group was chosen due to its high regio-selectivity. A bromination of 3-fluorophenol would lead to different dibrominated isomers. A key condition was the excess of bromine. In the purification process the di- and tri-brominated products were easier to separate from each other than the mono- and di-brominated congeners. The diazonium salt was decomposed.
Preparation and characterization of PBDE/F-PBDE dusts, PBDE/F-PBDE dusts
The NMDs were similar in that they ranged between 1 μm and 2 μm. However, the APS has reduced counting efficiency for particles smaller than 0.8 μm. Consequently, the actual NMD is likely to be smaller than that reported here because there may have been uncounted particles that were smaller than 0.8 μm. The fact that the size distributions were quite broad indicates that the powders were composed of a range of particles. This finding is consistent with the mechanical process that was used to prepare these powders. SEM images confirmed the broad size distributions and wide variety in the morphology of the particles. The observed size distributions of these pure powders may have implications for toxicity studies. Particle size and morphology will likely influence the route of exposure, uptake, solubility, and toxicity of the PBDEs. Thus, one would be unable to attribute toxic effects of a discrete size and particle morphology that elicits an adverse effect in a toxicity study. Additionally, the fact that these pure dusts are not associated with a backbone material probably renders them unlike a common environmental exposure in an indoor or occupational setting.
Dusts of F-PBDE/PBDE coated silica gel particles
Due to the fact that PBDEs are used as coating materials, we choose to coat defined spherical particles with F-PBDEs and PBDEs to gain the study materials for our ongoing biological studies. Four different coating procedures were applied to optimize the coating process under mechanical mixture with a rotavapour set-up. Procedure (B) gave satisfactory results for our study. Under these optimized conditions, coatings with percentages above 40% resulted in artifacts of crystals from PBDEs. The comparison of the two backbones showed that the adhesion of PBDEs and F-PBDEs was stronger with common silica gel. The endcapped material resulted in particles with somewhat greasy properties. This is due to the weak interactions of the C18 chains with the PBDEs and F-PBDEs leading to inhibition of a crystallization of the coating material on the surface. Comparing the particles of F-PBDEs/PBDEs with the coated spherical silica gel, a tremendous increase in the quality of the particles was observed. The particle size distribution was reduced to less than 2 μm and followed a symmetrical log normal distribution. The morphology was comparable for all particles, equally spherical and crystalline on the surface of the silica gel and amorphous on the C18 endcapped silica gel. These uniformly coated F-PBDE/PBDE particles are ideal test probes to investigate pulmonary exposure to particle bound PBDEs. In addition, this particle material allows us to validate the F-tagging concepts for monitoring, tracing and probing particular matter.
Proof of concept of the F-tagging tool for particle tracing
It is our hypothesis that F-tagging is a tool to trace particles. We introduce F-PBDEs as model compounds to prove the hypothesis. F-PBDEs could function as tracers in environmental, toxicological and pulmonary exposure studies aimed at monitoring absorption, distribution, excretion, bioaccumulation and magnification of PBDEs. From previous studies utilizing F-PBDEs (37,38) or F-tagged polychlorinated biphenyls (42,43) and polyaromatic hydrocarbons (44), we know that monofluoro-substitued aromatic compounds resemble closely their corresponding parent compounds from a chemical and physical point of view. However, this is the first time that F-tagging is employed to trace particles instead of single compounds. To investigate the effect of the solubility of solid particles versus homogeneous solutions, we spiked pooled rat blood serum with (i,ii) F-PBDE/PBDE coated silica gel and C18-endcapped silica gel particles and inverted prior to extraction, (iii, iv) F-PBDE/PBDE coated silica gel and C18-endcapped silica gel particles pre-treated with HCl and n-hexane and inverted prior to extraction. We normalized these values with prior dissolved stock F-PBDE/PBDEs in n-hexane. The employed work-up procedure is based on a procedure for blood analysis of polychlorinated biphenyls (40). One should note that standard operation conditions were used with no further attempt to optimize, because it was the mutual similarity of PBDE/F-PBDE pair data generally required for monitoring and mimicking purposes not the completeness of the extraction method was of interest. In this case, a low recovery, yields more interesting data. Analytical separation and quantification was carried out by GC-MS.
While major differences in the relative recoveries occurred between the exposure procedures, the fluoro-tagged analogues closely resembled the corresponding parent PBDEs. In general, the recovery in blood was low due to the high lipophilicity of the serum. However, recoveries were higher with solutions in n-hexane compared with particles, showing that the PBDEs/F-PBDEs adhered well to the backbones. While the recovery from the C18 endcapped backbone was lower compared with the silica gel, the finding becomes different after HCl treatment. The C18 chains functioned as a bonded liquid phase. The PBDEs/F-PBDEs were less stronger bound to the lipophilic C18 backbone. With HCl treatment the partitioning between the n-hexane and C18-phase changed. From chromatographic procedures it is known that the C18 chains fold together while exposed to low pH levels. This results in a decreased solubility for the PBDEs/F-PBDEs and release into the n-hexane solution. Figure 6B demonstrates the applicability of F-tagged analogues to serve as recovery standards for blood serum quantifications. Preliminary in vivo studies seem to confirm these findings. F-PBDE/PBDE pairs were detected in similar concentrations in blood serum taken from rats after pulmonary instillation of F-PBDE/PBDE-coated particles. The general outcome of the comparison is that F-PBDEs appear to be well suited for use as tracers in fate studies of PBDE particle exposure in biological systems. How meaningful toxicological studies are depends on the correct characterization of the xenobiotic and its correct administration (36). This is especially the case for dusts. Particle size, distribution, morphology, composition, surface area and chemistry and particle reactivity in solution are all important factors. PBDEs are released as fine particles and/or partition into the air. It is fundamental for toxicological and environmental studies investigating routes of exposure and the toxicity of PBDEs to characterize accurately the particulate matter.
Supplementary Material
Table S1: 1H NMR chemical shifts δ [ppm] and couplings nJ (1H1H, 1H19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S2: 19F NMR chemical shifts δ [ppm] and couplings nJ (1H1H, 1H19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S3: 13C NMR chemical shifts δ [ppm] and couplings nJ (13C19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S4: m/z values and relative abundance [%] of ions of 2,2′,4,4′-tertrabromo-diphenyl iodonium salt (1a), 3,3′,4,4′-tertrabromo-diphenyl iodonium salt (1b) and 2,2′,4,4′,5,5′-hexabromo-diphenyl iodonium salt (1c) measured by HPLC-MS.
Table S5: m/z values and relative abundance [%] of ions of PBDEs and F-PBDEs (3a–i) measured by GC-MS.
Acknowledgments
We would like to thank Jane Ross from the Central Microscopy Facility for her technical support and interest in our study. This publication was made possible by the Alexander von Humboldt Foundation, Bonn, Germany, by grant number P42 ES013661 from the National Institute of Environmental Health Sciences (NIEHS), by the University of Iowa Environmental Health Sciences Research Center, P30 ES05605, and United States Environmental Protection Agency grant RD-2902102. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Humboldt Foundation, NIEHS, or the US EPA.
Abbreviations
- APS
aerodynamic particle sizer
- BZL
Ballschmitter-Zell-Luthe
- 13C NMR
Carbon Nuclear Magnetic Resonance Spectroscopy
- F-PBDE 17-F5′
5′-fluoro-2,2′,4-tribromodiphenyl ether
- F-PBDE 25-F5′
5′-fluoro-2,3′,4-tribromodiphenyl ether
- F-PBDE 28-F3′
3′-fluoro-2,4,4′-tribromodiphenyl ether
- F-PBDE 35-F5′
5′-fluoro-3,3′,4-tribromodiphenyl ether
- F-PBDE 47-F3
3-fluoro-2,2′,4,4′-tetrabromodiphenyl ether
- F-PBDE 99-F3′
3′-fluoro-2,2′,4,4′,5-pentabromodiphenyl ether
- F-PBDE
monofluoro-substituted Polybrominated diphenyl ethers
- F-tagged
fluoro-tagged or substituted
- 1H NMR
Proton Nuclear Magnetic Resonance Spectroscopy
- PBDE 35
3,3′,4-tribromodiphenyl ether
- PBDE 47
2,2′,4,4′-tetrabromodiphenyl ether
- PBDE 99
2,2′,4,4′,5-pentabromodiphenyl ether
- PBDEs
Polybrominated diphenyl ethers
- PCBs
polychlorinated biphenyls
- PCDDs
polychlorinated dibenzo dioxins
- SEM
scanning electron microscopy
References
- 1.Butt CM, Diamond ML, Truong J, Ikonomou MG, Ter Schure AFH. Spatial distribution of polybrominated diphenyl ethers in southern Ontario as measured in indoor and outdoor window organic films. Environ Sci Technol. 2004;38:724–731. doi: 10.1021/es034670r. [DOI] [PubMed] [Google Scholar]
- 2.Sjödin A, Carlsson H, Thursesson K, Sjolin S, Bergman A, Ostman C. Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol. 2001;35:448–454. doi: 10.1021/es000077n. [DOI] [PubMed] [Google Scholar]
- 3.Ikonomou MG, Rayne S, Addison RF. Exponential Increases of the Brominated Flame Retardants, Polybrominated Diphenyl Ethers, in the Canadian Arctic from 1981–2000. Environ Sci Technol. 2002;36:1886–1892. doi: 10.1021/es011401x. [DOI] [PubMed] [Google Scholar]
- 4.Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DVC. Geographical distribution (2000) and temporal trends (1981–2000) of brominated diphenyl ethers in Great Lakes herring gull eggs. Environ Sci Technol. 2002;36:4783–4789. doi: 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- 5.Ryan J, Patry B, Mills P, Beaudoin NG. Recent trends in levels of brominated diphenyl ethers in human milks from Canada. Organohalogen Compd. 2002;58:173–176. [Google Scholar]
- 6.Norén K, Meironyté D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20 30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- 7.Strandberg B, Dodder NG, Basu I, Hites RA. Concentrations and spatial variations of polybrominated diphenyl ethers and other organohalogen compounds in Great Lakes air. Environ Sci Technol. 2001;35:1078–1083. doi: 10.1021/es001819f. [DOI] [PubMed] [Google Scholar]
- 8.Harner T, Ikonomou M, Shoeib M, Stern G, Diamond M. Passive Air Sampling results for PBDEs along an Urban-Rural Transect. Organohalogene Compounds. 2002;57:33–36. [Google Scholar]
- 9.Ikonomou MG, Rayne S, Fischer M, Fernandez MP, Cretney W. Occurrence and Congener Profiles of Polybrominated Diphenyl Ethers (PBDEs) in Environmental Samples from Western Canada. Chemosphere. 2002;46:649–663. doi: 10.1016/s0045-6535(01)00229-6. [DOI] [PubMed] [Google Scholar]
- 10.Environmental Health Criteria 162: Brominated dipheynyl ethers. World Health Organization (1994)
- 11.Sellström U, Kierkegaard A, de Wit C, Jansson B. Polybrominated diphenyl ethers (PBDE) and hexabromocyclododecane (HBCD) in sediment and fish from a Swedish river. Environ Toxicol Chem. 1998;17:1065–1072. [Google Scholar]
- 12.Allchin CR, Law RJ, Morris S. Polybrominated diphenylethers in sediments and biota downstream of potential sources in the UK. Environ Pollut. 1999;105:197–207. [Google Scholar]
- 13.Ter Schure AFH, Larsson P, Merila J, Jönsson KI. Latitudinal Fractionation of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Frogs (Rana temporaria) Environ Sci Technol. 2002;36:5057–5061. doi: 10.1021/es0258632. [DOI] [PubMed] [Google Scholar]
- 14.Raab U, Schwegler U, Preiss U, Albrecht M, Fromme H. Bavarian breast milk survey Pilot study and future developments. Int J Hygiene Environ Health. 2007;210:341–344. doi: 10.1016/j.ijheh.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ Res. 2003;93(2):186–194. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 16.Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish breast milk. A time-related trend study, 1972 1997. J Toxicol Environ Health A. 1999;58(6):329–341. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- 17.Hites R. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 18.Kalantzi OI, Martin FL, Thomas GO, Alcock RE, Tang HR, Drury SC, Carmichael PL, Nicholson JK, Jones KC. Different levels of polybrominated diphenyl ethers ( PBDEs ) and chlorinated compounds in breast milk from two U.K. Regions. Environ Health Perspect. 2004;112(10):1085–1091. doi: 10.1289/ehp.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilienthal H, Hack A, Roth-Haerer A, Grande SW, Talsness CE. Effects of developmental exposure to 2,2′,4,4′,5-pentabromodiphenyl ether ( PBDE-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114(2):194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muirhead EK, Skillman AD, Hook SE, Schultz IR. Oral Exposure of PBDE - 47 in Fish: Toxicokinetics and Reproductive Effects in Japanese Medaka (Oryzias latipes) and Fathead Minnows (Pimephales promelas) Environ Sci Technol. 2006;40(2):523–528. doi: 10.1021/es0513178. [DOI] [PubMed] [Google Scholar]
- 21.Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220(2–3):104–116. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154(1–2):11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman AA, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:340–399. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luthe G, Jacobus J, Robertson LW. Receptor interactions by polybrominated diphenyl ethers versus polychlorinated biphenyls: a theoretical Structure-activity assessment. Environ Toxicol Pharmacol. 2008;25:202–210. doi: 10.1016/j.etap.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh G, Bergman A, Bladh LG, Gillner M, Jakobsson E. Synthesis of p-hydroxybromodiphenyl ethers and binding to the thyroid. Organohalogene Compounds. 1998;37:305–308. [Google Scholar]
- 26.National Toxicology Program (NTP), (1986) Toxicology and carcinogenesis studies of decabromodiphenyl oxide (CAS No. 1163-19-5) in F344/N rats and B6C3F1 mice (feeding studies). US Department of Health and Human Services, NTP Technical Report 309, NIH Publication No. 86–2565.
- 27.Lind Y, Aune A, Atuma S, Becker W. Food intake of the brominated flame retardants PBDEs and HBCD in Sweden. Organohalogene Compounds. 2002;58:181–184. [Google Scholar]
- 28.Hagmar L, Bergman Å. Human exposure to BFPs in Europe. Proceedings of the second international workshop of brominated flame retardants, 14–16 May, 2001. Stockholm, Sweden: The Swedish Chemical Society; 2001. p. 107. [Google Scholar]
- 29.Gearhart J, Posselt H. Toxic at any Speed, Ecology Center. 2006 www.ecocenter.org.
- 30.Qu W, Bi X, Sheng G, Lu S, Fu J, Yuan J, Li L. Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ Int. 2007;33(8):1029–1034. doi: 10.1016/j.envint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Thuresson K, Bergman K, Rothenbacher K, Herrmann T, Sjölin S, Hagmar L, Päpke O, Jakobsson K. Polybrominated diphenyl ether exposure to electronics recycling workers--a follow up study. Chemosphere. 2006;64(11):1855–1861. doi: 10.1016/j.chemosphere.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Lyman GH, Priesler HD, Papahadjopoulos P. Membrane action of DMSO and other chemical inducers of Friend leukemic cell differentiation. Nature. 1976;262:360–363. doi: 10.1038/262360a0. [DOI] [PubMed] [Google Scholar]
- 33.Maggio B, Ahkong QF, Lucy JA. PEG surface potential and cell fusion. Biochem J. 1976;158:647–650. doi: 10.1042/bj1580647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 35.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warheit DB. How Meaningful are the Results of Nanotoxicity Studies in the Absence of Adequate Material Characterization? Toxicol Sci. 2008;101(2):183–185. doi: 10.1093/toxsci/kfm279. [DOI] [PubMed] [Google Scholar]
- 37.Luthe G, Leonards PG, Reijerink G, Liu H, Johansen J, Roberson RW. Monofluorinated Analogues of Polybrominated Diphenyl Ethers as Analytical Standards: Synthesis, NMR, and GC-MS Characterization and Molecular Orbital studies, Environ. Sci Technol. 2006;40:3023–3029. doi: 10.1021/es052410z. [DOI] [PubMed] [Google Scholar]
- 38.Klösener J, Swenson DC, Robertson LW, Luthe G. Electrostatic and aspheric influence of the fluoro-substitution of 4-bromodiphenyl ether (PBDE 3) Acta Cryst B. 2007;64:108–119. doi: 10.1107/S0108768107067079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia MJ, McClean MD, Webster TF. Human Exposure to PBDEs: Associations of PBDE Body Burdens with Food Consumption and House Dust Concentrations. Environ Sci Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- 40.Bergman Å, Norstrom K, Haraguchi H, Béland P. PCB and DDE methyl sulfones in mammals from Canada and Sweden. Environ Toxicol Chem. 1994;13:121–128. [Google Scholar]
- 41.Marsh G, Stenutz R, Bergman A. Synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers - Natural products and potential polybrominated diphenyl ether metabolites. Eur J Org Chem. 2003;14:2566–2574. [Google Scholar]
- 42.Luthe G, Schut BJ, Aaseng JE. Monofluorinated analogues of polychlorinated biphenyls (F-PCBs): Synthesis using the Suzuki-coupling, characterization, specific properties and intended use. Chemosphere. 2006;27:153–167. doi: 10.1016/j.chemosphere.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Luthe G, Swenson DC, Robertson LW. Influence of fluoro-substitution on the planarity of 4-chlorobiphenyl (PCB 3) Acta Cryst B. 2007;63:319–327. doi: 10.1107/S0108768106054255. [DOI] [PubMed] [Google Scholar]
- 44.Luthe G, Ariese F, Brinkman UATh. Monofluorinated Polycyclic Aromatic Hydrocarbons: Standards in environmental Chemistry and Biochemical Applications. In: Neilson AH, editor. Handbook of Environmental Chemistry: Organic Fluorine Compounds. Springer Verlag; Berlin, Germany: 2002. pp. 249–275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: 1H NMR chemical shifts δ [ppm] and couplings nJ (1H1H, 1H19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S2: 19F NMR chemical shifts δ [ppm] and couplings nJ (1H1H, 1H19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S3: 13C NMR chemical shifts δ [ppm] and couplings nJ (13C19F) [Hz] of F-PBDEs 35-F5′ (3d), 47-F3 (3g), 99-F3′ (3i), PBDEs 35 (3d), 47 (3f) and 99 (3h) in CDCl3.
Table S4: m/z values and relative abundance [%] of ions of 2,2′,4,4′-tertrabromo-diphenyl iodonium salt (1a), 3,3′,4,4′-tertrabromo-diphenyl iodonium salt (1b) and 2,2′,4,4′,5,5′-hexabromo-diphenyl iodonium salt (1c) measured by HPLC-MS.
Table S5: m/z values and relative abundance [%] of ions of PBDEs and F-PBDEs (3a–i) measured by GC-MS.