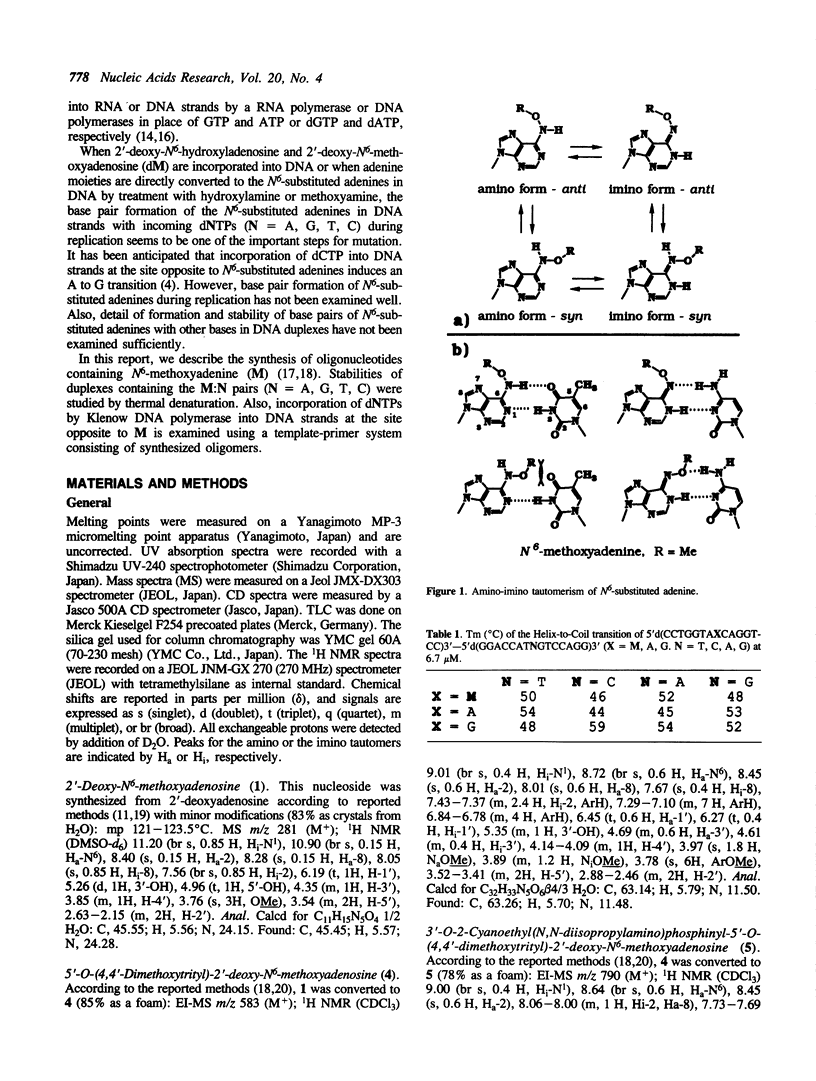
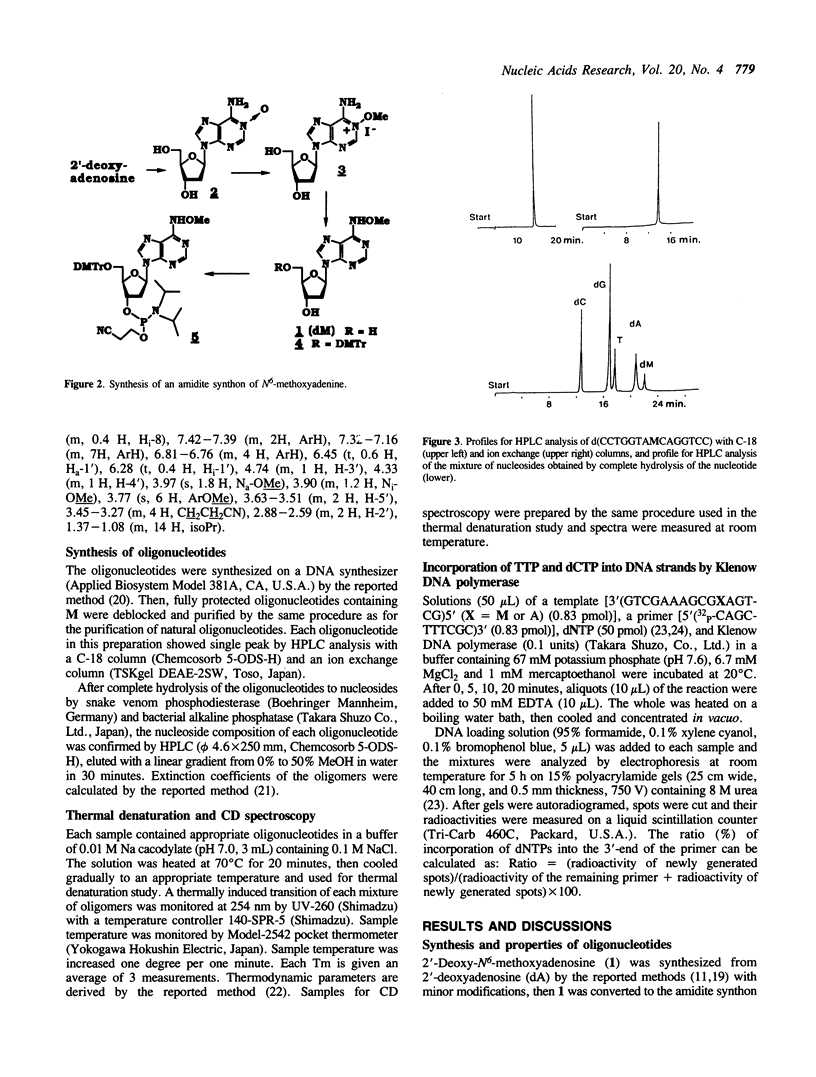
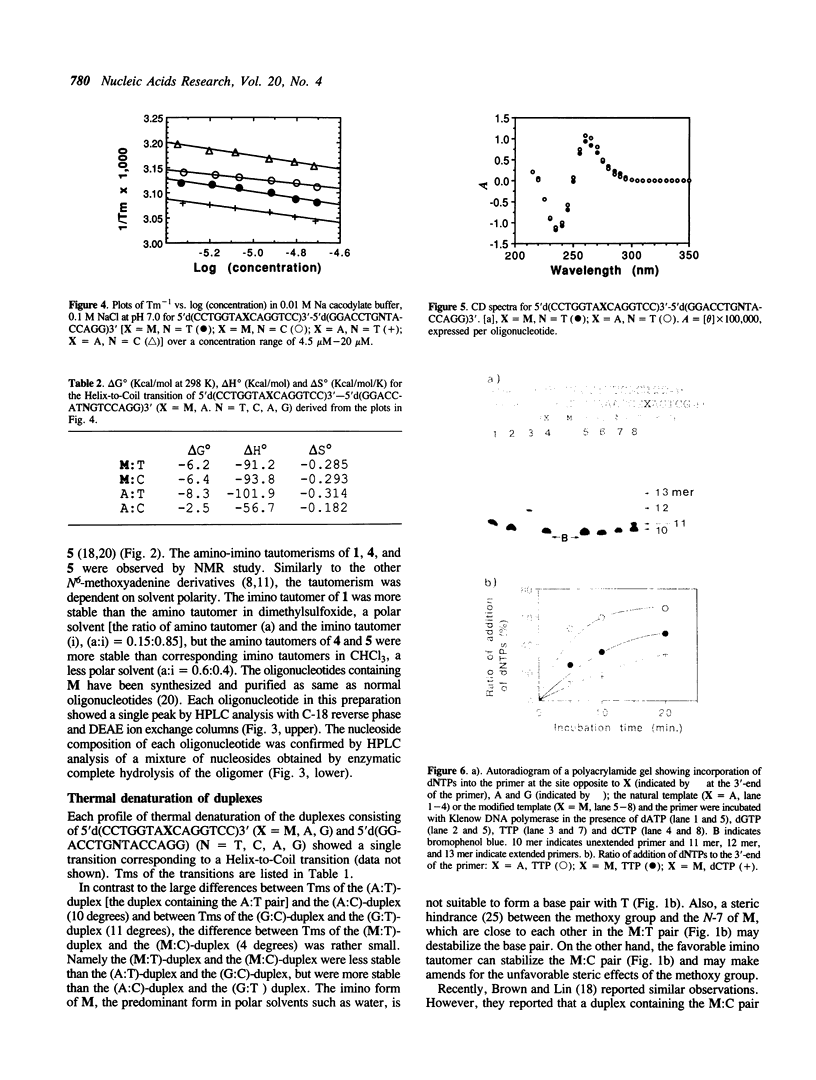
Abstract
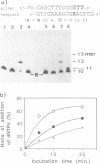
Oligodeoxyribonucleotides containing N6-methoxyadenine (M) have been synthesized. The order of stability of duplexes consisting of synthesized oligodeoxyribonucleotides, 5'd(CCTGGTAXCAGGTCC)3'-5'd(GGACCTGNTACCAGG)3' (X = M, A, G. N = A, G, T, C), was M: A (Tm = 52 degrees C) greater than M: T (50 degrees C) greater than M: G (48 degrees C) greater than M: C (46 degrees C) observed by thermal denaturation in a buffer of 0.01 M Na cacodylate, and 0.1 M NaCl at pH 7.0. The Tms are within a range of 6 degrees of difference, which is smaller than those of Tms of the duplexes containing A:N pairs (11 degrees) and G:N pairs (11 degrees). DNA replication study on a template-primer system, 5'd(32p-CAGCTTTCGC)3' 3'd(GTCGAAAGCGMAGTCG)5', showed that TTP and dCTP were incorporated into DNA strands at a site opposite to M by Klenow DNA polymerase, but dATP and dGTP were not.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Masih M. T., Bessman M. J. Biochemical studies on the mutagen, 6-N-hydroxylaminopurine. Synthesis of the deoxynucleoside triphosphate and its incorporation into DNA in vitro. J Biol Chem. 1986 Feb 15;261(5):2020–2026. [PubMed] [Google Scholar]
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N. N., Brown D. M., Salisbury S. A. The stability of oligodeoxyribonucleotide duplexes containing degenerate bases. Nucleic Acids Res. 1987 Oct 26;15(20):8167–8176. doi: 10.1093/nar/15.20.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum G. I., Kierdaszuk B., Shugar D. Tautomerism and conformation of the promutagenic analogue N6-methoxy-2',3',5'-tri-O-methyladenosine. Nucleic Acids Res. 1984 Mar 12;12(5):2447–2460. doi: 10.1093/nar/12.5.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M., Lin P. K. Synthesis and duplex stability of oligonucleotides containing adenine-guanine analogues. Carbohydr Res. 1991 Sep 2;216:129–139. doi: 10.1016/0008-6215(92)84156-m. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Spasokukotskaya T. N., Koudelka J. Mechanism of the mutagenic action of hydroxylamine. VIII. Functional properties of the modified adenosine residues. Biochim Biophys Acta. 1975 Apr 16;390(1):1–13. [PubMed] [Google Scholar]
- Eritja R., Horowitz D. M., Walker P. A., Ziehler-Martin J. P., Boosalis M. S., Goodman M. F., Itakura K., Kaplan B. E. Synthesis and properties of oligonucleotides containing 2'-deoxynebularine and 2'-deoxyxanthosine. Nucleic Acids Res. 1986 Oct 24;14(20):8135–8153. doi: 10.1093/nar/14.20.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley G. V., Téoule R., Guy A., Fritzsche H., Guschlbauer W. NMR studies on oligodeoxyribonucleotides containing the dam methylation site GATC. Comparison between d(GGATCC) and d(GGm6ATCC). Biochemistry. 1985 Aug 13;24(17):4540–4548. doi: 10.1021/bi00338a009. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Singer B. The chemical basis for the mutagenicity of hydroxylamine and methoxyamine. Biochim Biophys Acta. 1972 Mar 24;262(3):264–268. doi: 10.1016/0005-2787(72)90262-6. [DOI] [PubMed] [Google Scholar]
- Freese E. B. The mutagenic effect of hydroxyaminopurine derivatives on phage T4. Mutat Res. 1968 Mar-Apr;5(2):299–301. doi: 10.1016/0027-5107(68)90028-6. [DOI] [PubMed] [Google Scholar]
- Kierdaszuk B., Stolarski R., Shugar D. Hydroxylamine and methoxyamine mutagenesis: tautomeric equilibrium of the promutagenic, N6-methoxyadenosine in solvents of different polarities. Acta Biochim Pol. 1984;31(1):49–64. [PubMed] [Google Scholar]
- Kochetkov N. K., Budowsky E. I. The chemical modification of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1969;9:403–438. doi: 10.1016/s0079-6603(08)60773-4. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Jones S. S., Hackett N. R., Khorana H. G. Specific amino acid substitutions in bacterioopsin: Replacement of a restriction fragment in the structural gene by synthetic DNA fragments containing altered codons. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2285–2289. doi: 10.1073/pnas.81.8.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins M. J., Trip E. M. Sugar-modified N 6 -(3-methyl-2-butenyl)adenosine derivatives, N 6 -benzyl analogs, and cytokinin-related nucleosides containing sulfur or formycin. Biochemistry. 1973 Jun 5;12(12):2179–2187. doi: 10.1021/bi00736a001. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Stolarski R., Kierdaszuk B., Hagberg C. E., Shugar D. Hydroxylamine and methoxyamine mutagenesis: displacement of the tautomeric equilibrium of the promutagen N6-methoxyadenosine by complementary base pairing. Biochemistry. 1984 Jun 19;23(13):2906–2913. doi: 10.1021/bi00308a009. [DOI] [PubMed] [Google Scholar]
- Stolarski R., Kierdaszuk B., Hagberg C. E., Shugar D. Mechanism of hydroxylamine mutagenesis: tautomeric shifts and proton exchange between the promutagen N6-methoxyadenosine and cytidine. Biochemistry. 1987 Jul 14;26(14):4332–4337. doi: 10.1021/bi00388a022. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Negishi K., Hayatsu H. Proofreading of a mutagenic nucleotide, N4-aminodeoxycytidylic acid, by Escherichia coli DNA polymerase I. Biochem Biophys Res Commun. 1987 Feb 27;143(1):104–109. doi: 10.1016/0006-291x(87)90636-x. [DOI] [PubMed] [Google Scholar]
- Tsuchiyama H., Atsumi G., Matsuda A., Negishi K., Hayatsu H. Analysis of 2-amino-N6-hydroxyadenine-induced mutagenesis in phage M13mp2. Mutat Res. 1991 Aug;253(1):47–54. doi: 10.1016/0165-1161(91)90344-8. [DOI] [PubMed] [Google Scholar]