Abstract
Background
TRIM5α and TRIMCyp are cytoplasmic proteins that bind incoming retroviral capsids and mediate early blocks to viral infection. TRIM5 proteins form cytoplasmic bodies, which are highly dynamic structures. So far, TRIM5 proteins have been found only in the cytoplasm of cells. Interestingly, other proteins from the TRIM family localize to the nucleus. Therefore, we tested the possibility that TRIM5 proteins traffic to the nucleus and the impact of this trafficking on retroviral restriction.
Results
Here we report that the TRIM5α proteins of two Old World primates, humans and rhesus monkeys, are transported into the nucleus and are shuttled back to the cytoplasm by a leptomycin B-sensitive mechanism. In leptomycin B-treated cells, these TRIM5α proteins formed nuclear bodies that also contained TRIM19 (PML). Deletion of the amino terminus, including the linker 1 (L1) region, resulted in TRIM5α proteins that accumulated in nuclear bodies. Leptomycin B treatment of TRIM5α-expressing target cells only minimally affected the restriction of retrovirus infection.
Conclusions
We discovered the ability of human and rhesus TRIM5α to shuttle into and out of the nucleus. This novel trafficking ability of TRIM5α proteins could be important for an as-yet-unknown function of TRIM5α.
Keywords: Restriction factor, intracellular localization, retrovirus, leptomycin B
Background
Proteins of the tripartite motif (TRIM) family contain RING, B-Box and coiled-coil domains, and thus have been referred to as RBCC proteins [1]. Members of this family have been implicated in diverse processes such as cell proliferation, differentiation, development, oncogenesis and apoptosis [1,2]. TRIM proteins often self-associate and, when overexpressed, aggregate to form nuclear or cytoplasmic bodies [1].
TRIM5α is a cytoplasmic protein that is capable of restricting retrovirus infection in a species-dependent manner [3]. Variation among TRIM5α proteins in different primates accounts for the early, post-entry blocks to infection by particular retroviruses [3-7]. For example, TRIM5α proteins of Old World monkeys block human immunodeficiency virus (HIV-1) infection [3-5,7], whereas TRIM5α proteins of New World monkeys block infection by simian immunodeficiency virus (SIVmac) [8]. TRIM5α from humans (TRIM5αhu) is not as potent in restricting HIV-1 infection as Old World monkey TRIM5α, but TRIM5αhu potently restricts other retroviruses, e.g., N-tropic murine leukemia virus (N-MLV) and equine infectious anemia virus (EIAV) [3,4,6-8]. Owl monkeys, a New World monkey species, are unusual in not expressing a TRIM5α protein, but instead express TRIMCyp, in which the RBCC domains of TRIM5 are fused to a cyclophilin A moiety [9,10].
Variation in splicing of the TRIM5 primary transcript leads to the expression of TRIM5 isoforms, designated α, γ and δ [1]. The TRIM5α isoform contains, in addition to the RING, B-box 2 and coiled-coil domains, a carboxy-terminal B30.2(SPRY) domain. The B30.2(SPRY) domain is essential for the antiretroviral activity of TRIM5α [3]. In some cases, the differences in the ability of TRIM5α proteins from various primate species to restrict particular retroviruses are determined by sequences in the B30.2(SPRY) domain [11-19]. The B30.2(SPRY) domain in TRIM5α and the cyclophilin A domain in TRIMCyp allow these restriction factors to bind specifically to particular retroviral capsids [9,20-24]. Additional sequences in the B-box 2 domain contribute to higher-order self-association of TRIM5α, which allows higher avidity for the retroviral capsid [25-27]. TRIM5α proteins aggregate on the incoming retroviral capsid [28]; and, by as-yet-uncertain mechanisms, decrease the stability of the capsid [23,27,29,30].
Some TRIM proteins localize in the nucleus of cells. One example is TRIM19 (promyelocytic leukemia (PML) protein), which is a major component of nuclear domain 10 (ND10) bodies [31-33]. TRIM19 has been shown to interfere with the replication of several DNA and RNA viruses [34-41]. Both TRIM19 and TRIM5α can inhibit herpes simplex virus replication [34,40,41], and both proteins are induced by type I interferons [18,42,43]. Thus, both cytoplasmic (e.g., TRIM5α) and nuclear (e.g., TRIM19) TRIM proteins may be involved in innate resistance to viral infection.
Here we study the intracellular localization of different TRIM5α proteins and TRIMCyp after treatment of cells with leptomycin B. Leptomycin B is a specific inhibitor of the nuclear export factor CRM1 (exportin 1), which is critical for the export of proteins carrying a nuclear export sequence [44-49]. We document that TRIM5αhu and TRIM5αrh are actively shuttling between the cytoplasm and nucleus. By contrast, TRIM5α proteins from the squirrel monkey (a New World monkey) and the cow did not accumulate in the nucleus upon leptomycin B treatment. TRIMCyp from owl monkeys also localized in the cytoplasm upon treatment with leptomycin B. We investigated the contribution of the nuclear export of TRIM5α to the antiretroviral activity of the protein.
Results
Leptomycin B treatment results in nuclear accumulation of some TRIM5α proteins
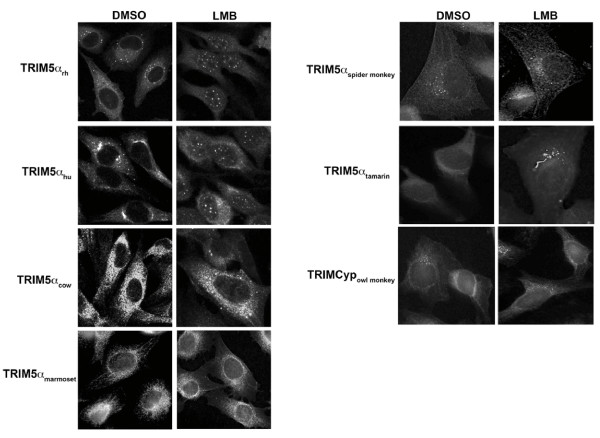
During the course of studying TRIM5α, we tested the effect of leptomycin B (LMB), a specific inhibitor of nuclear export [44-49], on TRIM5α localization. As dogs do not express a functional TRIM5 protein[14], we initially studied the localization of different TRIM5α variants in canine cells. LMB treatment of Cf2Th canine cells stably expressing TRIM5αhu or TRIM5αrh resulted in the accumulation of these proteins in the nucleus (Figure 1). Both proteins were found in nuclear bodies after LMB treatment. By contrast, TRIMCyp and the TRIM5α proteins from cows and several species of New World monkeys (squirrel monkeys, spider monkeys, marmosets and tamarins) remained localized in the cytoplasm after LMB treatment. These results suggest that TRIM5αhu and TRIM5αrh shuttle into the nucleus and require active transport via the CRM1 protein to achieve cytoplasmic localization.
Figure 1.
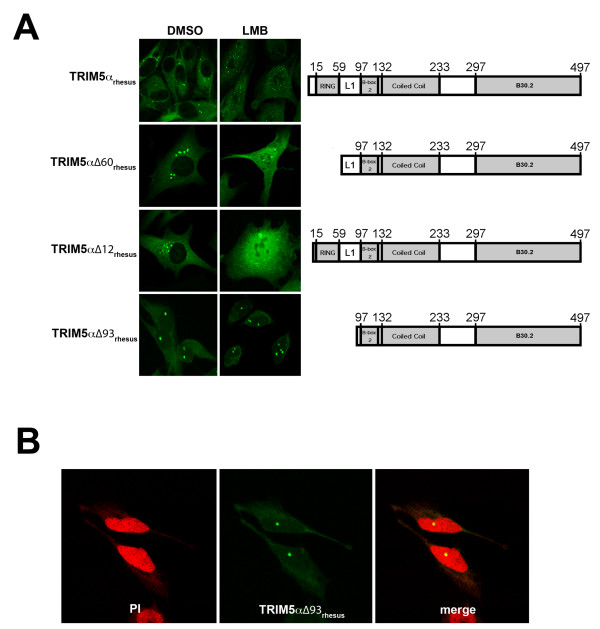
Retention of some TRIM5 variants in the nucleus after leptomycin B treatment. Cf2Th cells stably expressing the indicated HA-tagged TRIM5α proteins were treated with 5 ng/ml of leptomycin B (LMB) or DMSO for two hours. Treated cells were stained using anti-HA antibodies conjugated to FITC. Representative figures are shown.
Rapid accumulation of TRIM5αhu and TRIM5αrh in the nucleus after LMB treatment
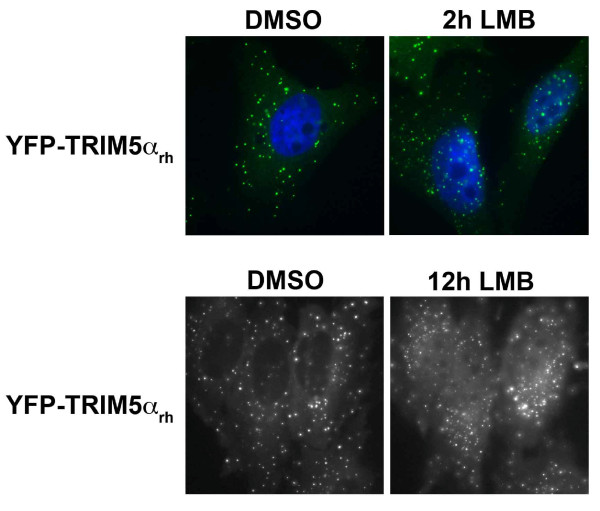
To understand the kinetics of TRIM5αrh movement into the nucleus, we performed time-lapse fluorescent microscopy using a HeLa cell line stably expressing a TRIM5αrh-yellow fluorescent protein (YFP) fusion. These experiments revealed that treatment of cells with LMB resulted in a rapid accumulation of TRIM5αrh-GFP in the nucleus (Figure 2). Nuclear bodies containing TRIM5αrh-GFP were evident by 2 hours following the initiation of LMB treatment.
Figure 2.
Time course of accumulation of YFP-TRIM5αrh fusion protein in the nucleus after leptomycin B treatment. HeLa cells stably expressing a YFP-TRIM5αrh fusion protein were treated with 5 ng/ml of LMB or DMSO for 2 and 12 hours. Treated cells were stained using anti-HA antibodies conjugated to FITC (green) and DAPI to stain the cell nucleus (blue). Representative figures are shown.
Nuclear TRIM5αhu and TRIM5αrh proteins localize to ND10 bodies with TRIM19
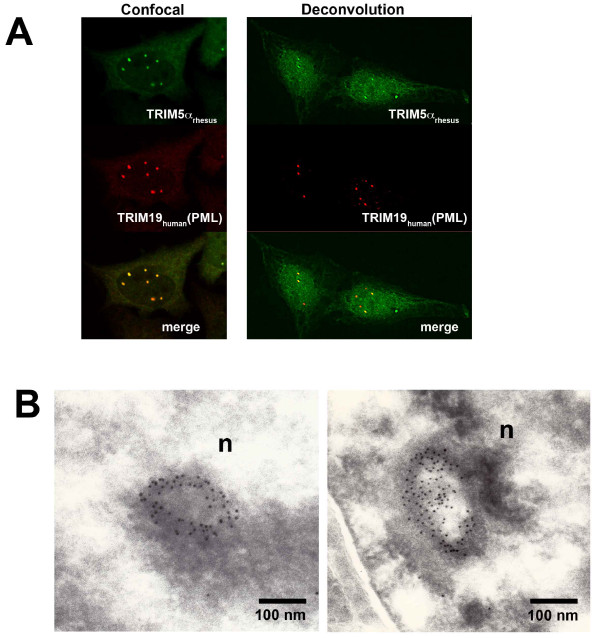
To examine whether TRIM5αrh localizes to the same ND10 bodies as TRIM19 after LMB treatment, LMB-treated human cells stably expressing TRIM5αrh were stained with antibodies directed against TRIM19 and the hemagglutinin (HA) epitope tag on TRIM5αrh. The nuclear TRIM5αrh colocalized with TRIM19 (Figure 3A). Gold-labeled antibodies directed against the HA epitope tag on TRIM5αrh were used to investigate the structure of the nuclear bodies. The TRIM5α-directed antibodies formed ring-like structures similar in appearance to those previously described for TRIM19 in ND10 bodies (Figure 3B) [31,33].
Figure 3.
Colocalization of TRIM5α and TRIM19 (PML) in leptomycin B-treated cells. HeLa cells stably expressing HA-tagged TRIM5αrh proteins were treated with 5 ng/ml of leptomycin B (LMB) for two hours. Cells were stained for TRIM5α using anti-HA FITC-conjugated antibodies. PML was stained using anti-PML antibodies and Cy3-conjugated anti-goat secondary antibodies (A). LMB-treated HeLa cells expressing TRIM5αrh were fixed. Ultrathin sections were labeled using an anti-HA antibody and Protein A-gold (10-nm particles). Ring-like structures (n, nuclear bodies) in the cell nucleus were labeled with the antibody (B).
Localization of a TRIM5αrh-pyruvate kinase fusion protein
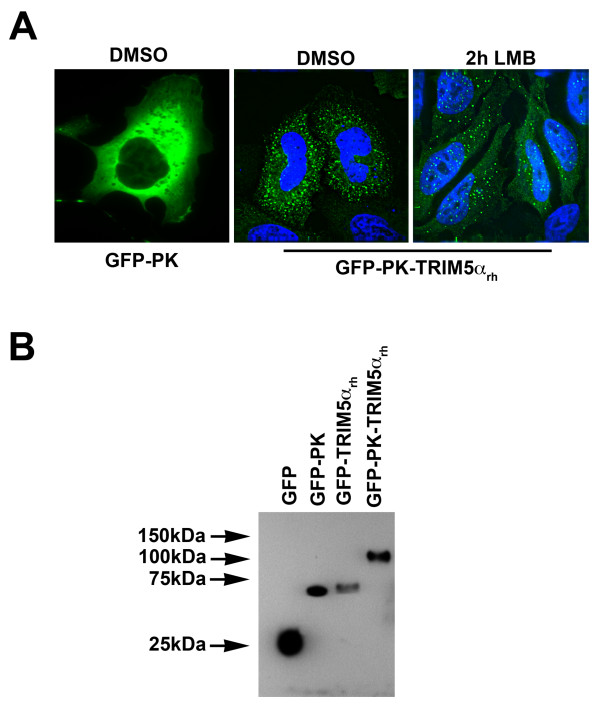
The diameter of the nuclear pore is approximately 0.9 nm, which allows globular proteins less than 60 kD to diffuse freely through the channel [50-52]. TRIM5α proteins (approximately 55 kD) are close to this diffusion limit. Moreover, TRIM5α forms a stable dimer [20,21]; however, we do not know if the majority of TRIM5α molecules that enter the nucleus are monomers or dimers. In addition, the molecular shape of TRIM5α is unknown. These uncertainties raised the possibility that TRIM5α is actively transported into the nucleus. To test this possibility, TRIM5αrh was fused to pyruvate kinase (PK), which is normally a cytoplasmic protein [53] and to the green fluorescent protein (GFP) to create the GFP-PK-TRIM5αrh chimeric protein. The GFP-PK-TRIM5αrh protein and a control GFP-PK protein were transiently expressed in HeLa cells (Figure 4). Localization of these proteins was examined in untreated and LMB-treated cells (Figure 4). After a two-hour treatment with 10 nM LMB, the GFP-PK-TRIM5αrh protein was detected in both the nucleus and the cytoplasm. By contrast, the GFP-PK protein was detected only in the cytoplasm of untreated and LMB-treated cells. These results are consistent with the active transport of TRIM5αrh to the nucleus.
Figure 4.
Localization of a GFP-PK-TRIM5α protein in leptomycin B-treated cells. HeLa cells transiently expressing the fusion constructs GFP-PK or GFP-PK-TRIM5αrh were treated with 5 ng/ml of LMB or with the equivalent concentration of DMSO for 2 hours (A). Protein expression levels of the different fusion constructs were measured by Western blot using anti-GFP antibodies (B).
Identification of TRIM5αrh regions modulating localization
Proteins that localize to the nucleus and shuttle to the cytoplasm often contain nuclear localization and nuclear export signals, respectively [44-48]. TRIM5αhu and TRIM5αrh lack an obvious nuclear localization signal [54,55], nor do they contain sequences motifs predicted to function as nuclear export signals [56]. To gain some insight into the TRIM5αrh sequences that modulate nuclear localization and export, a series of TRIM5αrh mutants with deletions in N-terminal components were studied. The TRIM5αrh Δ12 and TRIM5α Δ60 proteins behaved like wild-type TRIM5αrh with respect to localization in untreated cells (Figure 5A and Table 1). However, in the LMB-treated cells, TRIM5αrh Δ12 and TRIM5α Δ60 exhibited a bright, more diffuse pattern with fewer nuclear bodies when compared with wild-type TRIM5αrh (Figure 5A and Table 1). These results indicate that neither the immediate TRIM5αrh N-terminus nor the RING domain significantly influence nuclear localization and export. By contrast, the TRIM5αrh Δ93 mutant localized to nuclear bodies and to the cytosol, even in the absence of LMB treatment (Figure 5B and Table 1). This localization pattern did not change significantly upon LMB treatment. Thus, deletion of TRIM5αrh sequences between residues 60 and 93, in the Linker 1 (L1) region of the protein, appears to decrease the efficiency of nuclear export of TRIM5αrh.
Figure 5.
Localization of TRIM5αrh N-terminal deletion mutants in leptomycin B-treated cells. Cf2Th cells stably expressing wild-type TRIM5αrh or the indicated deletion mutant were treated with 5 ng/ml of LMB or DMSO for two hours. Treated cells were stained using anti-HA antibodies conjugated to FITC. TRIM5αrh domains are depicted for each variant, and the numbers of the amino acid residues at the boundaries of the different domains are shown (A). L1 represents the Linker 1 region. The TRIM5αrh∆93 protein bodies are located in the cellular nucleus (B). Cf2Th cells expressing TRIM5αrh∆93 were stained using using anti-HA antibodies conjugated to FITC (green) and propidium iodide for nuclear staining (red). A representative image is shown.
Table 1.
Number of TRIM5α cytoplasmic and nuclear bodies in LMB-treated cells
| Number of cytoplasmic and nuclear bodies per 100 cells | ||||||
|---|---|---|---|---|---|---|
| DMSO | LMB | |||||
| Cytoplasmic | Nuclear | Total | Cytoplasmic | Nuclear | Total | |
| TRIM5αrh | 448 | 2 | 450 | 7 | 543 | 550 |
| TRIM5αrh∆12 | 127 | 3 | 130* | 5 | 128 | 133* |
| TRIM5αrh∆60 | 202 | 8 | 210* | 2 | 78 | 80* |
| TRIM5αrh∆93 | 4 | 151 | 155 | 12 | 158 | 170 |
*Cytoplasmic and nuclear bodies of TRIM5αrh∆12 and ∆60 were on average larger than bodies observed for wt TRIM5αrh and TRIM5αrh∆93 proteins.
Contribution of nuclear export of TRIM5αhu and TRIM5αrh to retroviral restriction
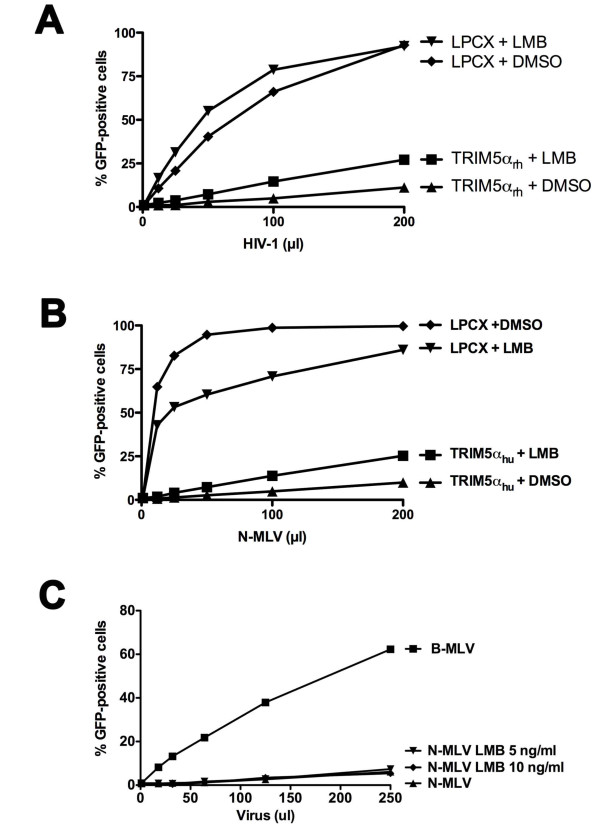
To study the contribution of TRIM5α nuclear export to retroviral restriction, we treated cells stably expressing TRIM5αrh and TRIM5αhu with LMB for two hours. Then the cells were challenged with recombinant HIV-1 and N-MLV expressing GFP. Treatment with LMB continued during the incubation of the cells with virus and overnight thereafter. LMB treatment exerted only minimal effects on the ability of TRIM5αrh to restrict HIV-1 infection and on the ability of TRIM5αhu to inhibit N-MLV infection (Figure 6).
Figure 6.
Effect of leptomycin B treatment of TRIM5α-expressing cells on retrovirus restriction. Cf2Th cells stably expressing TRIM5αrh or transduced with the empty vector LPCX were challenged with increasing amounts of HIV-1-GFP in the presence of 5 ng/ml of LMB or DMSO (A). Similarly, Cf2Th cells stably expressing TRIM5αhu were challenged with increasing amounts of N-MLV-GFP in the presence of 5 ng/ml of LMB or DMSO (B). TE671 cells, which naturally express TRIM5αhu, were challenged with B-MLV-GFP and N-MLV-GFP in the presence of the indicated concentration of LMB or the DMSO control (C). The x-axis indicates the volume of a stock of recombinant GFP-expressing virus added to the target cells. Forty-eight hours after infection, the percentage of infected cells was measured by counting the GFP-positive cells using a flow cytometer. Similar results were obtained in three independent experiments.
Discussion
All characterized TRIM5α proteins are located in the cytoplasm of expressing cells [15,28,57-59]. Here we report the surprising observation that some TRIM5α proteins are imported into the nucleus and then exported back into the cytoplasm by a CRM1-dependent mechanism. Of interest, this transient routing through the nucleus was observed for the TRIM5α proteins of two Old World primates, and not for the TRIM5α proteins of a cow or several New World monkeys, or for the TRIMCyp protein of another New World monkey (the owl monkey). This raises the possibility that nuclear shuttling represents a property that was gained by Old World primate TRIM5α proteins after the divergence from the New World monkeys.
Our results with the GFP-PK-TRIM5αrh fusion protein suggest that TRIM5αrh is actively transported into the nucleus, as the fusion protein is well above the size limit for passive diffusion of proteins through the nuclear pore [50-52]. Nonetheless, no typical nuclear localization motif is evident on TRIM5α [54,55]. The accumulation of TRIM5αhu and TRIM5αrh in the nucleus after LMB treatment implicates a CRM1-dependent process in the export of these TRIM5α proteins from the nucleus [44-49]. However, there are no classical nuclear export motifs in TRIM5α proteins [56]. It is possible that TRIM5α utilizes unusual motifs for interacting with nuclear pore proteins. Analysis of the localization of N-terminally truncated TRIM5αrh mutants suggests that deletion of residues 60-93, in the linker 1 (L1) region, disrupts the nuclear export of the protein. Whether this is a result of deletion of a non-canonical nuclear export signal or an indirect effect requires further investigation. As an example of the latter effect, the linker 1 (L1) regions could mediate the association of TRIM5αrh and TRIM5αhu with another factor that shuttles between the nuclear and cytoplasm.
Despite the accumulation of TRIM5αhu and TRIM5αrh proteins in the nucleus after LMB treatment, restriction of N-MLV and HIV-1, respectively, remained potent. Although it is possible that nuclear TRIM5αhu and TRIM5αrh can inhibit retrovirus infection, the specific recognition of the retroviral capsid, which does not enter the intact nucleus, is thought to be important for potent restriction [22,23]. A more likely explanation is that the residual TRIM5α protein in the cytoplasm of these overexpressing cells is sufficient to inhibit virus infection. Any newly synthesized TRIM5α in these cells that has not yet entered the nucleus is potentially available for capsid interaction.
One caveat of these studies is the use of exogenously expressed TRIM5α proteins to study nuclear shuttling. When better antibodies against endogenous TRIM5α become available, the shuttling behavior of the endogenously expressed TRIM5α protein can be examined.
What might be the possible advantage of having the Old World primate TRIM5α proteins shuttle into and out of the nucleus? If these TRIM5α proteins acquire post-translational modifications or binding partners in the process, our results suggest that such acquisition is apparently not necessary for HIV-1 or N-MLV restriction. The presence of TRIM5α in the nucleus could be important for other TRIM5α functions besides retroviral restriction. For example, Old World monkey TRIM5α proteins have recently been shown to inhibit the infection of herpes simplex viruses 1 and 2 [41]. The colocalization of nuclear TRIM5α in ND10 bodies with TRIM19, which also has anti-herpes virus activity [34,39,40], might have functional importance in this respect. Future studies should shed light on these interesting possibilities.
Conclusions
Here we discovered the ability of human and rhesus TRIM5α to shuttle into and out of the nucleus. Although not essential for retroviral restriction, this novel ability of TRIM5α might be involved in other functions such as the ability of TRIM5 to trigger NF-kB[38].
Methods
Plasmid construction
The plasmids used to establish cell lines stably expressing TRIM5α variants or TRIMCyp have been previously described [8,58]. The plasmids expressing mutant TRIM5αrh proteins with N-terminal deletions were constructed by polymerase chain reaction (PCR) amplification of TRIM5 cDNA, as previously described [3]. The amplified fragments were cloned into the EcoRI and Cla I sites of the pLPCX plasmid (Stratagene). All of the TRIM5α proteins have an epitope tag from influenza hemagglutinin (HA). Human TRIM5α has the HA tag at the carboxyl terminus, and all the other TRIM5α proteins have the HA tag at the amino terminus.
Creation of cells stably expressing TRIM5α and TRIMCyp variants
Retroviral vectors encoding TRIM5α or TRIMCyp proteins were created using the pLPCX vector plasmid [3]. Recombinant viruses were produced in 293T cells by cotransfecting the pLPCX plasmids with the pVPack-GP and pVPack-VSV-G packaging plasmids (Stratagene). The pVPack-VSV-G plasmid encodes the vesicular stomatitis virus (VSV) G envelope glycoprotein, which allows efficient entry into a wide range of vertebrate cells.
Protein analysis
Cellular proteins were extracted with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris, pH 7.4; 100 mM NaCl; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]; 1% NP-40; 2 mg of aprotinin/ml; 2 mg of leupeptin/ml; 1 mg of pepstatin A/ml; 100 mg of phenylmethylsulfonyl fluoride/ml). The cell lysates were analyzed by SDS-PAGE (10% acrylamide), followed by blotting onto nitrocellulose membranes (Amersham Pharmacia Biotech). Detection of protein by Western blotting utilized monoclonal antibodies that are specifically reactive with the HA epitope tag (Roche). Detection of proteins was performed by enhanced chemiluminescence (NEN Life Sciences Products).
Infection with recombinant viruses expressing green fluorescent protein (GFP)
Recombinant HIV-1 or N-MLV expressing GFP were prepared as described [3]. HIV-1 viral stocks were quantified by measuring reverse transcriptase (RT) activity. For infections, 3 × 104 HeLa human epithelial cells or Cf2Th canine cells seeded in 24-well plates were incubated in the presence of virus for 24 hours. Cells were washed and returned to culture for 48 hours, and then subjected to FACS analysis with a FACScan (Becton Dickinson).
Intracellular location of TRIM5 variants
Localization of TRIM5 variants was studied as previously described [60]. Briefly, cells were grown overnight on 12-mm-diameter coverslips and fixed in 3.9% paraformaldehyde (Sigma) in phosphate-buffered saline (PBS; Cellgro) for 30 minutes. In some experiments, cells were incubated with 5 ng/ml leptomycin B (LMB) in medium for 2-10 hours prior to fixation. Cells were washed in PBS, incubated in 0.1 M glycine (Sigma) for 10 minutes, washed in PBS, and permeabilized with 0.05% saponin (Sigma) for 30 minutes. Samples were blocked with 10% donkey serum (Dako, Carpinteria, CA) for 30 minutes, and incubated for 1 hour with antibodies. HA-tagged proteins were stained using an anti-HA FITC-conjugated antibody, clone 3F10 (Roche). The TRIM19 (PML) protein was stained with an antibody against PML, sc-9863 (Santa Cruz Biotechnology, CA) and anti-goat Cy3-conjugated antibodies(Jackson ImmunoResearch, PA). Subsequently, samples were mounted for fluorescence microscopy by using the ProLong Antifade Kit (Molecular Probes, Eugene, OR). Images were obtained with a BioRad Radiance 2000 laser scanning confocal microscope with Nikon 60X N.A.1.4 optics.
Detection of TRIM5α by electron microscopy
HeLa cells stably expressing HA-tagged TRIM5αrh treated with 5 ng/ml LMB for 2 h were removed from the tissue culture dish with 5 mM EDTA in PBS, pelleted, and resuspended in a small volume of 4% paraformaldehyde in 0.2 M sodium phosphate buffer, pH 7.4. Ultrathin sections were cut at -120˚C with a cryo-diamond knife. Sections were picked up from the knife with a loop dipped in a 1:1 mixture of 2.3 M sucrose and 2% methylcellulose and transferred to a carbon-coated copper grid. Grids were left floating on PBS with the section facing down. Grids were washed in PBS and blocked in 1% bovine serum albumin (BSA) in PBS for 15 min. Grids were then incubated with the anti-HA 3F10 antibody (Roche) in 1% BSA in PBS for 30 min and washed four times for 15 min in PBS. Then, the grids were incubated with Protein A-gold 10-nm particles (Jackson Immunoresearch) in 1% BSA in PBS for 20 min and washed four times for 15 min in PBS. Images were acquired using a transmission electron microscope JEOL 1200EX-80kV.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
FDG designed and performed experiments, wrote the manuscript. DEG designed and performed experiments. TJH designed and performed experiments. JS designed experiments and wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Felipe Diaz-Griffero, Email: felipe.diaz-griffero@einstein.yu.edu.
Daniel E Gallo, Email: dgallo1@gmail.com.
Thomas J Hope, Email: thope@northwestern.edu.
Joseph Sodroski, Email: joseph_sodroski@dfci.harvard.edu.
Acknowledgements
We thank Ms. Yvette McLaughlin and Ms. Elizabeth Carpelan for manuscript preparation and the National Institutes of Health (AI063987(JS), AI076094(JS), AI047770(TJH) and a Center for AIDS Research Award AI60354), the International AIDS Vaccine Initiative, the Bristol-Myers Squibb Foundation, and the late William F. McCarty-Cooper for research funding. F.D.-G. is a recipient of a K99/R00 Pathway to Independence Award from the National Institutes of Health (1K99MH086162-01), an American Foundation for AIDS Research Mathilde Krim fellowship in basic biomedical research (106987-43-RFHF), and a Claudia Adams Barr award from the Dana-Farber Cancer Institute. We also would like to thank the James B. Pendleton Charitable Trust. T.J.H. is funded by a P50 GM082545 from the NIH.
References
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S. et al. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799–808. doi: 10.1038/nrmicro1248. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Perez-Caballero D, Yang A, Cowan S, Bieniasz PD. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc Natl Acad Sci USA. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci USA. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron MJ, Stremlau M, Song B, Ulm W, Mulligan RC, Sodroski J. TRIM5alpha mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci USA. 2004;101:11827–11832. doi: 10.1073/pnas.0403364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Lynch C, Stoye JP. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci USA. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Javanbakht H, Perron M, Park DH, Stremlau M, Sodroski J. Retrovirus restriction by TRIM5alpha variants from Old World and New World primates. J Virol. 2005;79:3930–3937. doi: 10.1128/JVI.79.7.3930-3937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci USA. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- Song B, Gold B, O'Huigin C, Javanbakht H, Li X, Stremlau M, Winkler C, Dean M, Sodroski J. The B30.2(SPRY) domain of the retroviral restriction factor TRIM5alpha exhibits lineage-specific length and sequence variation in primates. J Virol. 2005;79:6111–6121. doi: 10.1128/JVI.79.10.6111-6121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Bozek K, Domingues FS, Shioda T, Nakayama EE. Impact of a single amino acid in the variable region 2 of the Old World monkey TRIM5alpha SPRY (B30.2) domain on anti-human immunodeficiency virus type 2 activity. Virology. 2009;388:160–168. doi: 10.1016/j.virol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Grutter C, de Castillia CS, Pertel T, Olivari S, Grutter MG, Luban J. An invariant surface patch on the TRIM5alpha PRYSPRY domain is required for retroviral restriction but dispensable for capsid binding. J Virol. 2009;83:3365–3373. doi: 10.1128/JVI.00432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci USA. 2005;102:2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D, Hatziioannou T, Yang A, Cowan S, Bieniasz PD. Human tripartite motif 5alpha domains responsible for retrovirus restriction activity and specificity. J Virol. 2005;79:8969–8978. doi: 10.1128/JVI.79.14.8969-8978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura S, Yap MW, Sheldon T, Stoye JP. All three variable regions of the TRIM5alpha B30.2 domain can contribute to the specificity of retrovirus restriction. J Virol. 2006;80:8554–8565. doi: 10.1128/JVI.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5alpha leads to HIV-1 restriction. Curr Biol. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Nakayama EE, Miyoshi H, Nagai Y, Shioda T. A specific region of 37 amino acid residues in the SPRY (B30.2) domain of African green monkey TRIM5alpha determines species-specific restriction of simian immunodeficiency virus SIVmac infection. J Virol. 2005;79:8870–8877. doi: 10.1128/JVI.79.14.8870-8877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AK, Diaz-Griffero F, Li Y, Li X, Sodroski J. Biochemical and biophysical characterization of a chimeric TRIM21-TRIM5alpha protein. J Virol. 2008;82:11669–11681. doi: 10.1128/JVI.01559-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier CR, Sandrin V, Eckert DM, Christensen DE, Chandrasekaran V, Alam SL, Aiken C, Olsen JC, Kar AK, Sodroski JG, Sundquist WI. Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol. 2008;82:11682–11694. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S, Luban J. TRIM5alpha selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Qin XR, Hayashi F, Kigawa T, Finzi A, Sarnak Z, Lienlaf M, Yokoyama S, Sodroski J. A B-box 2 Surface Patch Important for TRIM5{alpha} Self-Association, Capsid-binding Avidity and Retrovirus Restriction. J Virol. 2009. [DOI] [PMC free article] [PubMed]
- Li X, Sodroski J. The TRIM5alpha B-box 2 domain promotes cooperative binding to the retroviral capsid by mediating higher-order self-association. J Virol. 2008;82:11495–11502. doi: 10.1128/JVI.01548-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Kar A, Lee M, Stremlau M, Poeschla E, Sodroski J. Comparative requirements for the restriction of retrovirus infection by TRIM5alpha and TRIMCyp. Virology. 2007. [DOI] [PMC free article] [PubMed]
- Campbell EM, Perez O, Anderson JL, Hope TJ. Visualization of a proteasome-independent intermediate during restriction of HIV-1 by rhesus TRIM5alpha. J Cell Biol. 2008;180:549–561. doi: 10.1083/jcb.200706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Kar A, Perron M, Xiang SH, Javanbakht H, Li X, Sodroski J. Modulation of Retroviral Restriction and Proteasome Inhibitor-resistant Turnover by Changes in the TRIM5{alpha} B-box 2 Domain. J Virol. 2007. [DOI] [PMC free article] [PubMed]
- Diaz-Griffero F, Perron M, McGee-Estrada K, Hanna R, Maillard PV, Trono D, Sodroski J. A human TRIM5alpha B30.2/SPRY domain mutant gains the ability to restrict and prematurely uncoat B-tropic murine leukemia virus. Virology. 2008. [DOI] [PMC free article] [PubMed]
- Perron MJ, Stremlau M, Lee M, Javanbakht H, Song B, Sodroski J. The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol. 2007;81:2138–2148. doi: 10.1128/JVI.02318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett RD. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene. 2001;20:7266–7273. doi: 10.1038/sj.onc.1204759. [DOI] [PubMed] [Google Scholar]
- Negorev D, Maul GG. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene. 2001;20:7234–7242. doi: 10.1038/sj.onc.1204764. [DOI] [PubMed] [Google Scholar]
- Maul GG. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Everett RD, Maul GG. HSV-1 IE protein Vmw110 causes redistribution of PML. Embo J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal AS, Szekely L, Elgh F. Ebola virus infection inversely correlates with the overall expression levels of promyelocytic leukaemia (PML) protein in cultured cells. BMC Microbiol. 2003;3:6. doi: 10.1186/1471-2180-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla WV, Pinschewer DD, Klenerman P, Rousson V, Gaboli M, Pandolfi PP, Zinkernagel RM, Salvato MS, Hengartner H. Effects of promyelocytic leukemia protein on virus-host balance. J Virol. 2002;76:3810–3818. doi: 10.1128/JVI.76.8.3810-3818.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavani M, Rodas J, Lukashevich IS, Horejsh D, Pandolfi PP, Borden KL, Salvato MS. Role of the promyelocytic leukemia protein PML in the interferon sensitivity of lymphocytic choriomeningitis virus. J Virol. 2001;75:6204–6208. doi: 10.1128/JVI.75.13.6204-6208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen SU, Emerman M. Human Trim5alpha has additional activities that are uncoupled from retroviral capsid recognition. Virology. 2011;409:113–120. doi: 10.1016/j.virol.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroishi A, Bozek K, Shioda T, Nakayama EE. A single amino acid substitution of the human immunodeficiency virus type 1 capsid protein affects viral sensitivity to TRIM5 alpha. Retrovirology. 2010;7:58. doi: 10.1186/1742-4690-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama EE, Shioda T. Anti-retroviral activity of TRIM5 alpha. Rev Med Virol. 2010;20:77–92. doi: 10.1002/rmv.637. [DOI] [PubMed] [Google Scholar]
- Xu D, Holko M, Sadler AJ, Scott B, Higashiyama S, Berkofsky-Fessler W, McConnell MJ, Pandolfi PP, Licht JD, Williams BR. Promyelocytic leukemia zinc finger protein regulates interferon-mediated innate immunity. Immunity. 2009;30:802–816. doi: 10.1016/j.immuni.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthagena L, Parise MC, Ringeard M, Chelbi-Alix MK, Hazan U, Nisole S. Implication of TRIM alpha and TRIMCyp in interferon-induced anti-retroviral restriction activities. Retrovirology. 2008;5:59. doi: 10.1186/1742-4690-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994;269:6320–6324. [PubMed] [Google Scholar]
- Watanabe M, Fukuda M, Yoshida M, Yanagida M, Nishida E. Involvement of CRM1, a nuclear export receptor, in mRNA export in mammalian cells and fission yeast. Genes Cells. 1999;4:291–297. doi: 10.1046/j.1365-2443.1999.00259.x. [DOI] [PubMed] [Google Scholar]
- Wolff B, Sanglier JJ, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/S1074-5521(97)90257-X. [DOI] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/S0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Kuersten S, Ohno M, Mattaj IW. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 2001;11:497–503. doi: 10.1016/S0962-8924(01)02144-4. [DOI] [PubMed] [Google Scholar]
- Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Mohr D, Frey S, Fischer T, Guttler T, Gorlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO J. 2009;28:2541–2553. doi: 10.1038/emboj.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Baker RP, Bayliss R, Clayton L, Grant RP, Littlewood T, Matsuura Y. Molecular mechanism of translocation through nuclear pore complexes during nuclear protein import. FEBS Lett. 2001;498:145–149. doi: 10.1016/S0014-5793(01)02489-9. [DOI] [PubMed] [Google Scholar]
- Dabauvalle MC, Franke WW. Determination of the intracellular state of soluble macromolecules by gel filtration in vivo in the cytoplasm of amphibian oocytes. J Cell Biol. 1986;102:2006–2014. doi: 10.1083/jcb.102.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert SW, Lamb RA. Cell surface expression of glycosylated, nonglycosylated, and truncated forms of a cytoplasmic protein pyruvate kinase. J Cell Biol. 1988;107:865–876. doi: 10.1083/jcb.107.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brameier M, Krings A, MacCallum RM. NucPred--predicting nuclear localization of proteins. Bioinformatics. 2007;23:1159–1160. doi: 10.1093/bioinformatics/btm066. [DOI] [PubMed] [Google Scholar]
- Nair R, Rost B. LOC3D: annotate sub-cellular localization for protein structures. Nucleic Acids Res. 2003;31:3337–3340. doi: 10.1093/nar/gkg514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour T, Kiemer L, Molgaard A, Gupta R, Skriver K, Brunak S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel. 2004;17:527–536. doi: 10.1093/protein/gzh062. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Perez O, Melar M, Hope TJ. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology. 2007;360:286–293. doi: 10.1016/j.virol.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Li X, Javanbakht H, Song B, Welikala S, Stremlau M, Sodroski J. Rapid turnover and polyubiquitylation of the retroviral restriction factor TRIM5. Virology. 2006;349:300–315. doi: 10.1016/j.virol.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Song B, Diaz-Griffero F, Park DH, Rogers T, Stremlau M, Sodroski J. TRIM5alpha association with cytoplasmic bodies is not required for antiretroviral activity. Virology. 2005;343:201–211. doi: 10.1016/j.virol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Reszka N, Zhou C, Song B, Sodroski JG, Knipe DM. Simian TRIM5alpha proteins reduce replication of herpes simplex virus. Virology. 2010;398:243–250. doi: 10.1016/j.virol.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Griffero F, Hoschander SA, Brojatsch J. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J Virol. 2002;76:12866–12876. doi: 10.1128/JVI.76.24.12866-12876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]