Abstract
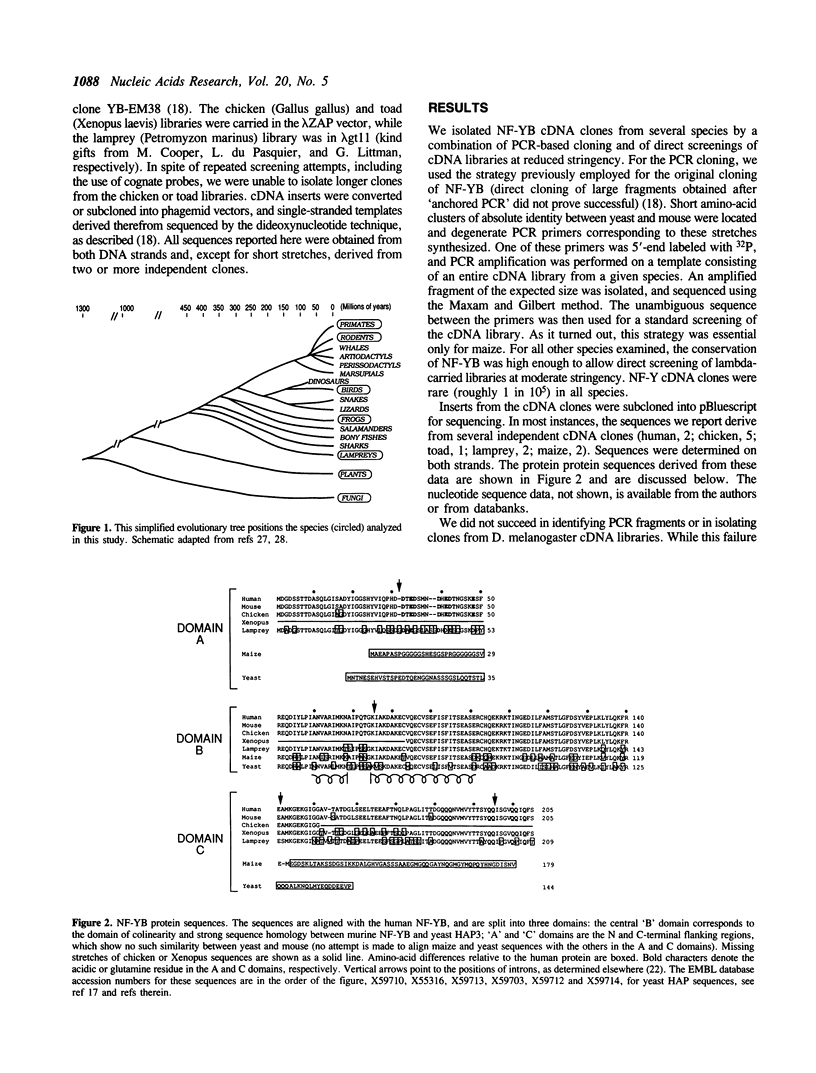
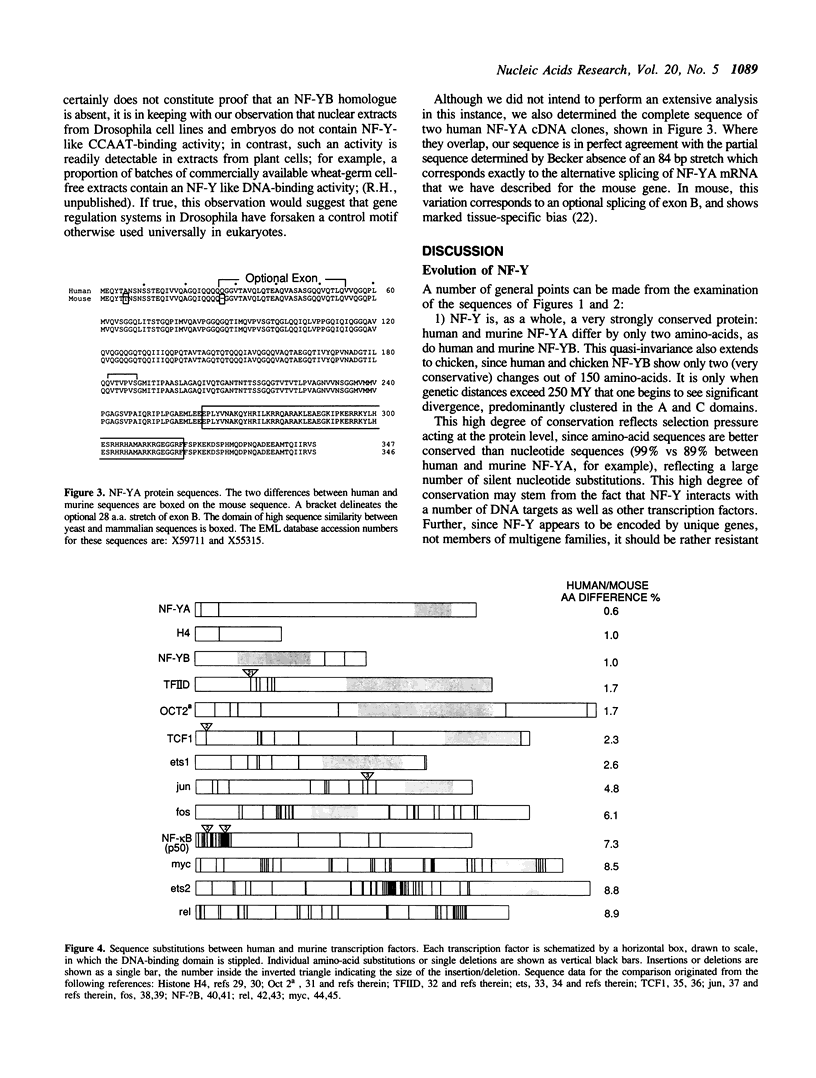
NF-Y is a CCAAT-specific transcription factor thought to be involved in the regulation of a variety of eukaryotic genes. It shows a striking sequence similarity with the yeast factor HAP2/3. In an attempt to trace back its evolutionary history, we succeeded in isolating NF-Y cDNA clones from a plant and from several species of vertebrates. The patterns of sequence conservation delineate potential functional domains: A central, highly conserved, domain likely responsible for DNA-binding and subunit interaction; more evolutionarily flexible flanking regions, in which variability is clustered, individualizing conserved glutamine or acidic amino-acids putatively involved in protein-protein contacts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Fikes J. D., Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Olesen J., Hahn S., Baldwin A. S., Guarente L., Sharp P. A. A yeast and a human CCAAT-binding protein have heterologous subunits that are functionally interchangeable. Cell. 1988 Apr 8;53(1):25–35. doi: 10.1016/0092-8674(88)90484-9. [DOI] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Grumont R. J., Gerondakis S. Structure of a mammalian c-rel protein deduced from the nucleotide sequence of murine cDNA clones. Oncogene Res. 1989;4(1):1–8. [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Hahn S., Guarente L. Yeast HAP2 and HAP3: transcriptional activators in a heteromeric complex. Science. 1988 Apr 15;240(4850):317–321. doi: 10.1126/science.2832951. [DOI] [PubMed] [Google Scholar]
- Hatamochi A., Golumbek P. T., Van Schaftingen E., de Crombrugghe B. A CCAAT DNA binding factor consisting of two different components that are both required for DNA binding. J Biol Chem. 1988 Apr 25;263(12):5940–5947. [PubMed] [Google Scholar]
- Hatzopoulos A. K., Stoykova A. S., Erselius J. R., Goulding M., Neuman T., Gruss P. Structure and expression of the mouse Oct2a and Oct2b, two differentially spliced products of the same gene. Development. 1990 Jun;109(2):349–362. doi: 10.1242/dev.109.2.349. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R. A., Bollekens J., Dorn A., Benoist C., Mathis D. Properties of a CCAAT box-binding protein. Nucleic Acids Res. 1987 Sep 25;15(18):7265–7282. doi: 10.1093/nar/15.18.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen R., Li X. Y., Black D., Matthes H., Benoist C., Mathis D. Co-evolution from yeast to mouse: cDNA cloning of the two NF-Y (CP-1/CBF) subunits. EMBO J. 1990 Oct;9(10):3119–3127. doi: 10.1002/j.1460-2075.1990.tb07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Kim C. G., Sheffery M. Physical characterization of the purified CCAAT transcription factor, alpha-CP1. J Biol Chem. 1990 Aug 5;265(22):13362–13369. [PubMed] [Google Scholar]
- Knight G. B., Gudas J. M., Pardee A. B. Cell-cycle-specific interaction of nuclear DNA-binding proteins with a CCAAT sequence from the human thymidine kinase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8350–8354. doi: 10.1073/pnas.84.23.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity S. N., Vuorio T., de Crombrugghe B. The B subunit of a rat heteromeric CCAAT-binding transcription factor shows a striking sequence identity with the yeast Hap2 transcription factor. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5378–5382. doi: 10.1073/pnas.87.14.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P. J., Dayhoff M. D. Eukaryotes versus prokaryotes: an estimate of evolutionary distance. Science. 1970 Jun 19;168(3938):1469–1471. doi: 10.1126/science.168.3938.1469. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Oikarinen J., Hatamochi A., de Crombrugghe B. Separate binding sites for nuclear factor 1 and a CCAAT DNA binding factor in the mouse alpha 2(I) collagen promoter. J Biol Chem. 1987 Aug 15;262(23):11064–11070. [PubMed] [Google Scholar]
- Olesen J. T., Guarente L. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 1990 Oct;4(10):1714–1729. doi: 10.1101/gad.4.10.1714. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Quitschke W. W., Lin Z. Y., DePonti-Zilli L., Paterson B. M. The beta actin promoter. High levels of transcription depend upon a CCAAT binding factor. J Biol Chem. 1989 Jun 5;264(16):9539–9546. [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Birnstiel M. L. Structure and expression in L-cells of a cloned H4 histone gene of the mouse. J Mol Biol. 1981 Oct 5;151(4):607–625. doi: 10.1016/0022-2836(81)90426-5. [DOI] [PubMed] [Google Scholar]
- Sierra F., Stein G., Stein J. Structure and in vitro transcription of a human H4 histone gene. Nucleic Acids Res. 1983 Oct 25;11(20):7069–7086. doi: 10.1093/nar/11.20.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B. Transcriptional activation. Acid blobs and negative noodles. Nature. 1988 May 19;333(6170):210–212. doi: 10.1038/333210a0. [DOI] [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Fujino I., Aoyama A., Horikoshi M., Hoffmann A., Roeder R. G., Muramatsu M., Mikoshiba K. Striking homology of the 'variable' N-terminal as well as the 'conserved core' domains of the mouse and human TATA-factors (TFIID). Nucleic Acids Res. 1991 Jul 25;19(14):3861–3865. doi: 10.1093/nar/19.14.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Vuorio T., Maity S. N., de Crombrugghe B. Purification and molecular cloning of the "A" chain of a rat heteromeric CCAAT-binding protein. Sequence identity with the yeast HAP3 transcription factor. J Biol Chem. 1990 Dec 25;265(36):22480–22486. [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin J., Mueller C., Schibler U. A ubiquitous CCAAT factor is required for efficient in vitro transcription from the mouse albumin promoter. J Mol Biol. 1990 Aug 20;214(4):865–874. doi: 10.1016/0022-2836(90)90341-I. [DOI] [PubMed] [Google Scholar]
- van Straaten F., Müller R., Curran T., Van Beveren C., Verma I. M. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen A. J., Massung R. F., Stein J. L., Stein G. S. Human H1 histone gene promoter CCAAT box binding protein HiNF-B is a mosaic factor. Biochemistry. 1988 Aug 23;27(17):6534–6541. doi: 10.1021/bi00417a051. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oosterwegel M., Dooijes D., Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991 Jan;10(1):123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


