Summary
Secreted Hedgehog (Hh) ligands signal through the canonical receptor Patched (Ptch1). However, recent studies implicate three additional Hh-binding, cell surface proteins, Gas1, Cdo and Boc, as putative co-receptors for Hh ligands. A central question is to what degree these co-receptors function similarly and their collective requirement in Hh signal transduction. Here we provide evidence that Gas1, Cdo, and Boc, play overlapping and essential roles during Hh-mediated ventral neural patterning of the mammalian neural tube. Specifically, we demonstrate two important roles for these molecules: an early role in cell fate specification of multiple neural progenitors, and a later role in motor neuron progenitor maintenance. Most strikingly, genetic loss-of-function experiments indicate an obligatory requirement for Gas1, Cdo and Boc in Hh pathway activity in multiple tissues.
Keywords: Mouse, Hedgehog, Neural Tube, Development, Gas1, Cdo, Boc, motor neuron
Introduction
Hh signaling is essential for the patterning of both invertebrate and vertebrate embryos (McMahon et al., 2003). In mammals, Sonic hedgehog (Shh) signaling regulates numerous developmental processes, including craniofacial development, digit specification in the limb, and patterning of the ventral neural tube (McMahon et al., 2003). Biochemical and genetic studies performed over a decade ago identified the twelve-pass transmembrane protein Patched 1 (Ptch1) as the canonical Hh receptor (Marigo et al., 1996; Stone et al., 1996). Several additional Hh ligand-binding proteins have been identified that modulate Hh pathway activity at the cell surface. These include the Hh antagonist Hh interacting protein (Hhip1; Chuang and McMahon, 1999), and three additional Hh-binding proteins, Growth arrest-specific 1 (Gas1), CAM-related/down-regulated by oncogenes (Cdo) and Brother of Cdo (Boc; Allen et al., 2007; Martinelli and Fan, 2007a; Tenzen et al., 2006; Yao et al., 2006; Zhang et al., 2006). Cdo and Boc are structurally related and conserved from Drosophila to mouse, whereas Gas1 is a distinct, vertebrate-specific Hh pathway component. Despite strong evidence that Gas, Cdo and Boc promote Hh signaling, the general requirement for their action remains to be determined.
Cdo and Boc are cell surface integral membrane proteins that have an extracellular domain comprised of a series of immunglobulin and fibronectin-like repeats; structural analyses have identified the fibronectin repeats as critical for Shh binding (McLellan et al., 2006; McLellan et al., 2008; Tenzen et al., 2006; Yao et al., 2006). Initial studies in Drosophila identified a role for ihog, a Drosophila homolog of Cdo and Boc in Hh signaling (Lum et al., 2003), while more recent studies indicate that ihog, together with boi (a second Cdo and Boc homolog) are essential for transducing the Hh signal in the developing wing imaginal disc (Camp et al., 2010; Zheng et al., 2010). In mice, Cdo mutant embryos display microform holoprosencephaly, a defect commonly associated with mutations in the Shh pathway (Cole and Krauss, 2003), while Boc mutants are defective in Shh-dependent commissural axon guidance (Okada et al., 2006). Gas1 is a GPI-anchored Hh-binding protein whose extracellular domain shares homology to GDNF receptors (Cabrera et al., 2006; Schueler-Furman et al., 2006; Stebel et al., 2000). Gas1 functions in Shh signaling in multiple tissues during embryogenesis (Allen et al., 2007; Lee et al., 2001; Martinelli and Fan, 2007a). Strikingly, mice deficient in both Gas1 and Cdo display severe patterning defects in Shh-dependent processes (Allen et al., 2007), suggesting that these molecules may cooperate in the promotion of Shh signaling during embryonic development.
Given the combined genetic data in flies and mice, we sought to assess the relative contribution of Gas1, Cdo and Boc to vertebrate Hh signal transduction during embryogenesis. Specifically, we examined Hh-dependent neural patterning in mice lacking Gas1, Cdo and Boc function individually, or in combination. We find that while removal of any one component individually has either no effect, or relatively mild effects on Shh-dependent neural patterning, removal of any two Hh pathway components severely affects both neural progenitor specification and subsequent maintenance of motor neuron progenitors. In contrast to Drososphila, Cdo and Boc alone are not essential for Shh signal transduction. Genetic removal of both Cdo and Boc activity abrogates Shh signaling in the developing neural tube; however, the loss of these components does not affect signaling in other Hh-responsive tissues, including the developing limb. Double mutant analyses indicate that in the limb, Gas1 and Boc, but not Cdo are required for an appropriate Hh response during digit specification. Strikingly, removal of all three Hh-binding proteins results in a near complete loss of Hh signaling, including a complete failure of ventral neural tube patterning, defective heart looping and early embryonic lethality, similar to Smo−/− embryos. Overall, these data support a model in which Gas1, Cdo and Boc function as essential Hh co-receptors in vertebrate Hh signal transduction. Further, our findings provide additional insights into the dynamic requirement for Shh signaling in neural patterning.
Results
Gas1, Cdo and Boc all promote ectopic Shh signaling during neural patterning
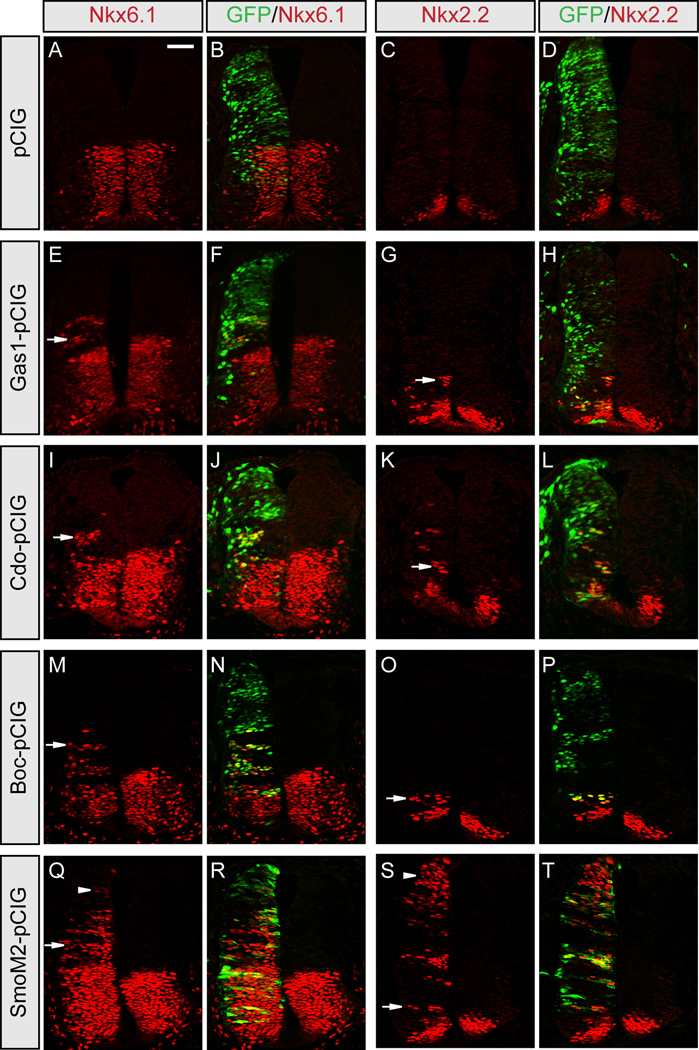
Previous studies suggested that Gas1, Cdo and Boc promote Shh signaling during neural patterning (Allen et al., 2007; Martinelli and Fan, 2007a; Tenzen et al., 2006). To confirm these data and directly compare the ability of Gas1, Cdo and Boc to activate Shh signaling, we performed overexpression studies in the developing chick neural tube (Figure 1). Ectopic expression of GFP alone has no effect on ventral neural patterning (Figures 1A–1D), while expression of Gas1 (Figures 1E–1H), Cdo (Figures 1I–1L) or Boc (Figures 1M–1P) all promote ectopic Shh-dependent ventral neural patterning in a cell-autonomous manner, including ectopic specification of the class-II transcription factor Nkx6.1 (Figures 1E–1F, 1I–1J, 1M–1N, and 1Q–1R), which requires low-level Hh signaling, and the V3 interneuron progenitor (pV3) marker Nkx2.2 (Figures 1G–1H, 1K–1L, 1O–1P, and 1S–1T), which requires high-level Hh signaling (Briscoe and Ericson, 2001). Notably, despite strong ectopic expression of Gas1, Cdo and Boc in the dorsal neural tube, ectopic Nkx6.1 (Figures 1F, 1J, and 1N) and Nkx2.2 (Figures 1H, 1L, and 1P) are spatially restricted either to the ventral neural tube, or the dorsal-ventral intersect in embryos expressing these constructs. In contrast, expression of a constitutively active Smo construct (SmoM2; Xie et al., 1998) that activates Hh signaling independent of ligand results in ectopic expression of Nkx6.1 and Nkx2.2 even in dorsal positions where Shh signaling is absent (Figures 1Q–1T, arrowheads). Taken together, these data suggest that Gas1, Cdo and Boc all share a similar ability to promote Shh signaling in a ligand-dependent manner.
Figure 1. Gas1, Cdo and Boc equally promote Shh-dependent specification of ventral neural progenitors.
HH stage 21–22 chick neural tubes electroporated with pCIG (A–D), Gas1-pCIG (E–H), Cdo-pCIG (I–L), Boc-pCIG (M–P), or SmoM2-pCIG (Q–T) were sectioned at the forelimb level and stained with antibodies raised against Nkx6.1 (red; A, E, I, M, Q) and Nkx2.2 (red; C, G, K, O, S). GFP expressing cells (green; B, F, J, N, R and D, H, L, P, T) indicate electroporated cells on one side, while the un-electroporated half of the neural tube serves as an internal negative control. Arrows denote ectopic expression of Nkx6.1 (E, I, M, Q) and Nkx2.2 (G, K, O, S). Arrowheads indicate ectopic Nkx6.1 and Nkx2.2 expression throughout the dorsal neural tube in embryos expressing SmoM2 (Q–T), whereas ectopic induction of these markers following Gas1 (E–H), Cdo (I–L) and Boc (M–P) electroporation was restricted to the ventral neural tube. Scale bar: A, 50µm.
Normal neural patterning in Boc−/− embryos
Given the functional equivalence of Gas1, Cdo and Boc in the promotion of Shh signaling in ovo, and previous work demonstrating a role for Gas1 and Cdo action in neural patterning in vivo (Allen et al., 2007), we examined whether genetic removal of Boc function also alters Shh-dependent specification of ventral neural progenitors. Surprisingly, Boc−/− mice are viable and fertile, with no overt defects in Hh-dependent tissues (data not shown), as reported for a different null mutant (Okada et al., 2006). Detailed examination of neural patterning in these mice at embryonic day 10.5 (E10.5) revealed no differences in Shh expression (Figures S1A, 1D, and 1G), or that of distinct targets linked to low (Nkx6.1 and Dbx1; Figures S1B, 1E, and 1H), moderate (Olig2, which denotes motor neuron progenitors; pMNs), or high (Nkx2.2 and the floor plate marker FoxA2; Figures S1C, 1F, and 1I) levels of Shh signaling. Earlier analysis at E8.5-E9.5 also failed to reveal any difference in Shh patterning between Boc−/−embryos and wild type (wt) embryos (data not shown).
Cdo−/−; Boc−/− double mutants display severe and unusual neural patterning defects
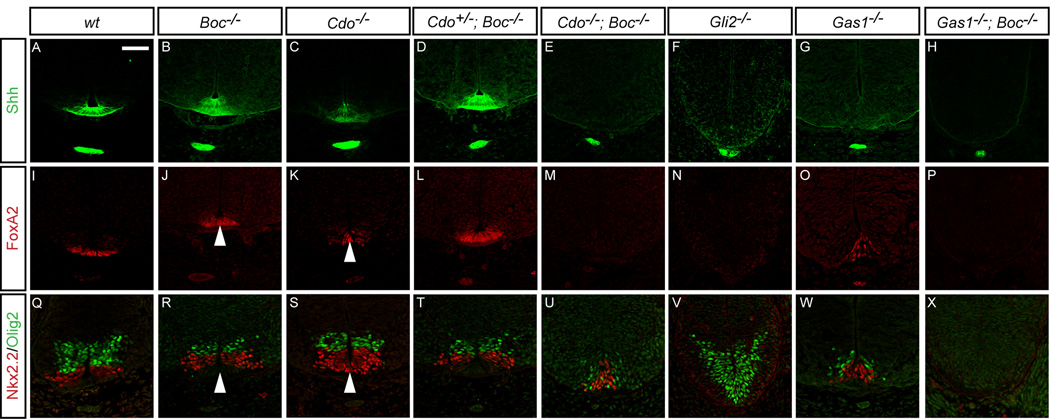
One explanation for the lack of a phenotype in Boc−/− mice is that the structurally and functionally similar protein Cdo compensates for the loss of Boc in the embryo. To test this, we examined neural patterning in Cdo−/−; Boc−/− double mutant embryos (Figure 2). Cdo−/− mutants display floor plate patterning defects at E10.5 (Tenzen et al., 2006), compared to wt embryos (Figures 2A, 2I, and 2Q), including reduced numbers of FoxA2+ cells, and increased expression of FoxA2 and Nkx2.2 double positive cells (Figures 2C, 2K, and 2S, arrowheads). Similarly to Boc−/− embryos (Figures 2B, 2J, and 2R), Cdo+/−; Boc−/− embryos appear phenotypically normal (Figures 2D, 2L, and 2T). Importantly, Cdo+/−; Boc−/− embryos are also viable and fertile, with no obvious defects (data not shown). In contrast, Cdo−/−; Boc−/− embryos display severe patterning defects, including the loss of Shh (Figure 2E), and FoxA2 (Figure 2M) in the floor plate. Additionally, Nkx2.2+ and Olig2+ populations were markedly reduced (Figure 2U), with Olig2+ pMNs more severely depleted than Nkx2.2+ V3 interneuron progenitors (pV3). Strikingly, this phenotype is almost identical that produced by reducing Shh levels in Gas1 mutant embryo (Gas1−/−; Shh+/−; Allen et al., 2007). Overall, these results suggest a significant, yet redundant role for Cdo and Boc function during neural patterning; however, unlike Drosophila (Camp et al., 2010; Zheng et al., 2010), genetic removal of Cdo and Boc in mice does not result in complete loss of Hh signaling, since some Shh-dependent neural progenitors are established in double mutant embryos (Figure 2U). Thus, the requirement for Cdo and Boc function in Hh signaling appears to be distinct from ihog and boi function in Drosophila.
Figure 2. Defective ventral neural patterning in E10.5Cdo−/−; Boc−/− and Gas1−/−; Boc−/− mouse embryos.
Immunofluorescent analysis of E10.5 forelimb level sections detects expression of Shh (green; A–H), FoxA2 (red; I–P), Nxk2.2 and Olig2 (red and green, respectively; Q–X) in wt (A, I, Q), Boc−/− (B, J, R), Cdo−/− (C, K, S), Cdo+/−; Boc−/− (D, L, T), Cdo−/−; Boc−/− (E, M, U), Gli2−/− (F, N, V), Gas1−/− (G, O, W), and Gas1−/−; Boc−/− (H, P, X) embryos. Arrowheads denote FoxA2 and Nkx2.2 double positive floorplate cells in Cdo−/− embryos (S), but not Boc−/− embryos (R). Despite the complete loss of FoxA2+ FP cells in both Cdo−/−; Boc−/−, and Gli2−/− embryos (M and N, respectively), there is a selective loss of Olig2+ but not Nkx2.2+ cells in Cdo−/−; Boc−/−embryos (U). In contrast, Gli2−/− embryos lack Nkx2.2+ cells, but maintain Olig2 expression (V). Gas1−/−; Boc−/− embryos display complete loss of FoxA2 (P), Nkx2.2 and Olig2 (X) at E10.5. Scale bar: A, 50µm. See Figure S1 for a more detailed analysis of neural patterning in Boc−/−embryos. Refer to Figure S2 for Gas1, Cdo and Boc protein distribution in E10.5 mouse neural tubes.
Gas1, Cdo and Boc are essential for Shh-dependent maintenance of motor neuron progenitors
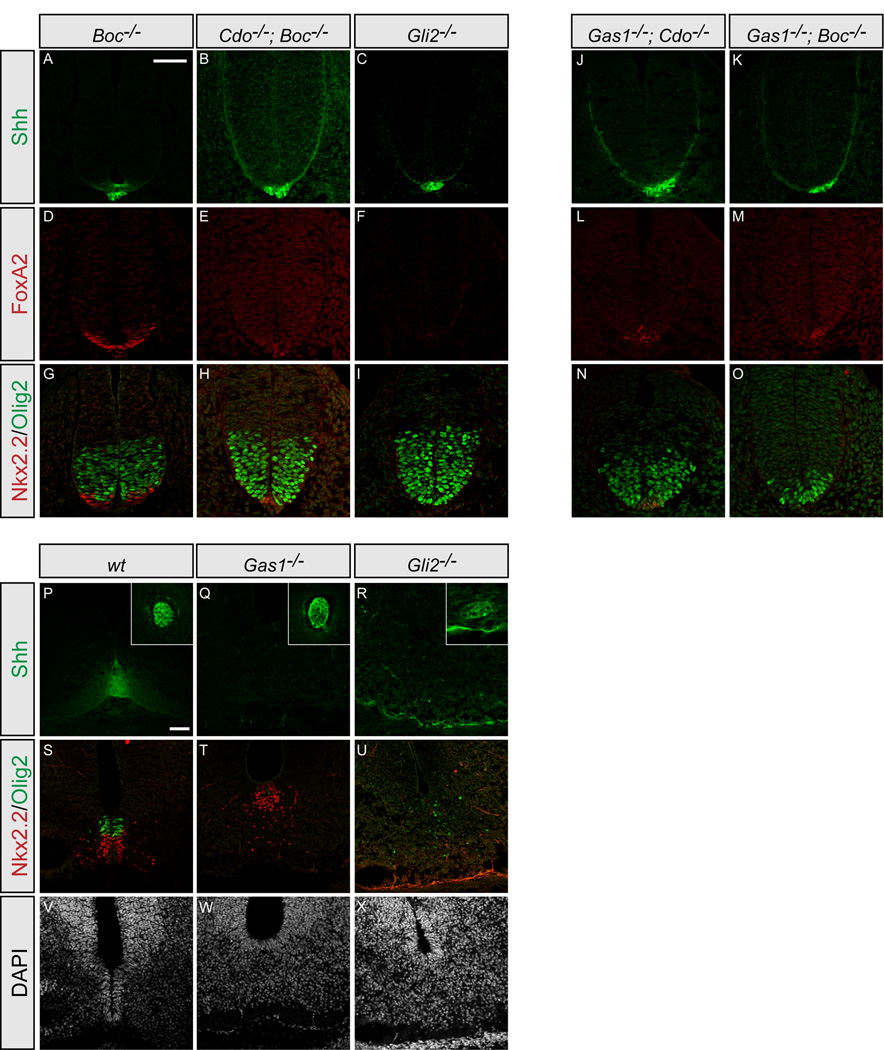
In vitro Shh patterning assays have clearly demonstrated a lower threshold requirement for Olig2+ versus Nkx2.2+ progenitor cell specification (Dessaud et al., 2007; Ericson et al., 1997). Therefore, the differential sensitivity of Olig2+ cells (Figure 2U) is quite surprising as this population is expected to be less affected by reduced Shh input. Since Cdo−/−; Boc−/− embryos lack Shh+ and FoxA2+ floor plate (Figures 2E and 2M), one possibility is that the lack of floor plate is sufficient to explain the phenotype of these mutants. However, at E10.5 Gli2−/− mutants that also lack floor plate (Figures 2F and 2N) still retain Olig2+ pMNs, but display a complete loss of Nkx2.2+ pV3 progenitors (Figure 2V). Thus, the phenotype of Cdo−/−; Boc−/− embryos cannot be explained by the loss of floor plate expression of Shh alone and is not reconciled with a simple concentration dependent patterning model. A second possibility is that Cdo and Boc double mutant phenotypes reveal changing roles for Shh signaling in the developing neural tube. To test this hypothesis, we examined neural patterning a day earlier in Cdo−/−; Boc−/− embryos.
Analysis of Cdo−/−; Boc−/− embryos at E9.5 reveals several intriguing results (Figure 3). Compared to Boc−/− embryos (Figures 3A, 3D, and 3G), where neural patterning is normal, Cdo−/−; Boc−/− double mutants resemble Gli2−/− mutants and lack expression of Shh or FoxA2 in the presumptive floor plate (Figures 3B–3C and 3E–3F). Surprisingly, although Nkx2.2+ pV3 progenitors are reduced in Cdo−/−; Boc−/− embryos as observed at E10.5, Olig2+ pMN progenitor specification appears quite normal (Figure 3H). Thus Cdo and Boc are required at early stages to promote Shh-dependent specification of Nkx2.2+ pV3 progenitors, and at later stages to mediate Shh-dependent maintenance of Olig2+ pMN progenitors after their initial specification. Importantly, these data are consistent with previously published analyses suggesting an ongoing role for Shh signaling in motor neuron progenitor maintenance (Allen et al., 2007; Dessaud et al., 2010; Ericson et al., 1996).
Figure 3. Motor neuron progenitors are specified, but not maintained in embryos with reduced Shh signaling.
Antibody detection of Shh (green; A–C, J–K), FoxA2 (red; D–F, L–M), Nkx2.2 and Olig2 (red and green, respectively; G–I, N–O) in forelimb level sections of E9.5 Boc−/− (A, D, G), Cdo−/−; Boc−/− (B, E, H), Gli2−/− (C, F, I), Gas1−/−; Cdo−/− (J, L, N), and Gas1−/−; Boc−/−(K, M, O) embryos. At E9.5 Cdo−/−; Boc−/− embryos contain similar numbers of Olig2+ progenitors to Boc−/− or Gli2−/− embryos (G and I, respectively) in marked contrast to E10.5 embryos. Note the variable presence of a few FoxA2+ and Nkx2.2+ cells in Cdo−/−; Boc−/−, Gas1−/−; Cdo−/− and Gas1−/−; Boc−/− embryos at the forelimb level (E, H, L–O). Neither cell type appears to be specified in Gli2−/− embryos (F, I). Immunofluorescent detection of Shh (green; P–R), Nkx2.2 and Olig2 (red and green, respectively; S–U) in forelimb level sections of E12.5 wt (P, S, V), Gas1−/− (Q, T, W), and Gli2−/− (R, U, X) embryos. Nuclei are identified with DAPI (G–I). Insets (A–C) indicate notochord expression of Shh. Note that in Gas1−/− embryos (E), only a few Olig2+ cells are present, while the number of Nkx2.2+ cells is comparable to wt (D). Olig2 + cells are reduced and fail to cluster normally in Gli2−/− embryos (F). Scale bar: A,P, 50µm.
Since previous work also indicated a potential role for Gas1 in motor neuron maintenance, and that Gas1 and Cdo cooperate to promote Shh-dependent neural patterning (Allen et al., 2007), we generated Gas1−/−; Boc−/− mice to determine whether Gas1 and Boc might also cooperate in these processes (Figure 2). Gas1, Cdo and Boc proteins are expressed in largely overlapping domains in the neural tube of E10.5 mouse embryos (Figures S2A, 2C, and 2I), although Cdo protein is also detected in the notochord and floorplate (Figure S2C, arrow and asterisk). In agreement with previous data (Allen et al., 2007), and similar to Cdo−/−; Boc−/−embryos (Figures 2E, M, U), Gas1−/− embryos display a loss of Shh+ floor plate (Figure 2G), and co-expression of Nkx2.2 and FoxA2 (Figures 2O and 2W) in E10.5 Gas1−/− embryos. Additionally, the number of Olig2+ cells is also significantly reduced in these embryos (Figure 2W). As with Cdo+/−; Boc−/− mice, Gas1+/−; Boc−/− embryos are viable and fertile with no overt phenotype (data not shown). In contrast, Gas1−/−; Boc−/− embryos have a more severe phenotype (Figures 2H, 2P, and 2X); in addition to a loss of Shh (Figure 2H) and FoxA2 (Figure 2P), there is a complete absence of Nkx2.2+ and Olig2+ cells (Figure 2X) at E10.5, phenocopying the neural patterning deficiency of Gas1−/−; Cdo−/− embryos at this developmental stage (Allen et al., 2007).
Although Gas1−/−; Cdo−/− and Gas1−/−; Boc−/− embryos have no Nkx2.2+ or Olig2+ cells at E10.5, the phenotype could result from either a failure of initial specification of these progenitors or ongoing maintenance of specified cell types. To distinguish between these alternative explanations, we examined neural patterning at E9.5 in both Gas1−/−; Cdo−/− and Gas1−/−; Boc−/−embryos (Figure 3). As with Cdo−/−; Boc−/− embryos (Figure 3B), Gas1−/−; Cdo−/− and Gas1−/−; Boc−/−embryos do not express Shh in the presumptive floor plate (Figures 3J and 3K, respectively), though a few FoxA2+ cells (Figures 3L and 3M), and Nkx2.2+ cells (Figures 3N and 3O) are specified, indicating some initial Shh input, but insufficient signaling for continued ventral specification (cf. Figures 2H, 2P, and 2X). Consistent with these results, examination of Olig2 expression at E9.5 indicates relatively normal numbers of Olig2+ cells in Gas1−/−; Cdo−/− embryos (Figure 3N), and a reduced, but significant number of Olig2+ pMN cells in Gas1−/−; Boc−/−embryos (Figure 3O). From these data we can conclude that the complete loss of Olig2+ cells in Gas1−/−; Cdo−/− and Gas1−/−; Boc−/− embryos at E10.5 is due to a failure to maintain these progenitors following specification. Further, removal of any two Shh-binding proteins (Cdo and Boc, Gas1 and Cdo, or Gas1 and Boc) results in severe patterning defects, but is not sufficient to abolish the Hh response at early developmental stages.
The significant reductions in the number of Olig2+ pMNs from E9.5 to E10.5 in Gas1, Cdo and Boc double mutant embryos raises the question of whether the requirement for Shh in motor neuron progenitor maintenance is restricted to the E9.5/E10.5 time window, or if this requirement extends throughout embryogenesis. To test this, we examined neural patterning at later developmental stages in Gas1−/− and Gli2−/− embryos (Figures 3P–3X). Olig2+ pMNs are present in both Gas1−/− and Gli2−/− embryos at E10.5 (Figures 2V and 2W). In wt embryos at E12.5, Shh is still strongly expressed in both the notochord and FP (Figure 3P). In contrast, in both Gas1−/− and Gli2−/− embryos, Shh is only present in the notochord (Figures 3Q and 3R). Compared with wt embryos (Figure 3S), which still display significant numbers of Olig2+ cells, examination of Gas1−/− embryos reveals that Nkx2.2+ pV3 progenitors are maintained, while only a few Olig2+pMN progenitors remain at E12.5 (Figure 3T). Similarly, reduced numbers of Olig2+ pMNs are also observed in Gli2−/− embryos (Figure 3U); the lack of Nkx2.2+ pV3 progenitors in Gli2−/− embryos is due to the earlier failure to initially specify these cells (cf. Figure 2V and Figure 3I; Matise et al., 1998; Mo et al., 1997). Overall, these data suggest that the requirement for Shh signaling in motor neuron progenitor maintenance extends for at least several days during embryogenesis, and that Gas1, Cdo and Boc all play a role in this process.
Late stage inhibition of Shh signaling during neural patterning specifically affects motor neuron, but not pV3 progenitor cell maintenance
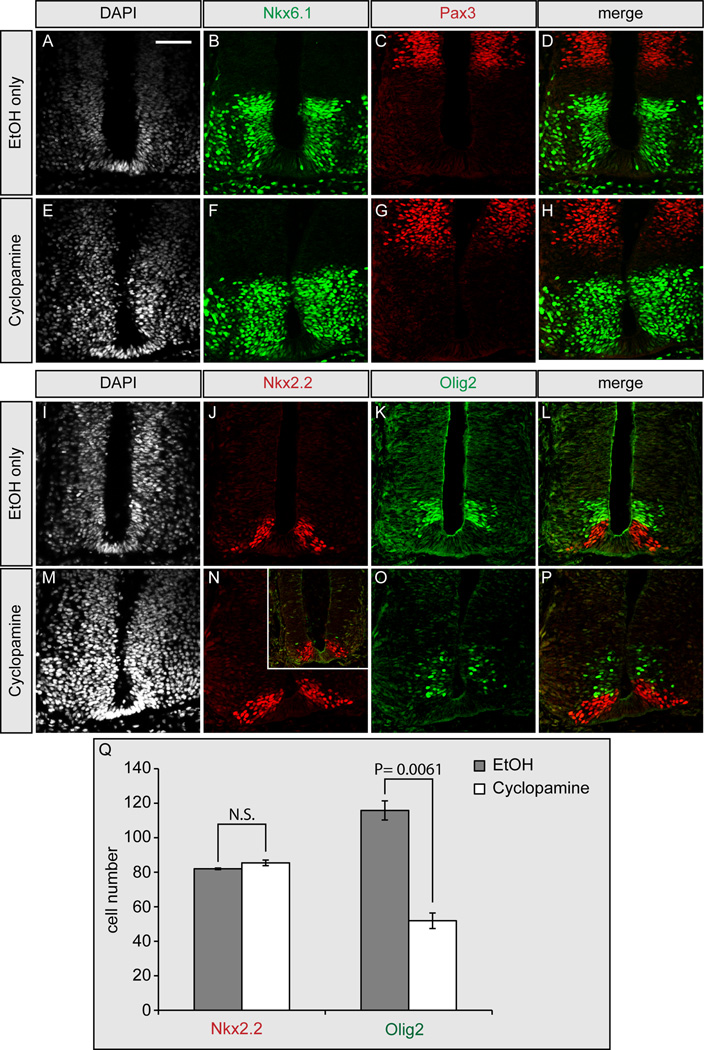
Despite strong evidence from Gas1, Cdo and Boc double mutant analyses that there is a selective effect of prolonged Shh signaling on motor neuron maintenance, an indirect effect of Gas1, Cdo and Boc on other pathways that regulate motor neuron progenitors cannot be ruled out. To directly test a distinct requirement for ongoing Shh signaling in the maintenance of motor neuron progenitors, we examined the effects of blocking Hh signaling after the initial specification of neural progenitors in the developing chick neural tube, using the selective Hh pathway inhibitor, cyclopamine (Cooper et al., 1998). Cyclopamine treatment of developing chick embryos at early embryonic stages (i.e. Hamburger-Hamilton [HH] stage 10–11) effectively blocks Hh-dependent neuronal specification (Incardona et al., 1998). We examined inhibition of Hh signaling at later embryonic stages (Figure 4) through cyclopamine administration at HH stage 17–18, a developmental stage where initial patterning events, including specification of Olig2+ cells has already occured (Novitch et al., 2001). Vehicle (ethanol) treated embryos display normal activation of the low level Hh target Nkx6.1 and repression of Pax 3 (Figures 4A–4D), as do cyclopamine treated embryos (Figures 4E–4H). Additionally, specification of the high level target, Nkx2.2, is unaffected in ethanol (Figure 4J) and cyclopamine treated embryos (Figure 4N). Strikingly, however, the number of Olig2+ pMN cells is significantly reduced in cyclopamine (Figure 4O), but not ethanol treated embryos (Figure 4K). In fact, some cyclopamine treated embryos display near total loss of Olig2+ cells (Figure 4N, inset), with no significant affect on Nkx2.2 cell number. Quantitation of less severely affected embryos (Figure 4Q) reveals a highly significant loss of Olig2+ pMN progenitors in late stage cyclopamine-treated embryos, confirming an ongoing and specific role for Shh in the maintenance of motor neuron progenitors that is conserved across species. Importantly, identical results are obtained following treatment with a second Hh pathway antagonist, SANT-1 (Figure S3), and these data are corroborated by recent studies in chick and mouse (Dessaud et al., 2010).
Figure 4. Delayed cyclopamine administration selectively affects motor neuron maintenance in HH stage 22 chick embryos.
Cyclopamine (E–H, M–P) or ethanol (A–D, I–L) were administered to HH stage 17–18 chick embryos for 24 hours, followed by immunofluorescent detection of nuclei (DAPI; A, E, I, M), Nkx6.1 (green; B, D, F, H), Pax3 (red; C, D, G, H), Nkx2.2 (red; J, L, N, P), and Olig2 (green; K, L, O, P). Note the normal maintenance of Nkx6.1, Pax3, and Nkx2.2 in cyclopamine-treated embryos (F–H, N–P). In contrast, there is a significant reduction in the number of Olig2+ cells following cyclopamine administration (N–P). The number of Olig2 cells varies from moderate (O, P) to severe (inset, N). Comparison of Nkx2.2 and Olig2 cell numbers in EtOH-treated embryos and moderately affected cyclopamine-treated embryos is quantitated in Q. Error bars represent the mean +/− SD calculated from analysis of sections from three different embryos. P-values calculated by two-tailed Student’s t-test are listed. NS, not significant. Scale bar: A, 50µm. Refer to Figure S3 for a similar analysis with a second Hh pathway antagonist, SANT-1.
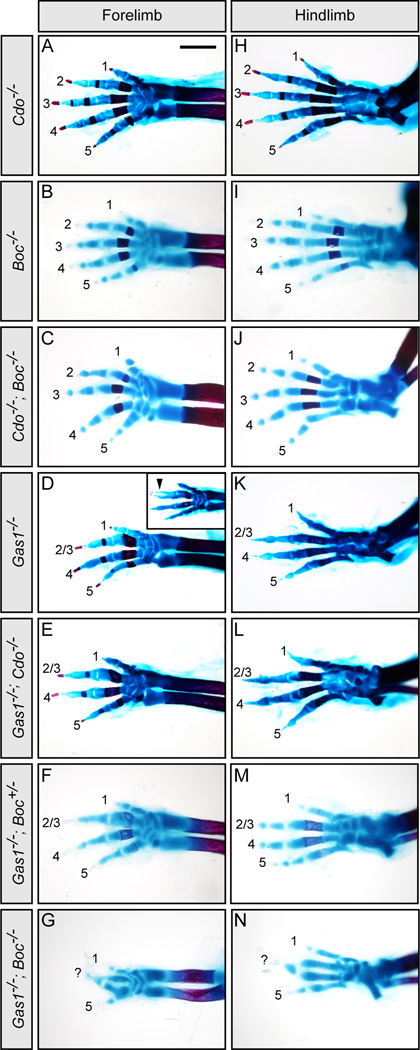
Gas1 and Boc, but not Cdo affect Shh-dependent digit specification during limb patterning
To determine whether Gas1, Cdo and Boc play equally important roles in other Hh-responsive tissues, we examined Shh-dependent digit specification in the developing limb at E18.5. Gas1, Cdo and Boc display overlapping expression in the anterior two-thirds of the forelimb bud in E10.5 mouse embryos (Figures S4B–S4D; Tenzen et al., 2006). Although digit specification is overtly normal in Cdo−/− (Figures 5A and 5H), Boc−/− (Figures 5B and 5I), and Cdo−/−; Boc−/− E18.5 embryos (Figures 5C and 5J), examination of limb patterning in Gas1−/−; Boc−/− embryos reveals an unexpected phenotype. As previously reported (Allen et al., 2007; Martinelli and Fan, 2007a), Gas1−/− embryos lack digit 2 or 3 in both the forelimb and hindlimb (Figures 5D and 5K); an identical phenotype is seen in Gas1−/−; Cdo−/− embryos (Figures 5E and 5L). However, while Gas1−/−; Boc+/− embryos appear identical to Gas1−/− mutants (Figures 5F and 5M), Gas1−/−; Boc−/− embryos (Figures 5G and 5N) display a more severe defect in digit patterning; these embryos not only appear to lack digit 2 but also have an apparent fusion of digits 3 and 4 in both the forelimb and hindlimb, and a digit pattern where anterior and posterior halves exhibit a near mirror image symmetry most notably in the forelimb. These digit specification and digit patterning defects correlate with decreased expression of the Hh pathway targets Ptch1 and Gli1 in the forelimbs of E11.5 Gas1−/−; Boc−/− embryos (Figures S4H–S4M). Significantly, there are no overt effects on Shh transcript levels in Gas1−/−; Boc−/− double mutants (Figures S4E–S4G). Overall, these results indicate that Gas1 and Boc play a major role in Shh-dependent organization of the mammalian limb.
Figure 5. Analysis of Cdo−/−; Boc−/− and Gas1−/−; Boc−/− limb development.
Forelimbs (A–G) and hindlimbs (H–N) of E18.5 embryos stained with alcian blue and alizarin red to visualize cartilage and bone, respectively, in the limb skeleton. Numbers denote specific digits (1 most anterior, and 5 most posterior). Cdo−/− (A, H), Boc−/− (B, I) and Cdo−/−; Boc−/− (C, J) embryos display normal digit patterning. In contrast, Gas1−/− embryos display fusion and loss of digits 2/3 (D, K). A similar phenotype is seen in Gas1−/−; Cdo−/− (E, L) and Gas1−/−; Boc+/− (F, M). In contrast, Gas1−/−; Boc−/− embryos display a significantly more severe digit patterning defect; only digits 1 and 5 are identifiable in both the forelimb (G) and hindlimb (N), and a third digit (labeled as “?”), possibly a fusion of digits 3 and 4, is at the anterior-posterior intersect. Scale bar: A, 1mm. Gas1, Cdo and Boc expression in the developing limb is provided in Figure S4, along with analysis of Shh, Ptch1, and Gli1 transcript levels in E10.5 Gas1−/−; Boc−/− forelimb buds.
Gas1, Cdo and Boc are essential co-receptors for Shh signaling during neural patterning
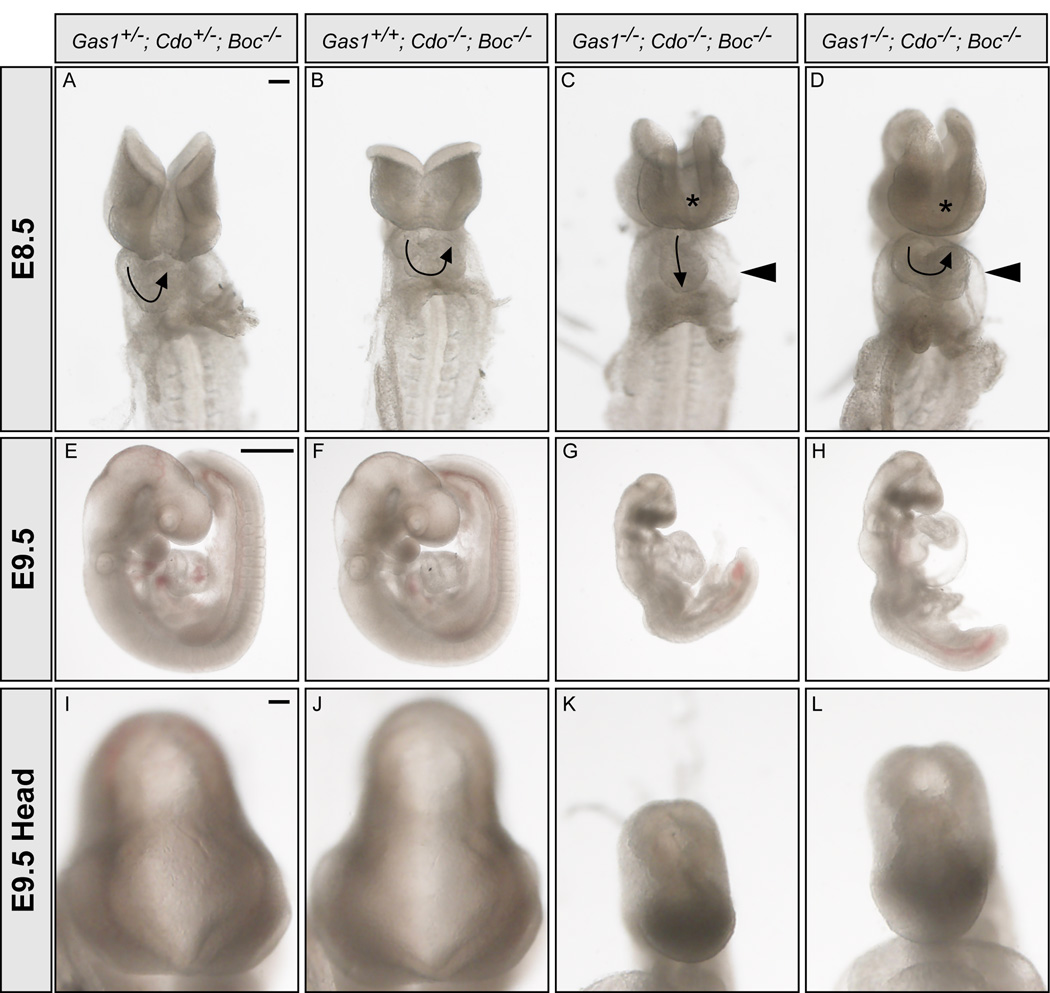
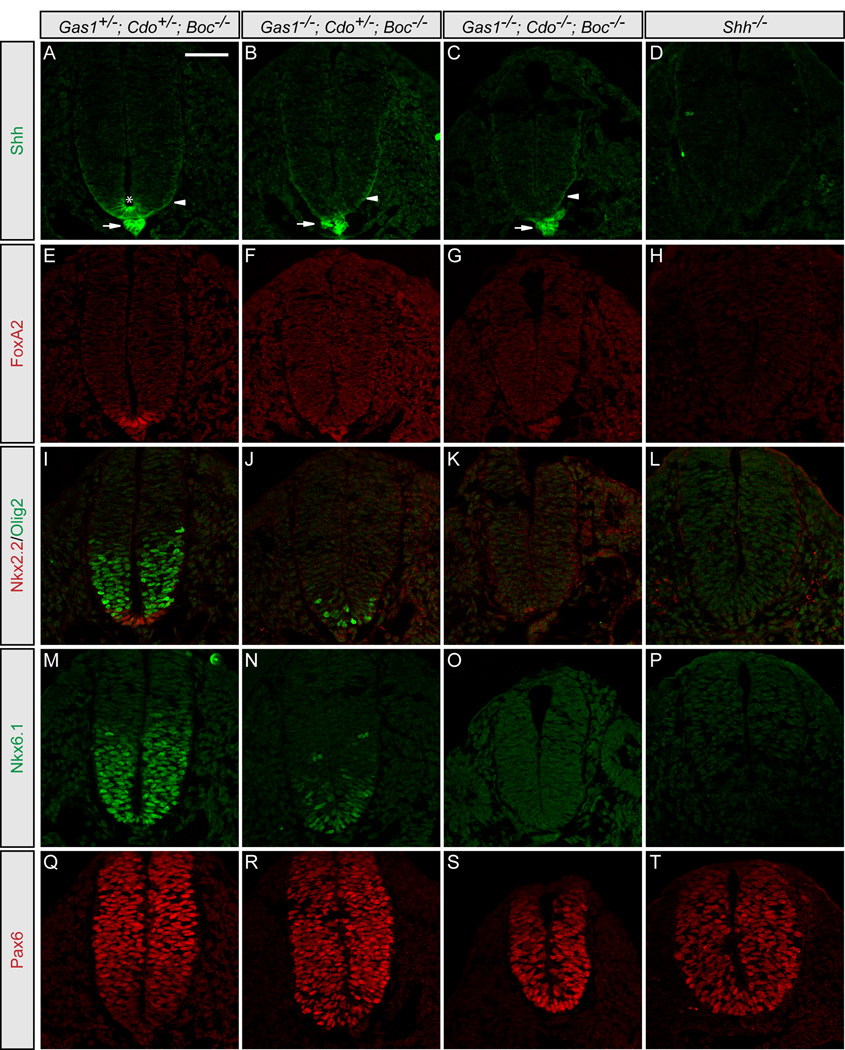
The striking neural patterning defects observed in Cdo−/−; Boc−/−, Gas1−/−; Cdo−/− and Gas1−/−; Boc−/− embryos suggest that all three cell surface Hh pathway components play a role in mediating the Hh response during neural patterning. Importantly, however, Shh-dependent patterning still occurs to some extent in these embryos (Figure 3). One possibility is that Gas1, Cdo and Boc act as necessary, but partially redundant co-factors to promote Shh signaling. To test this model, we generated mice lacking all Gas1, Cdo and Boc activity. Importantly, Gas1+/−; Cdo+/−; Boc−/− mice are viable and fertile with no overt phenotype (data not shown). Embryos with only one allele of either Cdo or Gas1 (Gas1−/−; Cdo+/−; Boc−/− or Gas1+/−; Cdo−/−; Boc−/−) survive to birth and resemble double mutants (data not shown). In contrast, Gas1−/−; Cdo−/−; Boc−/−embryos die by E9.5 (Figure 6), and display severe forebrain and cardiovascular defects at E8.5 (Figures 6C and 6D), including heart looping defects and pericardial edema. Triple mutant embryos fail to complete the turning process (Figures 6G and 6H), and additionally display cyclopia, and holoprosencephaly (Figures 6K and 6L), similar to Shh−/−; Ihh−/− double mutant embryos and Smo−/− embryos (Zhang et al., 2001). Consistent with the gross morphological defects of these embryos, detailed examination of E9.5 Gas1−/−; Cdo−/−; Boc−/− neural tubes reveals severe defects in ventral cell fate specification (Figure 7). Shh protein is expressed in the notochord of E9.5 Gas1−/−; Cdo−/−; Boc−/− embryos (Figure 7C), and secreted Shh protein can be detected in the lateral neural tube (Figure 7C, arrowhead). However, within the neural tube no Shh, FoxA2, Nkx2.2, Olig2, or Nkx6.1 are present (Figures 7C, 7G, 7K, and 7O), and Pax6, normally repressed by Shh signaling, extends to the ventral limit of the neural tube (Figure 7S). Strikingly, the neural patterning defects observed in Gas1−/−; Cdo−/−; Boc−/− embryos phenocopy Shh−/− embryos (Figures 7D, 7H, 7L, 7P, and 7T). Overall, the data presented here suggest that Gas1, Cdo and Boc indeed function as essential co-receptors for Hh signaling in multiple Hh-responsive tissues.
Figure 6. Cyclopia, holoprosencephaly and heart looping defects in E8.5 and E9.5 Gas1−/−; Cdo−/−; Boc−/− embryosGas1+/−; Cdo+/−; Boc−/− (A, E, I), Gas1+/+; Cdo−/−; Boc−/− (B, F, J) and two Gas1−/−; Cdo−/−; Boc−/−.
(C–D, G–H, K–L) embryos are shown. En face images of E8.5 (10–12 somite) embryos (A–D). Arrows indicate the direction of heart looping, while arrowheads denote pericardial edema that is present in Gas1−/−; Cdo−/−; Boc−/− embryos (C, D). 50% of Gas1−/−; Cdo−/−; Boc−/− embryos display a linear heart tube (N = 4/8 embryos). Asterisks indicate abnormal forebrain development that is a hallmark of the failure to specify ventral midline cell fates. Examination of embryos of the same genotype at E9.5 (20–25 somites; E–H) demonstrates a failure to complete the turning process in Gas1−/−; Cdo−/−; Boc−/− embryos (G, H). Higher magnification views of the heads of E9.5 embryos (I–L) reveal holoprosencephaly in Gas1−/−; Cdo−/−; Boc−/− embryos (K, L). Scale bars: A, 100um; E, 500µm; I, 100µm.
Figure 7. Simultaneous genetic removal of Gas1, Cdo and Boc results in complete loss of Shh-dependent ventral cell specification.
Antibody detection of Shh (green; A–D), FoxA2 (red; E–H), Nkx2.2 and Olig2 (red and green, respectively; I–L), Nkx6.1 (green; M–P), and Pax6 (red; Q–T) in Gas1+/−; Cdo+/−; Boc−/− (A, E, I, M, Q), Gas1−/−; Cdo+/−; Boc−/− (B, F, J, N, R), Gas1−/−; Cdo/-; Boc−/− (C, G, K, O, S), and Shh−/− (D, H, L, P, T) E9.5 embryos. Arrows (A–C) indicate Shh expression in the notochord. Arrowheads (A–C) denote secreted Shh protein. Shh expression in the floorplate is detected only in Gas1+/−; Cdo+/−; Boc−/− embryos (asterisk in A). Scale bar: A, 50µm.
Discussion
Ongoing requirement for Shh signaling and the co-receptors Gas1, Cdo and Boc in motor neuron maintenance
The specification of Olig2+ pMN progenitors during neural patterning is a highly regulated and complex process that requires inputs from not only Shh (Roelink et al., 1994), but also Retinoic Acid (RA) and Fibroblast Growth Factors (FGFs; Novitch et al., 2003). Later, combinatorial Hox gene expression regulates the subsequent generation of motor neuron diversity along the rostro-caudal axis (Dasen et al., 2003; Dasen et al., 2005). These data suggest that multiple signaling pathways are required at specific times and discrete locations for the proper formation of motor neurons. Early studies from the Jessell lab indicated two roles for Shh in motor neuron specification: an early role in motor neuron progenitor specification and a later role in progenitor differentiation into motor neurons (Ericson et al., 1996). Here we present evidence for a third role of Shh in the maintenance of Olig2+ motor neuron progenitors during later stages of neural patterning. Our data suggest that Shh pathway activity is selectively required to maintain Olig2 expression over several days (E9.5-E12.5) of embryogenesis, and that Gas1, Cdo and Boc play significant roles in mediating this signaling.
Recent work from Dessaud et al. also examined the maintenance of Shh-dependent ventral cell identities during neural patterning (Dessaud et al., 2010); through floor plate-specific deletion of Shh around E9.5 (following initial specification of Olig2 and Nkx2.2), they demonstrated that Olig2 maintenance requires ongoing Shh signaling. Significantly, they also identified defects in Nkx2.2 maintenance, a result not duplicated in Gas1−/− embryos at the same stage (E12.5). Several possible explanations for this discrepancy include differences in the level of notochord derived Shh between these mice, the timing and completeness of Shh loss in the neural tube, or an altered response to Shh ligand in Nkx2.2+ cells in Gas1−/− embryos.
Notably, at later developmental stages (from E12.5 onward), Olig2+ pMNs no longer give rise to motor neurons, but instead produce oligodendrocytes (Ligon et al., 2006; Rowitch, 2004). Thus, our data implicate Gas1, Cdo and Boc not only as regulators of motor neuron formation, but also as mediators of oligodendrocyte formation during later embryogenesis. Importantly, these data are consistent with genetic analyses of Shh−/−; Gi3−/− embryos that link Shh signaling to the precise timing of oligodendrocyte appearance during embryogenesis (Oh et al., 2005; Oh et al., 2009).
Gas1, Cdo and Boc are essential Hh co-receptors
The recent identification of Gas1, Cdo and Boc as ligand-binding components of the Hh pathway raises the question of their combinatorial roles in Shh signaling. This study demonstrates an absolute requirement for Gas1, Cdo and Boc in Shh-dependent neural patterning. Our data indicate that Gas1, Cdo and Boc are all equally capable of promoting Shh signaling during neural patterning; overexpression of any individual component results in ectopic ventral cell fate specification in a cell-autonomous and ligand-dependent manner. Additionally, while genetic removal of Gas1, Cdo or Boc individually has only modest effects on Shh signaling, removal of any two components results in significantly reduced Shh-dependent ventral neural patterning. Most strikingly, simultaneous removal of Gas1, Cdo and Boc results in a complete loss of Shh-dependent neural progenitors, mirroring the loss of Shh itself. Additionally, Gas1−/−; Cdo−/−; Boc−/− embryos display phenotypes such as cyclopia, holoprosencephaly and linearized heart tubes, phenotypes observed in Shh−/−; Ihh−/− double mutant embryos, and Smo−/−embryos, suggesting that most Hh signaling is abrogated when all co-receptors are absent (Zhang et al., 2001). However, that the linearized heart tube phenotype is not completely penetrant in Gas1−/−; Cdo−/−; Boc−/− embryos, suggests that some transient, early Hh signaling is possible in the absence of these receptors. Whether this early Hh signal is transduced solely through Shh binding to Ptch1 is an open question. Weak interactions of Shh with other extracellular factors such as vitronectin may be sufficient to promote some basal level of Hh signaling through Ptch1 (Pons and Marti, 2000; Pons et al., 2001).
Importantly, the findings presented here are corroborated by an independent study examining Hh-dependent cerebellar progenitor proliferation (Izzi et al., submitted); this work demonstrates an equally vital role for Gas1, Cdo and Boc in reception of the Hh signal in cerebellar granular neuron progenitor proliferation. Additionally, this study explores physical interactions between Gas1, Cdo and Boc with the canonical Hh receptor, Ptch1. Together these studies support a model in which Gas1, Cdo and Boc are essential co-receptors that mediate multiple cellular responses to Hh ligands in multiple tissues, and at multiple developmental stages.
Differential requirement for Hh co-receptors in vertebrates
Recent data suggest that the Drosophila homologs of Cdo and Boc (ihog and boi) are essential co-receptors for Hh in the developing wing imaginal disc (Camp et al., 2010; Zheng et al., 2010). However, despite many highly conserved core elements of the Hh pathway across species, significant differences exist in Hh signal transduction between vertebrates and invertebrates. Perhaps the most striking of these is the requirement for the primary cilium in vertebrate Hh signaling (Huangfu et al., 2003). Other differences exist though, including the function of a vertebrate-specific Hh antagonist Hhip1 (Chuang and McMahon, 1999), and, of greatest relevance to this study, the mode of Hh ligand binding to ihog/boi in flies, and Cdo/Boc in mice (McLellan et al., 2008). This report highlights two additional differences in Hh signaling. First, genetic data presented here indicate that mice lack an absolute requirement for Cdo and Boc function during embryogenesis. In fact, while genetic removal of Cdo and Boc function does result in significant neural patterning defects, Shh-mediated digit specification occurs normally in Cdo−/−; Boc−/− embryos; thus, Cdo and Boc are dispensable for normal limb development. These data are corroborated by a recent study examining the spectrum of holoprosencephaly in Cdo and Boc mutant mice (Zhang et al., 2010). Second, we present evidence that, in mice, Gas1 plays a significant role in reception of the Shh signal, and that together, Gas1, Cdo and Boc function as essential co-receptors for the vertebrate Hh pathway.
An outstanding question is what role, if any does Gas1 play in Hh signal reception in Drosophila? Recent work has identified structural similarities between Gas1 and glial cell derived neurotrophic factor receptors (GFRs; Cabrera et al., 2006). While Drosophila lacks both GDNF ligands (GFLs), as well as a clear Gas1 homolog, a Gas1 homolog does exist in Honey bees (Apis mellifora). There is also a single GDNF receptor-like (GFRL) homolog present in Drosophila (Airaksinen et al., 2006; Hatinen et al., 2007; Schueler-Furman et al., 2006). Future studies will be important to determine whether this GFRL protein plays any role in mediating Hh signaling in Drosophila or if it functions more similarly to GFRs as a modifier of Drosophila Ret signaling (Ibanez, 2010).
Additional cell surface proteins in Hh signal transduction
Gas1, Cdo and Boc are essential components of the Hh pathway that promote Shh signaling in a cell-autonomous manner through direct interactions with ligand. Since Gas1 is a GPI anchored protein (Stebel et al., 2000), and previous work demonstrated that the cytoplasmic domains of Cdo and Boc are dispensable for the promotion of Shh signaling (Tenzen et al., 2006), these proteins must utilize other cell surface or membrane-associated molecules to transduce extracellular Hh signals across the plasma membrane. A likely model, that Gas1, Cdo and Boc form a physical complex with Ptch1, and that engagement of this complex by Hh ligand is essential for signal transduction is supported by recent biochemical data (Zheng et al., 2010), and by functional data indicating that ligand binding is necessary, but not sufficient for the Hh-promoting function of Cdo and Boc (Allen et al., 2007; Tenzen et al., 2006).
While recent structural studies address the precise physical interactions of Hh ligands with Cdo and Boc (reviewed by Beachy et al., 2010), future work assessing the co-receptor function of Gas1, Cdo and Boc must also consider a growing number of additional Hh cell surface proteins. This includes the Hh-binding cell surface antagonists Ptch2 and Hhip1 (Carpenter et al., 1998; Chuang et al., 2003; Chuang and McMahon, 1999; Motoyama et al., 1998) and Hh pathway components that regulate the trafficking and turnover of Hh ligands, as has been described for megalin (McCarthy and Argraves, 2003; McCarthy et al., 2002) and recently proposed for Dispatched1 (Etheridge et al., 2010). Recent studies have also identified opposing roles for different glypican family members (six glypicans exist in mouse and human) in Hh signal transduction (Capurro et al., 2008; Williams et al., 2010); whether Gas1, Cdo and Boc functionally cooperate or compete with these proteins for Hh binding at the cell surface is unknown. Finally, a number of secreted and extracellular matrix proteins also regulate Shh, including Drosophila Shifted, (Glise et al., 2005; Gorfinkiel et al., 2005), zebrafish Scube2 (Glise et al., 2005; Hollway et al., 2006; Kawakami et al., 2005; Woods and Talbot, 2005), Drosophila trol (Park et al., 2003), and vitronectin (Martinez-Morales et al., 1997; Pons and Marti, 2000; Pons et al., 2001). Taken together, the total number of cell surface regulators of the Hh pathway consists of well over a dozen components, highlighting the complex and tightly regulated nature of Hh signaling during embryogenesis.
Experimental Procedures
Mice
Gas1LacZ (Martinelli and Fan, 2007b), CdoLacZ-2 (Cole and Krauss, 2003), Gli2zfd (Mo et al., 1997) and Shh (St-Jacques et al., 1998) mice have all been described previously. The generation of BocAP-2 (referred to as Boc) mice is described elsewhere (Zhang et al., 2010). Gas1, Cdo and Shh mice were maintained on a C57BL/6 background. Gli2 mice were maintained on a mixed C57BL/6; 129S4/SvJaeJ background. Gas1; Cdo; Boc mice were maintained on a mixed C57BL/6; 129S6/SvEvTac background. Noon of the day on which a vaginal plug was detected was considered as E0.5. For all subsequent analyses, a minimum of three embryos of each genotype was examined; representative images are shown.
In situ hybridization and immunofluorescence
Whole-mount digoxigenin in situ hybridization was performed as described (Wilkinson, 1992). Immunofluorescent analyses of mouse neural tubes were performed essentially as described previously (Jeong and McMahon, 2005). The antibodies used were as follows: 1:20 Mouse IgG1 anti Nkx6.1 (DSHB; F55A10), 1:20 mouse IgG2b anti-Nkx2.2 (DSHB; 74.5A5), 1:20 mouse IgG1 anti-Shh (DSHB; 5E1), 1:20 mouse IgG1 anti-FoxA2 (DSHB; 4C7), 1:20 mouse IgG2a Pax3 (DSHB), 1:20 mouse IgG1 anti-Pax6 (DSHB); Rabbit IgG anti-Olig2 antibody was purchased from Millipore (AB9610) and used at a dilution of 1:1000; Rabbit IgG anti-Dbx1 antibody was a gift from Yasushi Nakagawa and used at a dilution of 1:1000. Gas1, Cdo and Boc were detected with the following antibodies: 1:1000 goat IgG anti-Gas1 (R&D systems), 1:1000 goat IgG anti-Cdo (R&D systems), and 1:1000 goat IgG anti-Boc (R&D systems). All secondary antibodies were obtained from Invitrogen and used at a dilution of 1:500. Primary antibodies were incubated overnight at 4°C, followed by incubation with secondary antibodies for 1hr at room temperature. Images were collected with a Zeiss LSM 510 confocal microscope and Leica SP5X confocal microscope.
Chick electroporations
Chick electroporations were performed essentially as described previously (Tenzen et al., 2006). Briefly, pCIG, Gas1-pCIG, Cdo-pCIG, Boc-pCIG, and SmoM2-pCIG were injected into the neural tubes of Hamburger-Hamilton (HH) stage 10–12 chicken embryos at concentrations of 1.0µg/µl in PBS with 50 ng/µl Fast Green. Approximately 48 hours following electroporation embryos were recovered and fixed in 4% paraformaldehyde for subsequent immunofluorescent analysis. Fertile eggs were obtained from both Charles River and the Michigan State University Poultry Farm.
Hh antagonist administration
Cyclopamine (purchased from both Sigma and Alexis Biochemicals) was dissolved to a concentration of 1mg/ml in 100% ethanol. SANT-1 was purchased from Tocris Biosciences, dissolved in DMSO to a concentration of 10mM, and used at a concentration of 1uM. Neural tubes of HH stage 18 chick embryos were injected with each compound, ethanol alone, or untreated, followed by incubation for 24 hours. Embryos were then dissected and processed for subsequent immunofluorescent analysis of neural patterning.
Skeletal preparations
Skeletons of E18.5 mouse embryos were prepared according to a modified Alcian Blue (Sigma; A5268) and Alizarin Red (Sigma; A5533) staining protocol (Kessel et al., 1990; Wallin et al., 1994). Briefly, E18.5 embryos were dissected, skinned and eviscerated. Subsequently, embryos were fixed in ethanol, followed by acetone and stained for four days in an Alcian Blue/Alizarin red stain solution. Remaining tissue was digested with 1% potassium hydroxide, and embryos were cleared by incubation with progressively increasing concentrations of glycerol.
Supplementary Material
Acknowledgments
We are grateful to Dr. A. Joyner for the Gli2 mutant. We also thank Dr. Y. Nakagawa for the Dbx-1 antibody. Shh, FoxA2, Nkx2.2, Nkx6.1, Pax3, Pax6, and Pax7 antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA. We thank James Briscoe for critical comments and helpful discussions. Work in A.P.M.’s laboratory was supported by a grant from the NIH (R37 NS033642). R.S.K. is supported by grants from the NIH (R01AR46207) and March of Dimes (6-FY08-255). F.C. is funded by the Canadian Cancer Society Research Institute (CCSRI) and the Canadian Institutes of Health Research (CIHR). L.I. is supported by a Fonds de Recherche en Santé du Québec (FRSQ) post-doctoral training award. F.C. is an FRSQ research scientist. B.L.A. gratefully acknowledges support from The University of Michigan Biological Sciences Scholars Program and The Endowment for Basic Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Holm L, Hatinen T. Evolution of the GDNF family ligands and receptors. Brain Behav Evol. 2006;68:181–190. doi: 10.1159/000094087. [DOI] [PubMed] [Google Scholar]
- Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Cabrera JR, Sanchez-Pulido L, Rojas AM, Valencia A, Manes S, Naranjo JR, Mellstrom B. Gas1 is related to the glial cell-derived neurotrophic factor family receptors alpha and regulates Ret signaling. J Biol Chem. 2006;281:14330–14339. doi: 10.1074/jbc.M509572200. [DOI] [PubMed] [Google Scholar]
- Camp D, Currie K, Labbe A, van Meyel DJ, Charron F. Ihog and Boi are essential for Hedgehog signaling in Drosophila. Neural Dev. 2010;5:28. doi: 10.1186/1749-8104-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell. 2008;14:700–711. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci U S A. 1998;95:13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- Cole F, Krauss RS. Microform holoprosencephaly in mice that lack the Ig superfamily member Cdon. Curr Biol. 2003;13:411–415. doi: 10.1016/s0960-9822(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, Sasai N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8:e1000382. doi: 10.1371/journal.pbio.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Etheridge LA, Crawford TQ, Zhang S, Roelink H. Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development. 2010;137:133–140. doi: 10.1242/dev.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glise B, Miller CA, Crozatier M, Halbisen MA, Wise S, Olson DJ, Vincent A, Blair SS. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev Cell. 2005;8:255–266. doi: 10.1016/j.devcel.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Sierra J, Callejo A, Ibanez C, Guerrero I. The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Dev Cell. 2005;8:241–253. doi: 10.1016/j.devcel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Hatinen T, Holm L, Airaksinen MS. Loss of neurturin in frog--comparative genomics study of GDNF family ligand-receptor pairs. Mol Cell Neurosci. 2007;34:155–167. doi: 10.1016/j.mcn.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Hollway GE, Maule J, Gautier P, Evans TM, Keenan DG, Lohs C, Fischer D, Wicking C, Currie PD. Scube2 mediates Hedgehog signalling in the zebrafish embryo. Dev Biol. 2006;294:104–118. doi: 10.1016/j.ydbio.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Ibanez CF. Beyond the cell surface: new mechanisms of receptor function. Biochem Biophys Res Commun. 2010;396:24–27. doi: 10.1016/j.bbrc.2010.01.136. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Jeong J, McMahon AP. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development. 2005;132:143–154. doi: 10.1242/dev.01566. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nojima Y, Toyoda A, Takahoko M, Satoh M, Tanaka H, Wada H, Masai I, Terasaki H, Sakaki Y, et al. The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr Biol. 2005;15:480–488. doi: 10.1016/j.cub.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Kessel M, Balling R, Gruss P. Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell. 1990;61:301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Lee CS, Buttitta L, Fan CM. Evidence that the WNT-inducible growth arrest-specific gene 1 encodes an antagonist of sonic hedgehog signaling in the somite. Proc Natl Acad Sci U S A. 2001;98:11347–11352. doi: 10.1073/pnas.201418298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Olig gene function in CNS development and disease. Glia. 2006;54:1–10. doi: 10.1002/glia.20273. [DOI] [PubMed] [Google Scholar]
- Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007a;21:1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM. The role of Gas1 in embryonic development and its implications for human disease. Cell Cycle. 2007b;6:2650–2655. doi: 10.4161/cc.6.21.4877. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Barbas JA, Marti E, Bovolenta P, Edgar D, Rodriguez-Tebar A. Vitronectin is expressed in the ventral region of the neural tube and promotes the differentiation of motor neurons. Development. 1997;124:5139–5147. doi: 10.1242/dev.124.24.5139. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Argraves WS. Megalin and the neurodevelopmental biology of sonic hedgehog and retinol. J Cell Sci. 2003;116:955–960. doi: 10.1242/jcs.00313. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem. 2002;277:25660–25667. doi: 10.1074/jbc.M201933200. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Yao S, Zheng X, Geisbrecht BV, Ghirlando R, Beachy PA, Leahy DJ. Structure of a heparin-dependent complex of Hedgehog and Ihog. Proc Natl Acad Sci U S A. 2006;103:17208–17213. doi: 10.1073/pnas.0606738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455:979–983. doi: 10.1038/nature07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Takabatake T, Takeshima K, Hui C. Ptch2, a second mouse Patched gene is co-expressed with Sonic hedgehog. Nat Genet. 1998;18:104–106. doi: 10.1038/ng0298-104. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oh S, Huang X, Chiang C. Specific requirements of sonic hedgehog signaling during oligodendrocyte development. Dev Dyn. 2005;234:489–496. doi: 10.1002/dvdy.20422. [DOI] [PubMed] [Google Scholar]
- Oh S, Huang X, Liu J, Litingtung Y, Chiang C. Shh and Gli3 activities are required for timely generation of motor neuron progenitors. Dev Biol. 2009;331:261–269. doi: 10.1016/j.ydbio.2009.05.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJ, McDermott S, Datta S. Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Pons S, Marti E. Sonic hedgehog synergizes with the extracellular matrix protein vitronectin to induce spinal motor neuron differentiation. Development. 2000;127:333–342. doi: 10.1242/dev.127.2.333. [DOI] [PubMed] [Google Scholar]
- Pons S, Trejo JL, Martinez-Morales JR, Marti E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development. 2001;128:1481–1492. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, et al. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Schueler-Furman O, Glick E, Segovia J, Linial M. Is GAS1 a co-receptor for the GDNF family of ligands? Trends Pharmacol Sci. 2006;27:72–77. doi: 10.1016/j.tips.2005.12.004. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stebel M, Vatta P, Ruaro ME, Del Sal G, Parton RG, Schneider C. The growth suppressing gas1 product is a GPI-linked protein. FEBS Lett. 2000;481:152–158. doi: 10.1016/s0014-5793(00)02004-4. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 2006;10:647–656. doi: 10.1016/j.devcel.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. In situ hybridization : a practical approach. Oxford ; New York: IRL Press at Oxford University Press; 1992. [Google Scholar]
- Williams EH, Pappano WN, Saunders AM, Kim MS, Leahy DJ, Beachy PA. Dally-like core protein and its mammalian homologues mediate stimulatory and inhibitory effects on Hedgehog signal response. Proc Natl Acad Sci U S A. 2010;107:5869–5874. doi: 10.1073/pnas.1001777107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods IG, Talbot WS. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol. 2005;3:e66. doi: 10.1371/journal.pbio.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Zhang W, Hong M, Bae GU, Kang JS, Krauss RS. Boc modifies the holoprosencephaly spectrum of Cdo mutant mice. Dis Model Mech. 2010 doi: 10.1242/dmm.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell. 2006;10:657–665. doi: 10.1016/j.devcel.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. [PubMed] [Google Scholar]
- Zheng X, Mann RK, Sever N, Beachy PA. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010;24:57–71. doi: 10.1101/gad.1870310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.