Abstract
Mutant superoxide dismutase-1 (SOD1) has an unidentified toxic property that provokes ALS. Several ALS-linked SOD1 mutations cause long C-terminal truncations, which suggests that common cytotoxic SOD1 conformational species should be misfolded and that the C-terminal end cannot be involved. The cytotoxicity may arise from interaction of cellular proteins with misfolded SOD1 species. Here we specifically immunocaptured misfolded SOD1 by the C-terminal end, from extracts of spinal cords from transgenic ALS model mice. Associated proteins were identified with proteomic techniques. Two transgenic models expressing SOD1s with contrasting molecular properties were examined: the stable G93A mutant, which is abundant in the spinal cord with only a tiny subfraction misfolded, and the scarce disordered truncation mutant G127insTGGG. For comparison, proteins in spinal cord extracts with affinity for immobilized apo G93A mutant SOD1 were determined. Two-dimensional gel patterns with a limited number of bound proteins were found, which were similar for the two SOD1 mutants. Apart from neurofilament light, the proteins identified were all chaperones and by far most abundant was Hsc70. The immobilized apo G93A SOD1, which would populate a variety of conformations, was found to bind to a considerable number of additional proteins. A substantial proportion of the misfolded SOD1 in the spinal cord extracts appeared to be chaperone-associated. Still, only about 1% of the Hsc70 appeared to be associated with misfolded SOD1. The results argue against the notion that chaperone depletion is involved in ALS pathogenesis in the transgenic models and in humans carrying SOD1 mutations.
Keywords: Amyotropic Lateral Sclerosis (Lou Gehrig's Disease), Heat Shock Protein, Neurodegeneration, Protein-Protein Interactions, Superoxide Dismutase (SOD), Transgenic Mice
Introduction
Amyotrophic lateral sclerosis (ALS)2 is characterized by degeneration of motor neurons in the motor cortex, the brainstem, and the spinal cord. This results in progressive muscular atrophy and the patients usually succumb to respiratory failure within a few years. About 10% of ALS cases cluster in families (1), and in some of these the disease is linked to mutations in the gene of the antioxidant enzyme superoxide dismutase-1 (SOD1).
Overall, about 6% of all cases with ALS show SOD1 mutations, and more than 160 such mutations have been identified (2). The mutations confer a cytotoxic gain of function of unknown character to the enzyme (3–4). The ALS-linked mutant SOD1s, including nine with long C-terminal truncations, are likely to cause ALS by essentially the same mechanism. In the shortest of these the native sequence ends with Leu-117 followed by four novel amino acids and a premature stop codon. The truncated mutants lack the stabilizing Cys-57—Cys-146 disulfide bond and β-strand 8 of the β-barrel core of the protein, and should show limited native folding. Thus, any cytotoxic conformational species common to the ALS-linked SOD1 mutations should be misfolded. Furthermore, the C-terminal end cannot be involved in the cytotoxicity. One conceivable mechanism by which such misfolded SOD1 species might provoke the disease is through interaction with essential cellular proteins that could become inactivated, depleted, or erroneously activated.
We have previously shown that misfolded SOD1 species are enriched in spinal cords from mice expressing mutant human SOD1s (hSOD1s) (5). These species account for minute subfractions of the stable hSOD1 mutants G93A and D90A, and major portions of the small amounts of the unstable G85R and G127insTGGG (G127X) mutant hSOD1s present in spinal cords. Here we used novel antibodies to specifically capture misfolded hSOD1 species via their C-terminal ends from spinal cord extracts taken from transgenic model mice expressing mutant hSOD1s of widely different physical properties. Proteins associated with the captured hSOD1s were identified by two-dimensional gel electrophoresis separation and matrix-assisted laser desorption ionization time of flight mass spectrometry (two-dimensional gel/MALDI-TOF/MS) analysis.
EXPERIMENTAL PROCEDURES
Transgenic Mice
The G127X hSOD1 mouse model was developed in our laboratory and backcrossed with C57/BL6BomTac mice for >25 generations (6). Mice expressing the G93A mutant (G93AGur) and wild-type hSOD1 (4) were obtained from the Jackson Laboratory, and were backcrossed with C57/BL6BomTac for >20 generations. The mice used were around 100 days old. The use and maintenance of the mice and the experimental protocol described in this article were approved by the Ethics Committee for Animal Research at Umeå University.
Extraction of Tissue
Mice were killed by intraperitoneal injection of pentobarbital. The spinal cord was dissected by cutting the vertebral column at the base of the skull and just above the hip bone. A syringe was inserted at the lower opening and the spinal cord was flushed out with 0.15 m NaCl. Rat spinal cords were dissected in the same way. The spinal cords were homogenized with an Ultraturrax apparatus (IKA, Staufen, Germany) for 1 min and sonication for 1 min at 4 °C in 5 volumes of ice-cold PBS (10 mm potassium phosphate, pH 7.0, in 0.15 m NaCl) supplemented with 1.8 mm EDTA, 1 mm dithiothreitol, 1% Triton X-100, and the antiproteolytic mixture Complete® without EDTA (Roche Diagnostics, Basel, Switzerland). The homogenates were centrifuged at 20,000 × g for 30 min, and the supernatants collected.
To determine the proportion of hSOD1 that was pelleted in the centrifugation, 500 μl of homogenates from three 100-day-old G93A and G127X hSOD1 transgenic mice were centrifuged at 20,000 × g for 30 min, and the supernatants carefully removed. 1.5 ml of homogenization buffer was gently added to the tubes to remove any residual supernatant with soluble SOD1 without disrupting the pellets. There was no resuspension and washing of the pellet with homogenization buffer since the aim was to measure the primarily pelleted hSOD1. This step was repeated twice. The pellets were then suspended in 100 μl of homogenization buffer by sonication, mixed with SDS/PAGE sample buffer to a final SDS concentration of 2%, boiled and subjected to Western immunoblotting together with the supernatants.
Antibodies and Immunocapture
Antibodies to peptides corresponding to amino acids 131–153 in hSOD1 and amino acids 123–132 in the G127X mutant hSOD1 variant were raised in rabbits as previously described (6–7). After purification with protein A-Sepharose (GE Healthcare, Uppsala, Sweden) and Sulfolink peptide affinity purification (Pierce), the antibodies were coupled to CNBr-activated Sepharose-Fast Flow (GE Healthcare) at 2 mg/g wet gel. Typically, 100 mg wet weight of specific ab-Sepharose was incubated with an extract of two spinal cords for 30 min at 4 °C with gentle agitation. Prior to this incubation, the extract had been pre-cleared with the same amount of non-immune IgG-Sepharose. After centrifugation at 12,000 × g for 2 min, the supernatant with unbound proteins was removed. The ab-Sepharose gel beads were then washed twice, first with 50 ml of PBS containing 1% Triton X-100 and then with 50 ml of PBS, and then gently pelleted by centrifugation at 500 × g. The bound SOD1 was released by 60 min of incubation at 23 °C with 1 ml PBS containing 30 μg of the peptide used for immunization. The Sepharose beads were removed by filtration through an Ultrafree-CL centrifugal filter (Millipore, Bedford, MA). The proteins released were then precipitated with 10% trichloroacetic acid overnight at 4 °C, washed with ice-cold acetone, and dissolved in 200 μl of DeStreak Rehydration Solution (GE Healthcare).
Binding of Spinal Cord Proteins to Immobilized hSOD1s
Recombinant hSOD1s were expressed in Escherichia coli together with the copper chaperone for superoxide dismutase (CCS) and purified as described elsewhere (8). They carried cysteine to alanine mutations at positions 6 and 111 to avoid disulfide oligomerization during experiments. For immobilization, wild-type hSOD1 or G93A mutant hSOD1 were dialyzed with 0.1 m NaHCO3, 0.5 m NaCl, pH 8.3, at 4 °C overnight and were immobilized on CNBr-activated Sepharose at 6 mg/g wet gel according to the manufacturer's instructions. After washing, the remaining active groups on the gel were blocked with 1 m ethanolamine for 60 min at 23 °C. To remove electrostatically bound proteins, the gel beads were washed four cycles with 0.1 m Tris-HCl in 0.5 m NaCl, pH 8.5, followed by 0.1 m sodium acetate in 0.5 m NaCl, pH 4.5. To render the hSOD1s in apo form, the gel beads were treated for 4 h with 4 m guanidinium-Cl and 25 mm EDTA in 33 mm HEPES buffer, pH 6.7, at 23 °C and then washed with large volumes of PBS.
Gels with bound holo- or apo-hSOD1 were incubated for 90 min at 4 °C with 20 volumes of rat spinal cord homogenate, typically 5 ml of homogenate to 250 mg of wet gel beads. The extracts used were pre-cleared with the same amount ethanolamine-blocked CNBr-Sepharose. The gels were then washed twice with 100 ml of PBS containing 0.05% Triton X-100. The gel beads were recovered by fast filtration through a 5-μm nylon filter (Spectrum Laboratories, Rancho Dominguez, CA). The gels were dried in a SpeedVac apparatus (Thermo Scientific, Waltham, MA) at low temperature, and the bound proteins were released by incubation with DeStreak buffer for 90 min at 23 °C.
Two-dimensional Electrophoresis
Samples were separated by two-dimensional gel electrophoresis as described elsewere (9) except that 10–14.5% or 12.5% Criterion precast gels (Bio-Rad) were used for the second dimension. The gels were stained with Coomassie G-250 for protein identification, or silver stained for pattern analysis as described elsewhere (10).
Protein Identification
Protein identification was carried out as described elsewhere (11). Briefly, spots were excised from Coomassie G-250 stained gels and digested with trypsin (V511a: 2 μg/ml; Promega, Madison, WI). Peptide spectra were recorded in a Voyager DE-STR mass spectrometer (Applied Biosystems). Peptide peak lists generated in Data Explorer software version 4 (Applied Biosystems) were used to identify proteins by searching the Swiss-Prot database (version 50.9) using an in-house Mascot server that was licensed to Umeå University by Matrix Science (Boston, MA). Search parameters were set to allow mass deviations of ±50 ppm and one or two missed cleavage sites, as well as the fixed modification carbamidomethylation of cysteine and the variable modification oxidation of methionine. Identifications that were statistically significant (with a Mowse score of at least 60 and a p value of < 0.05) and that showed a gel position close to the theoretical mass and isoelectric point were regarded as positive.
Immunoblots and Quantifications
The Western immunoblots were generally carried out as previously described (6) using an antibody raised in chicken against a peptide corresponding to amino acids 57–72 of the hSOD1 sequence. For quantification of hSOD1 in pellets an antibody raised in rabbits against a peptide corresponding to amino acids 24–39 of the hSOD1 sequence was used. For Hsc70, a rat monoclonal antibody (ab19136; Abcam, Cambridge, UK) was used. The chemiluminescence of the blots was recorded in a ChemiDoc apparatus and analyzed with Quantity One software (Bio-Rad).
RESULTS
The aim of the present study was to identify proteins that bind to misfolded hSOD1 in spinal cord extracts from mice expressing two different mutant hSOD1s. The G93A hSOD1 transgenic model shows the highest levels of misfolded hSOD1 in the spinal cords of the transgenic models examined so far (5). Even so, only about 3% of the G93A mutant hSOD1 is misfolded. The truncated G127X hSOD1 is completely disordered structurally and is present at a somewhat lower level in the spinal cord than misfolded hSOD1 in G93A mice (5). To capture misfolded hSOD1 from the G93A mice, we used an antibody raised to a peptide sequence at the C-terminal end of hSOD1 (amino acids 131–153). The antibody shows almost complete specificity for misfolded hSOD1 species (Fig. 1A). For the G127X mice, an anti-peptide antibody was similarly used (raised to amino acids 123–132 at the C-terminal end; 128–132 being a novel sequence caused by the 4 nucleotide insertion in the G127X SOD1 mutation). This antibody does not recognize folded or misfolded hSOD1 with the native C-terminal sequence (6). The antibodies were covalently coupled to Sepharose and incubated with spinal cord extracts. The bound misfolded SOD1 was then released from the beads by incubation with the corresponding immunization peptides. The advantage of this mild procedure is that proteins that non-specifically adhere to the beads are released only to a limited extent, resulting in relatively clean two-dimensional gel patterns. Because both of these antibodies recognize parts of the hSOD1 sequence that is absent in some of the ALS-linked truncation mutants, they should not interfere with any protein interaction relevant to ALS pathogenesis.
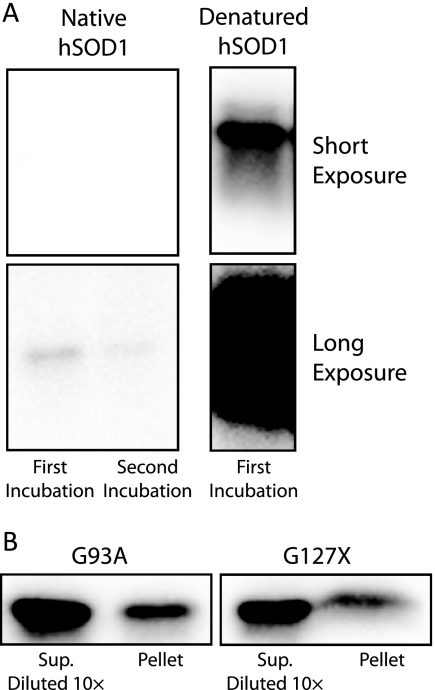
FIGURE 1.
A, immunoblots of native and denatured hSOD1 captured by the antibody raised against the peptide corresponding to amino acids 131–153 in hSOD1. Antibodies were immobilized on protein-A-Sepharose at 2 mg/g wet gel, and incubated with native hSOD1 or denatured hSOD1 (treated with 6 m guanidinium-Cl and 10 mm EDTA). Two consecutive incubations were done with native hSOD1 to control for trace amounts of denatured hSOD1 in the preparation. After washing, the captured SOD1 was released by boiling in SDS-PAGE sample buffer and visualized on Western immunoblots. The captured denatured hSOD1 sample was diluted 5 times more than the native sample. The capacity to bind denatured SOD1 was 1,500-fold higher than the capacity to bind native SOD1. B, quantification of hSOD1 present in 20,000 × g pellets. Pellets from three experiments each of G93A and G127X were solubilized in 1/5 of the centrifuged volume. Dilutions of supernatants and solubilized pellets were visualized on Western immunoblots and quantified. The proportions hSOD1 in pellets were 5.4 ± 1.2% and 5.5 ± 0.3%, respectively.
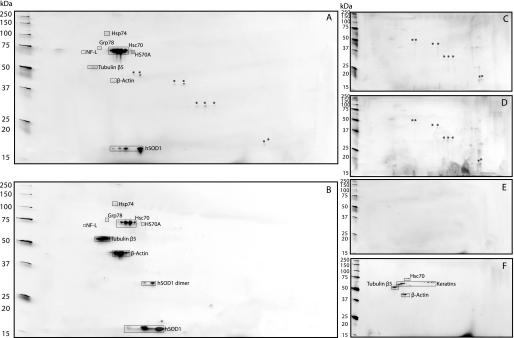
Fig. 2A shows the silver-stained two-dimensional gel patterns of G93A spinal cord extract proteins bound to and released from 131–153-abSepharose beads. In addition to hSOD1, several other protein spots were found. To control for unspecific protein adhesion and release from the beads, the 131–153-abSepharose was also incubated with spinal cord extracts from C57Bl6 control mice and G127X transgenic mice (Fig. 2, C and D). In a further control, we incubated a pre-immune IgG-Sepharose preparation with G93A spinal cord extracts (Fig. 2E). Typically, a few proteins/spots were seen in control experiments, most of them being apparently the same. The 131–153 antibody did bind some unspecific proteins, as they appeared in both controls with 131–153-abSepharose but not with IgG-Sepharose or the G127X hSOD1-specific antibody. These spots are marked with an asterisk in Fig. 2, A, C, and D. Despite pre-clearing with IgG-Sepharose gel, extensive washing, and use of different detergents, some spots remained on the control gels. These proteins (spots) were not considered to specifically bind to misfolded SOD1, and they were not considered further.
FIGURE 2.
Two-dimensional gel patterns of proteins captured by abSepharoses from mouse spinal cord extracts. A, G93A mouse spinal cord extract captured with 131–153-abSepharose. B, G127X mouse spinal cord extract captured with antibodies raised against the neopeptide in the C-terminal end of G127X coupled to Sepharose. Proteins identified with MALDI-TOF/MS are shown. As controls for the C-terminal 131–153 antibody, extracts from non-transgenic C57Bl6 control spinal cords and transgenic G127X mice were captured with 131–153-abSepharose in panels C and D, respectively. In panel E, IgG purified from rabbits before immunization was coupled to IgG and the Sepharose matrix, and incubated with G93A spinal cord extract to test for nonspecific binding of proteins to IgG and the Sepharose matrix. Proteins that bound non-specifically to the 131–153 antibody are marked with an asterisk. As a control for the G127X-specific antibody, it was incubated with spinal cord extracts from G93A mice (panel F). Minute amounts of Hsc70 that could be detected, as well as contaminating tubulin β5, β-actin, and keratins, are marked on the gel.
The binding proteins identified are marked on the two-dimensional gel image (Fig. 2A) and are listed in Table 1. The train of spots at about 16 kDa is hSOD1. By far the most abundant interacting protein was found to be Hsc70. It is notable that 3 of the other proteins found are also chaperones: Hsp74 and HS70A, which are cytosolic, and the endoplasmatic reticulum-resident Grp78. Next to Hsc70, β-tubulin was the strongest spot in the gel. However, both β-tubulin and β-actin showed highly variable contents in the immunocapture experiments, and they were also present in control incubations to a variable extent, sometimes stronger in the control gels than in the gels with specific antibodies used for capture (Fig. 2F). Thus, we cannot say with any certainty whether they specifically bind to misfolded SOD1. Hsc70 spots were also seen in Fig. 2F, but at levels far below those seen when misfolded SOD1 was captured (Fig. 2, A and B). We thus consider the interaction between Hsc70 and misfolded SOD1 to be real and specific.
TABLE 1.
Proteins binding to misfolded SOD1 in extracts of spinal cord of transgenic mice
Misfolded hSOD1 was immunocaptured from spinal cord extracts with antibodies against the C-terminal ends. Bound hSOD1 was then released with the peptides used for the antibody generation. Released hSOD1 and adherent proteins were separated by two-dimensional gel electrophoresis and proteins identified with MALDI-TOF-MS.
| Protein | Abbreviation | Accession no. (UniProtKB) | Sequence coverage (%) | Score/no. of peptides |
|---|---|---|---|---|
| Heat shock cognate 70 kDa protein | Hsc70 | P63017 | 36 | 199/20 |
| Heat shock 70 kDa protein 4 | Hsp74 | Q61316 | 12 | 63/10 |
| Heat shock 70 kDa protein 1A | HS70A | Q61696 | 23 | 86/11 |
| 78 kDa glucose-regulated protein | Grp78 | P20029 | 21 | 99/11 |
| Neurofilament light polypeptide | NF-L | P08551 | 30 | 103/12 |
| Tubulin β5 | P99024 | 27 | 168/12 | |
| β-Actin | P60710 | 17 | 65/6 |
In the study of G127X mice using the G127X hSOD1-specific antibody, the two-dimensional patterns were very similar to those seen with G93A extracts (e.g. Fig. 2B). Incubation of the G127X-specific antibody with a G93A spinal cord extract gave results that resembled those of previous controls (Fig. 2F).
To determine the distribution of hSOD1 between the soluble supernatant phase and the 20,000 × g pellets, the hSOD1 content in three pellets each from G93A and G127X experiments were determined. The pellets were found to contain 5.4 ± 1.2% and 5.5 ± 0.3%, respectively, of the total hSOD1 in the homogenates (Fig. 1B). Thus relatively small proportions of the total hSOD1 are present in non-disrupted tissue, debris, and in aggregates in the 100-day-old mice examined. At 100 days the G127X mice are far from symptomatic, whereas mild symptoms sometimes are present in G93A mice. Still, only small amounts of aggregates are present in G93A mice at that stage (12).
Spinal Cord Proteins Binding to hSOD1 Immobilized on Sepharose
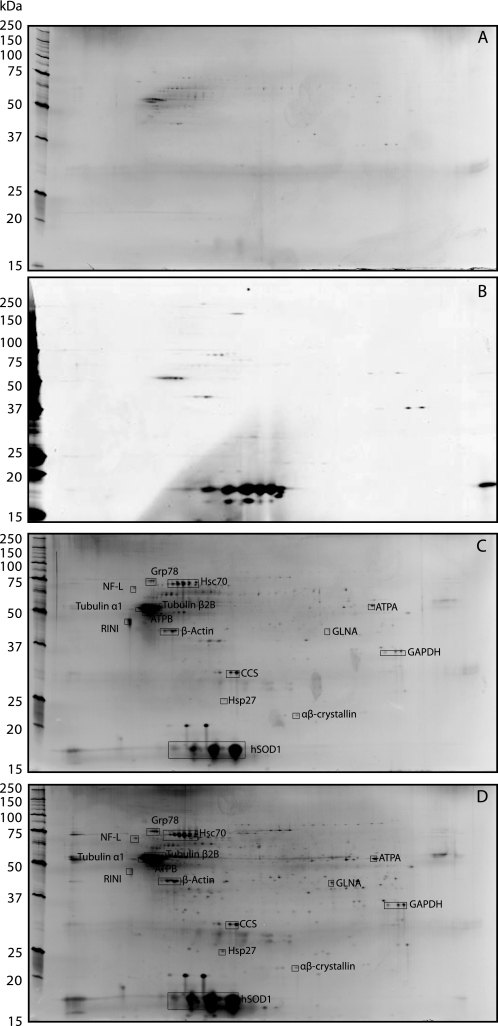
For comparison, we analyzed proteins that bound to recombinant G93A mutant or to recombinant wild-type hSOD1 immobilized on CNBr-activated Sepharose. The preparations were incubated with extracts of rat spinal cord. Rats transgenically expressing mutant hSOD1s also develop ALS (13). Thus, any ALS-causing interactions between hSOD1 and spinal cord proteins should also occur in the rat. First, proteins binding to holo hSOD1s were determined. Few interacting proteins were found and large amounts of hSOD1 were released with the interacting proteins (Fig. 3B). Holo hSOD1 is a dimer and hSOD1 bound to the Sepharose gel via only one subunit can easily be released. Another uncertainty is that the covalent coupling as well as the harsh post-coupling treatments might have rendered a fraction of the hSOD1 molecules un/misfolded. This fraction might have interacted with some of the proteins. Because of this uncertainty and the evidence that un/misfolded hSOD1 species might be more relevant for ALS pathogenesis we focused on immobilized apo hSOD1s. These were prepared by exposure of the gel beads to 4 m guanidinium-Cl and the chelator EDTA. The resulting beads should contain apo hSOD1 bound in random positions populating a variety of conformations from complete disorder to native folding (14). These preparations were incubated with rat spinal cord extracts in the same way. Following washing, bound proteins were analyzed by two-dimensional gel separation and MALDI-TOF/MS analysis (Fig. 3). The patterns seen with G93A mutant hSOD1 and wild-type hSOD1 were qualitatively similar, but the amounts of bound protein were greater with the mutant. Despite the considerably larger amounts of hSOD1 and extracted proteins involved, only moderately more protein appeared to be present on the gels compared with the immunocapture experiments with extracts from G93A transgenic mice (Fig. 2). The patterns were, however, different with markedly less Hsc70 and a far greater number of protein spots. Especially with the apoG93A hSOD1, several spots were below the MALDI-TOF/MS detection limit (Fig. 3). All proteins seen in the immunocapture experiments were identified, except Hsp74 and HS70A (Table 2). All protein spots seen in the experiments with immobilized holo hSOD1 (Fig. 3B) appeared to be present in the studies with apo hSOD1s.
FIGURE 3.
Two-dimensional gel patterns of rat spinal cord extract proteins binding to immobilized holo- and apo-hSOD1. A, proteins bound to ethanolamine-blocked, B, wild-type holo-hSOD1-bound C, wild-type apo-hSOD1-bound, and D, G93A apo-hSOD1-bound Sepharose gels (see “Experimental Procedures”) were visualized by two-dimensional electrophoresis and silver staining. Proteins identified with MALDI-TOF/MS are shown. In A, C, and D, 10–14.5% gels were used for the second dimension and a 12.5% gel in B.
TABLE 2.
Proteins in rat spinal cord extracts interacting with immobilized apoG93A hSOD1
Recombinant apo G93A mutant hSOD1 were immobilized on CNBr-Sepharose. The gels were incubated with rat spinal cord extracts and protein binding to hSOD1 were isolated, separated by two-dimensional gel electrophoresis and identified with MALDI-TOF-MS.
| Protein | Abbreviation | Accession no. (UniProtKB) | Sequence coverage (%) | Score/no. of peptides |
|---|---|---|---|---|
| Heat shock cognate 70 kDa protein | Hsc70 | P63018 | 24 | 143/14 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | P04797 | 50 | 160/12 |
| β-Actin | P60711 | 40 | 163/12 | |
| Tubulin β2B | P04691 | 42 | 172/24 | |
| Tubulin α1a | P68370 | 40 | 170/13 | |
| 78 kDa glucose-regulated protein | Grp78 | P06761 | 33 | 161/18 |
| Copper chaperone for superoxide dismutase | CCS | Q9JK72 | 33 | 172/12 |
| Glutamine synthetase | GLNA | P09606 | 51 | 269/23 |
| ATP synthase subunit β | ATPB | P10719 | 22 | 68/10 |
| Heat shock 27 kDa protein | Hsp27 | P42930 | 37 | 126/8 |
| Ribonuclease inhibitor | RINI | P29315 | 32 | 104/10 |
| ATP synthase subunit α | ATPA | P15999 | 28 | 143/16 |
| αβ-crystallin | P23928 | 49 | 79/10 | |
| Neurofilament light polypeptide | NF-L | P19527 | 46 | 204/25 |
The Hsc70 Interaction
The major interacting protein was Hsc70. To determine the proportion of spinal cord Hsc70 that bound misfolded hSOD1, 131–153-abSepharose was added to spinal cord extracts from G93A and wild-type hSOD1 transgenic mice. In the case of G127X mice, the mutant-specific 123–132 antibody was used. Following washing, the gel beads were boiled in sample buffer, and bound Hsc70 was quantified against dilutions of a G93A spinal cord extract (Fig. 4). The proportions detected for the mutants barely reached 1% of the total amount of Hsc70. Some Hsc70 was bound to misfolded SOD1 in spinal cord tissue from wild-type hSOD1 transgenic mice (0.2%) but none could be detected in non-transgenic littermates (not shown). The precise degree of association between Hsc70 and hSOD1 is difficult to assess, because of limitations in the experiments with the immobilized polyclonal antibodies. Some of the bound hSOD1 is likely to be lost from the antibody gels during the washing. To get an idea of the magnitude, the amount of G127X hSOD1 present in the sample buffer extract from the 123–132-abSepharose was compared with the total amount of mutant protein in the spinal cord extract before and after immunocapture. All the G127X hSOD1 was found to be captured from the extract, but only about 50% of that was recovered in the sample buffer extract. Thus, the true figures for the proportion of Hsc70 that bound to misfolded hSOD1 could, broadly speaking, be double those in Fig. 4. Notably, there might also be some reversal of Hsc70 binding to hSOD1 during the washing of the gel beads.
FIGURE 4.
Quantification of Hsc70 bound to hSOD1. Hsc70 bound to misfolded SOD1 in spinal cord extracts from wild-type hSOD1, G93A hSOD1, and G127X hSOD1 transgenic mice was quantified by comparison with the total amount of Hsc70 in dilutions of a G93A spinal cord extract. The figures are the means of results from four mice.
DISCUSSION
In this study, we used immunocapture of misfolded SOD1 and general proteomic techniques to search for interacting proteins without preconceived ideas. The aim was to identify the proteins that interacted with soluble misfolded hSOD1 in spinal cords from two strains of transgenic ALS mice. At the time point chosen (100-day-old) the G93A mice sometimes show mild symptoms where as the G127X mice are presymtomatic. However, very little hSOD1 is aggregated at this time point (Fig. 1B). Thus, in the present study any potentially noxious protein interactions with hSOD1 are likely to take place in the soluble phase. We found that the major proteins that interact with soluble misfolded hSOD1 in murine spinal cords are all chaperones (Table 1). The fact that they were the same for G93A and G127X mice shows that the 26 C-terminal amino acids are not involved in the interactions. By far the most important was Hsc70, which appeared to complex with a substantial proportion of the misfolded hSOD1. Association of misfolded hSOD1 in spinal cord extracts with Hsc70 has also been observed previously (15–16) and also in cell culture models (17) using proteomic methods. Hsc70 has also been found to co-aggregate with hSOD1 in inclusions in transgenic animal models (9). The inclusions also contained Grp78 and NF-L, suggesting that proteins that interact with soluble misfolded SOD1 also tend to co-aggregate. The constitutively expressed Hsc70 is enriched in the nervous system relative to levels found in peripheral tissues (18). The levels are higher in neurons than in other CNS cells, and are particularly high in motor neurons (19). Still, the shielding with Hsc70 is not sufficient to prevent hSOD1-induced cytotoxicity. This inability is not related to depletion of chaperoning capacity, because only a small proportion of the Hsc70 was found to be occupied in binding of misfolded hSOD1. A caveat is that we analyzed whole tissue in this study. Ventral horns account for 20% of the murine spinal cord (20), and motor neurons constitute 20% of the volume of ventral horns (21). If the major proportion of the complexing between Hsc70 and misfolded hSOD1 takes place in motor neurons, a depletion situation might exist. It is, however, well established that mutant hSOD1 has noxious effects on multiple cell types in the motor areas (22). Other transgenic models have lower levels of misfolded SOD1 (5) and should sequester a smaller proportion of the Hsc70. Finally, considerably less would be occupied in humans who express the mutant hSOD1s at some 50-fold lower rates than the transgenic murine models (23). This suggests that depletion of chaperone capacity is not a feature of mutant hSOD1-provoked ALS, as has been proposed (24). Accordingly, overexpression of Hsp70 (a functionally similar homolog of Hsc70) does not ameliorate disease caused by mutant hSOD1s in transgenic mice (25). The heat shock protein coinducer arimoclomol, which induces several chaperones concomitantly, does however prolong survival of G93A mice (26). It has not been tested in mice with lower levels of misfolded hSOD1. A clinical trial with arimoclomol is currently being conducted in ALS patients carrying SOD1 mutations (27), to evaluate its role in humans. Most of the immunocaptured proteins were molecular chaperones in the present study (Table 1); no interaction partner for misfolded SOD1 was found that could explain the cytotoxicity of mutant SOD1 in ALS.
In addition to those found here, more than 30 proteins have been reported to interact with mutant SOD1s (16, 28). A number of reasons for why they were not found can be suggested. We wanted to detect associations that did not involve the C-terminal end, and that only involved misfolded SOD1 species, because we considered that only those would be relevant. Speculatively, some of the previous findings could have involved associations with C-terminal segments as well as with more natively folded hSOD1 species. In the study with immobilized apo G93A, many more protein spots were indeed visible and identified (Fig. 3 and Table 2), some of which have already been shown to bind SOD1: actin, (16, 29), tubulin (16, 30), CCS (31), αβ-crystallin (32), Hsp27 (24), ATPA, ATPB, GAPDH (16), and Hsp70, the inducible homolog of Hsc70 (16, 24, 33–34). RINI has apparently not been described to bind hSOD1 before. Conspicuously absent in the immunocapture experiments was CCS, which was one of the major protein spots found in the experiment with apoG93A (Fig. 3 and Table 2). One likely explanation is that a significant interaction between SOD1 and CCS involves the C-terminal end of SOD1 (35). The systems studied have also varied; findings in cultured cells are not necessarily relevant to complex tissues such as spinal cord.
Sensitivity is another consideration. Despite the fact that all the misfolded hSOD1 from 2 spinal cords was captured in each experiment, it is apparent that the sensitivity of the present proteomic approach is insufficient to detect proteins complexed in very small amounts. Many of the previous studies have focused on preconceived candidate interacting proteins, allowing the use of specific antibodies for highly sensitive detection. A combination of the present C-terminal immunocapture protocol with high-resolution, high-sensitivity mass spectrometry might lead to the identification of further low-abundance interaction partners for misfolded SOD1.
In summary misfolded soluble hSOD1, both wild-type protein and two different mutants, was found to a large extent to be bound to chaperones in mouse spinal cords. The most abundant interacting protein was Hsc70, but only about 1% of the total cellular Hsc70 was sequestered. Chaperone insufficiency is therefore not likely to be cytotoxic in ALS. No other conceivable explanation for the cytotoxicity of mutant SODs was apparent from the other interactions identified.
Acknowledgments
We thank Eva Bern, Karin Hjertkvist, Ann-Charloth Nilsson, Ulla-Stina Spetz, and Agneta Öberg for excellent technical assistance, and Drs. Thomas Kieselbach and Vaibhav Srivastava for helpful discussions.
This work was supported by the Swedish Science Council, the Swedish Brain Fund/Hållsten Fund, the Swedish Medical Society including the Björklund Fund for ALS Research, the ALS Association, the Swedish Association of Persons with Neurological Disabilities, Västerbotten County Council, the Kempe Foundations, and the King Gustaf V and Queen Victoria Foundation.
- ALS
- amyotrophic lateral sclerosis
- SOD1
- superoxide dismutase-1
- MALDI-TOF/MS
- matrix-assisted laser desorption ionization time of flight mass spectrometry
- CCS
- copper chaperone for superoxide dismutase.
REFERENCES
- 1. Haverkamp L. J., Appel V., Appel S. H. (1995) Brain 118, 707–719 [DOI] [PubMed] [Google Scholar]
- 2. Wroe R., Wai-Ling Butler A., Andersen P. M., Powell J. F., Al-Chalabi A. (2008) Amyotroph. Lateral. Scler. 9, 249–250 [DOI] [PubMed] [Google Scholar]
- 3. Andersen P. M., Nilsson P., Ala-Hurula V., Keränen M. L., Tarvainen I., Haltia T., Nilsson L., Binzer M., Forsgren L., Marklund S. L. (1995) Nat. Genet. 10, 61–66 [DOI] [PubMed] [Google Scholar]
- 4. Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. (1994) Science 264, 1772–1775 [DOI] [PubMed] [Google Scholar]
- 5. Zetterström P., Stewart H. G., Bergemalm D., Jonsson P. A., Graffmo K. S., Andersen P. M., Brännström T., Oliveberg M., Marklund S. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14157–14162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jonsson P. A., Ernhill K., Andersen P. M., Bergemalm D., Brännström T., Gredal O., Nilsson P., Marklund S. L. (2004) Brain 127, 73–88 [DOI] [PubMed] [Google Scholar]
- 7. Forsberg K., Jonsson P. A., Andersen P. M., Bergemalm D., Graffmo K. S., Hultdin M., Jacobsson J., Rosquist R., Marklund S. L., Brännström T. (2010) PLoS. One. 5, e11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahl I. M., Lindberg M. J., Tibell L. A. (2004) Protein. Expr. Purif. 37, 311–319 [DOI] [PubMed] [Google Scholar]
- 9. Bergemalm D., Forsberg K., Srivastava V., Graffmo K. S., Andersen P. M., Brännström T., Wingsle G., Marklund S. L. (2010) J. Neurochem. 114, 408–418 [DOI] [PubMed] [Google Scholar]
- 10. Heukeshoven J., Dernick R. (1985) Electrophoresis 6, 103–112 [Google Scholar]
- 11. Bergemalm D., Forsberg K., Jonsson P. A., Graffmo K. S., Brännström T., Andersen P. M., Antti H., Marklund S. L. (2009) Mol. Cell Proteomics. 8, 1306–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karch C. M., Prudencio M., Winkler D. D., Hart P. J., Borchelt D. R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7774–7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagai M., Aoki M., Miyoshi I., Kato M., Pasinelli P., Kasai N., Brown R. H., Jr., Itoyama Y. (2001) J. Neurosci. 21, 9246–9254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindberg M. J., Tibell L., Oliveberg M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16607–16612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J., Farr G. W., Zeiss C. J., Rodriguez-Gil D. J., Wilson J. H., Furtak K., Rutkowski D. T., Kaufman R. J., Ruse C. I., Yates J. R., 3rd, Perrin S., Feany M. B., Horwich A. L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1392–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe Y., Morita E., Fukada Y., Doi K., Yasui K., Kitayama M., Nakano T., Nakashima K. (2008) PLoS. One 3, e3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi J. S., Cho S., Park S. G., Park B. C., Lee D. H. (2004) Biochem. Biophys. Res. Commun. 321, 574–583 [DOI] [PubMed] [Google Scholar]
- 18. Manzerra P., Rush S. J., Brown I. R. (1997) J. Cell. Physiol. 170, 130–137 [DOI] [PubMed] [Google Scholar]
- 19. Chen S., Brown I. R. (2007) Cell Stress Chaperones 12, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonsson P. A., Graffmo K. S., Brännström T., Nilsson P., Andersen P. M., Marklund S. L. (2006) J. Neuropathol. Exp. Neurol. 65, 1126–1136 [DOI] [PubMed] [Google Scholar]
- 21. Burke R. E., Marks W. E. (2002) Computational Neuroanatomy: Principles and Methods, 48th Ed., Humana, Totowa, NJ [Google Scholar]
- 22. Ilieva H., Polymenidou M., Cleveland D. W. (2009) J. Cell Biol. 187, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jonsson P. A., Graffmo K. S., Andersen P. M., Brännström T., Lindberg M., Oliveberg M., Marklund S. L. (2006) Brain 129, 451–464 [DOI] [PubMed] [Google Scholar]
- 24. Okado-Matsumoto A., Fridovich I. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9010–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J., Shinobu L. A., Ward C. M., Young D., Cleveland D. W. (2005) J. Neurochem. 93, 875–882 [DOI] [PubMed] [Google Scholar]
- 26. Kieran D., Kalmar B., Dick J. R., Riddoch-Contreras J., Burnstock G., Greensmith L. (2004) Nat. Med. 10, 402–405 [DOI] [PubMed] [Google Scholar]
- 27. Lanka V., Wieland S., Barber J., Cudkowicz M. (2009) Expert. Opin. Investig. Drugs. 18, 1907–1918 [DOI] [PubMed] [Google Scholar]
- 28. Jain M. R., Ge W. W., Elkabes S., Li H. (2008) Proteomics Clin. Appl. 2, 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takamiya R., Takahashi M., Park Y. S., Tawara Y., Fujiwara N., Miyamoto Y., Gu J., Suzuki K., Taniguchi N. (2005) Am. J. Physiol. Cell. Physiol. 288, C253–9 [DOI] [PubMed] [Google Scholar]
- 30. Kabuta T., Kinugawa A., Tsuchiya Y., Kabuta C., Setsuie R., Tateno M., Araki T., Wada K. (2009) Biochem. Biophys. Res. Commun. 387, 121–126 [DOI] [PubMed] [Google Scholar]
- 31. Casareno R. L., Waggoner D., Gitlin J. D. (1998) J. Biol. Chem. 273, 23625–23628 [DOI] [PubMed] [Google Scholar]
- 32. Shinder G. A., Lacourse M. C., Minotti S., Durham H. D. (2001) J. Biol. Chem. 276, 12791–12796 [DOI] [PubMed] [Google Scholar]
- 33. Ezzi S. A., Urushitani M., Julien J. P. (2007) J. Neurochem. 102, 170–178 [DOI] [PubMed] [Google Scholar]
- 34. Urushitani M., Kurisu J., Tateno M., Hatakeyama S., Nakayama K., Kato S., Takahashi R. (2004) J. Neurochem. 90, 231–244 [DOI] [PubMed] [Google Scholar]
- 35. Lamb A. L., Torres A. S., O'Halloran T. V., Rosenzweig A. C. (2001) Nat. Struct. Biol. 8, 751–755 [DOI] [PubMed] [Google Scholar]