Abstract
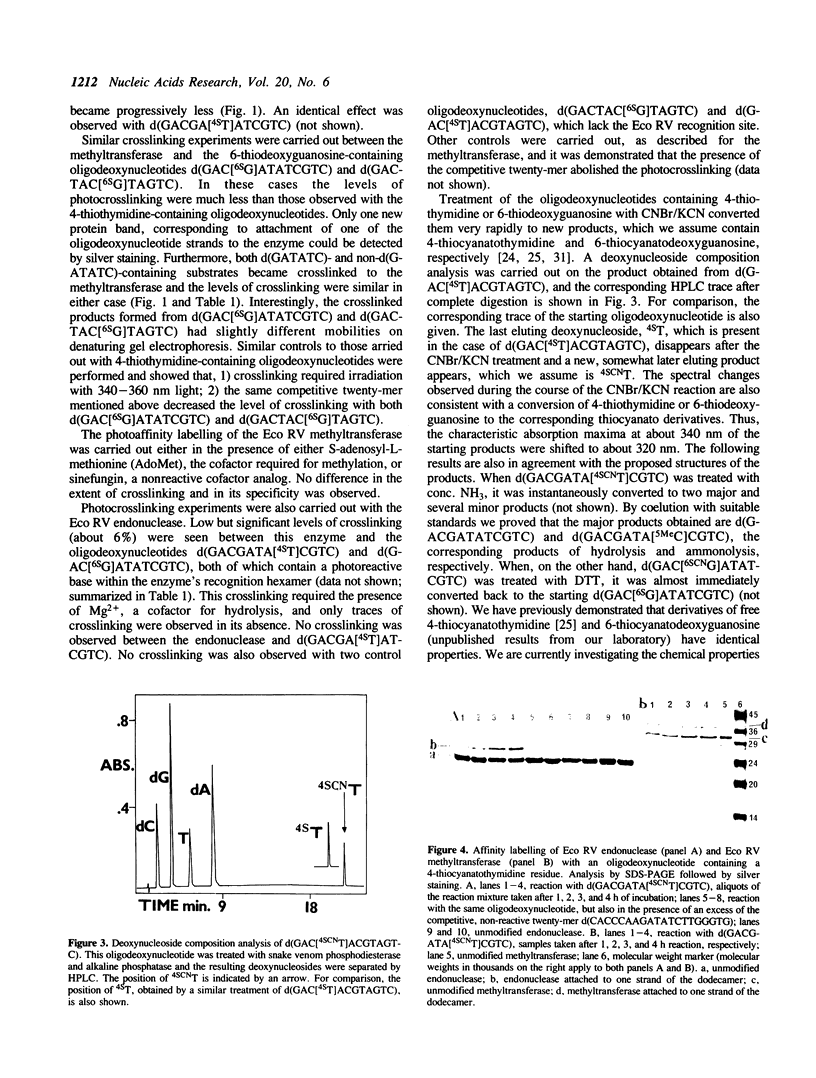
4-Thiothymidine and 6-thiodeoxyguanosine were incorporated into synthetic dodecamers containing the recognition site d(GATATC) of the enzymes Eco RV endonuclease and Eco RV methyltransferase. Upon irradiation with long wavelength UV light (340-360 nm), these oligodeoxynucleotides were photochemically crosslinked to both enzymes. The yields were up to 35% with the methyltransferase, but lower (up to 6%) with the endonuclease. Oligodeoxynucleotides containing 4-thiothymidine generally gave higher yields of crosslinking than those containing 6-thiodeoxyguanosine. Although both specific (i.e. those containing the d(GATATC) sequence) and non-specific (lacking this sequence) photoreactive oligodeoxynucleotides gave rise to crosslinked products, the use of a non-reactive, competitive substrate oligodeoxynucleotide suppressed the crosslinking, indicating that the reaction takes place at the enzymes' active sites. Oligodeoxynucleotides containing 4-thiocyanatothymidine or 6-thiocyanatodeoxyguanosine were also prepared by treatment of the title oligomers with CNBr and KCN. The dodecamers containing 4-thiocyanatothymidine were found to covalently modify both enzymes under study, with levels of crosslinking reaching up to 42% with the endonuclease and up to 12% with the methyltransferase. No crosslinking was observed with oligodeoxynucleotides containing 6-thiocyanatodeoxyguanosine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catalano C. E., Allen D. J., Benkovic S. J. Interaction of Escherichia coli DNA polymerase I with azidoDNA and fluorescent DNA probes: identification of protein-DNA contacts. Biochemistry. 1990 Apr 17;29(15):3612–3621. doi: 10.1021/bi00467a004. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Newman P. C. Synthesis and properties of oligonucleotides containing 4-thiothymidine, 5-methyl-2-pyrimidinone-1-beta-D(2'-deoxyriboside) and 2-thiothymidine. Nucleic Acids Res. 1989 Jul 11;17(13):4957–4974. doi: 10.1093/nar/17.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcy A., Brown R. S., Zabeau M., van Resandt R. W., Winkler F. K. Purification and crystallization of the EcoRV restriction endonuclease. J Biol Chem. 1985 Feb 25;260(4):1987–1990. [PubMed] [Google Scholar]
- Dontsova O., Kopylov A., Brimacombe R. The location of mRNA in the ribosomal 30S initiation complex; site-directed cross-linking of mRNA analogues carrying several photo-reactive labels simultaneously on either side of the AUG start codon. EMBO J. 1991 Sep;10(9):2613–2620. doi: 10.1002/j.1460-2075.1991.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. K., Johnson J. D., Haley B. E. 5-Azido-2'-deoxyuridine 5'-triphosphate: a photoaffinity-labeling reagent and tool for the enzymatic synthesis of photoactive DNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5382–5386. doi: 10.1073/pnas.83.15.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar Y. J., Evans R. K., Beach C. M., Coleman M. S. Interactions of photoactive DNAs with terminal deoxynucleotidyl transferase: identification of peptides in the DNA binding domain. Biochemistry. 1991 Mar 26;30(12):3075–3082. doi: 10.1021/bi00226a014. [DOI] [PubMed] [Google Scholar]
- Fiser I., Scheit K. H., Stöffler G., Kuechler E. Identification of protein S 1 at the messenger RNA binding site of the Escherichia coli ribosome. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1112–1118. doi: 10.1016/0006-291x(74)90427-6. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Scheit K. H. Affinity labeling of E. coli RNA polymerase with substrate and template analogues. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1227–1233. doi: 10.1016/0006-291x(73)90596-2. [DOI] [PubMed] [Google Scholar]
- Godovikova T. S., Grachev M. A., Kutyavin I. V., Tsarev I. G., Zarytova V. F., Zaychikov E. F. Studies of the functional topography of Escherichia coli RNA polymerase. Affinity labelling of RNA polymerase in a promoter complex by phosphorylating derivatives of primer oligonucleotides. Eur J Biochem. 1987 Aug 3;166(3):611–616. doi: 10.1111/j.1432-1033.1987.tb13557.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Photochemical attachment of lac repressor to bromodeoxyuridine-substituted lac operator by ultraviolet radiation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):947–951. doi: 10.1073/pnas.71.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralikrishna P., Cooperman B. S. A photolabile oligodeoxyribonucleotide probe of the peptidyltransferase center: identification of neighboring ribosomal components. Biochemistry. 1991 Jun 4;30(22):5421–5428. doi: 10.1021/bi00236a014. [DOI] [PubMed] [Google Scholar]
- Newman P. C., Nwosu V. U., Williams D. M., Cosstick R., Seela F., Connolly B. A. Incorporation of a complete set of deoxyadenosine and thymidine analogues suitable for the study of protein nucleic acid interactions into oligodeoxynucleotides. Application to the EcoRV restriction endonuclease and modification methylase. Biochemistry. 1990 Oct 23;29(42):9891–9901. doi: 10.1021/bi00494a020. [DOI] [PubMed] [Google Scholar]
- Newman P. C., Williams D. M., Cosstick R., Seela F., Connolly B. A. Interaction of the EcoRV restriction endonuclease with the deoxyadenosine and thymidine bases in its recognition hexamer d(GATATC). Biochemistry. 1990 Oct 23;29(42):9902–9910. doi: 10.1021/bi00494a021. [DOI] [PubMed] [Google Scholar]
- Nwosu V. U., Connolly B. A., Halford S. E., Garnett J. The cloning, purification and characterization of the Eco RV modification methylase. Nucleic Acids Res. 1988 May 11;16(9):3705–3720. doi: 10.1093/nar/16.9.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R., Gilbert W. Contacts between the lac repressor and the thymines in the lac operator. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4973–4976. doi: 10.1073/pnas.74.11.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashev I. G., Dimitrov S. I., Angelov D. Crosslinking proteins to nucleic acids by ultraviolet laser irradiation. Trends Biochem Sci. 1991 Sep;16(9):323–326. doi: 10.1016/0968-0004(91)90133-g. [DOI] [PubMed] [Google Scholar]
- Pleiss M. G., Cerutti P. A. Phototransformation of 4-thiouridine in Escherichia coli valine transfer ribonucleic acid to uridine, cytidine, and N 4 -methylcytidine. Biochemistry. 1971 Aug 3;10(16):3093–3099. doi: 10.1021/bi00792a017. [DOI] [PubMed] [Google Scholar]
- Pleiss M., Ochiai H., Cerutti P. A. Photochemically induced transition of 4-thiouridine to uridine or uridine and cytidine in E. coli transfer ribonucleic acid. Biochem Biophys Res Commun. 1969 Jan 6;34(1):70–76. doi: 10.1016/0006-291x(69)90530-0. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Chihara G. Synthetic nucleosides and nucleotides. I. On synthesis and properties of several thiocyanate derivatives of purines and their ribonucleosides. Chem Pharm Bull (Tokyo) 1967 Jul;15(7):909–914. doi: 10.1248/cpb.15.909. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Alteration of codon recognition of Escherichia coli transfer RNA by modification with cyanogen bromide. Biochim Biophys Acta. 1967 Aug 22;145(1):208–210. doi: 10.1016/0005-2787(67)90680-6. [DOI] [PubMed] [Google Scholar]
- Sawada F. Covalent attachment of 4-thiouridylic acid to ribonuclease A by near-ultraviolet radiation. Biochem Biophys Res Commun. 1975 May 5;64(1):311–316. doi: 10.1016/0006-291x(75)90254-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. D., Badcoe I. G., Clarke A. R., Halford S. E. EcoRV restriction endonuclease binds all DNA sequences with equal affinity. Biochemistry. 1991 Sep 10;30(36):8743–8753. doi: 10.1021/bi00100a005. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The assembly of newly replicated DNA into chromatin. Cold Spring Harb Symp Quant Biol. 1974;38:247–256. doi: 10.1101/sqb.1974.038.01.028. [DOI] [PubMed] [Google Scholar]
- Wolfes H., Fliess A., Winkler F., Pingoud A. Cross-linking of bromodeoxyuridine-substituted oligonucleotides to the EcoRI and EcoRV restriction endonucleases. Eur J Biochem. 1986 Sep 1;159(2):267–273. doi: 10.1111/j.1432-1033.1986.tb09863.x. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Favre A., Barrell B. G. Structure of transfer RNA. Evidence for interaction between two non-adjacent nucleotide residues in tRNA from Escherichia coli. Nature. 1969 Sep 27;223(5213):1331–1333. doi: 10.1038/2231331a0. [DOI] [PubMed] [Google Scholar]