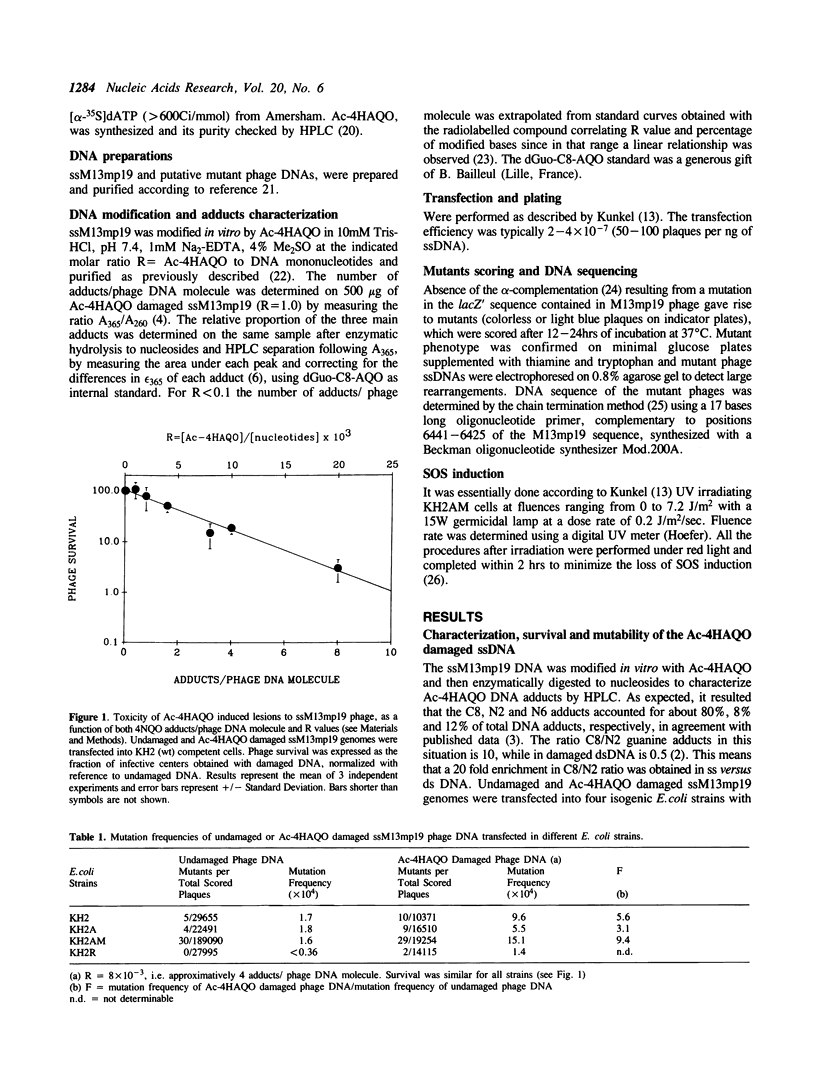
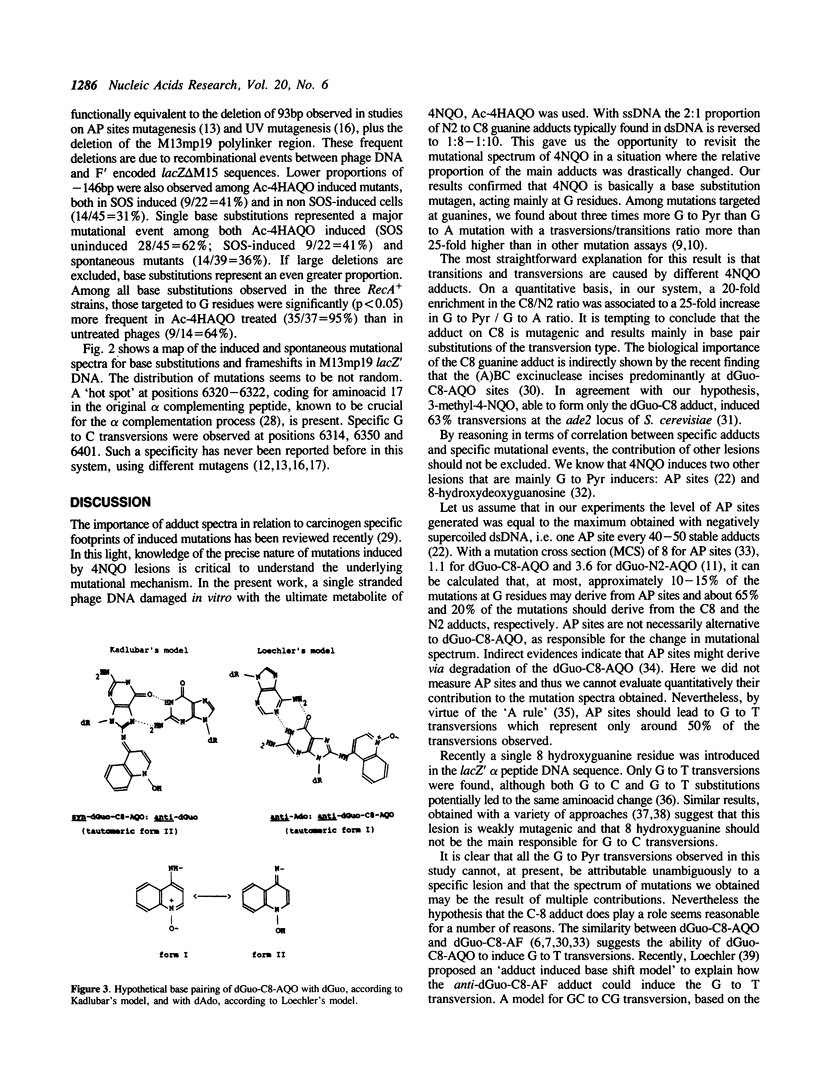
Abstract
4-Nitroquinoline-1-oxide is a potent mutagen and carcinogen which induces two main guanine adducts at positions C8 and N2. In ds or ss damaged DNA the ratio C8/N2 adducts is 1:2 and 8-10:1, respectively. In bacteria and yeast 4NQO has been shown to be a base substitution mutagen acting at G residues inducing mainly G to A transitions. We determined the mutational spectrum induced by the 4NQO metabolite, acetoxy-4-aminoquinoline 1-oxide, in the M13lacZ'/E. coli lacZ delta M15 alpha complementation assay using ssDNA. Among 68 Ac-4HAQO induced mutants, G to Pyr transversion was the most frequent base substitution observed. By comparison with dsDNA based systems, our data suggest that dGuo-C8-AQO induces G to Pyr transversions. A mechanism to explain how this lesion may induce transversions is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Daubersies P., Galiègue-Zouitina S., Loucheux-Lefebvre M. H. Molecular basis of 4-nitroquinoline 1-oxide carcinogenesis. Jpn J Cancer Res. 1989 Aug;80(8):691–697. doi: 10.1111/j.1349-7006.1989.tb01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Galiègue-Zouitina S., Loucheux-Lefebvre M. H. Conformations of poly(dG-dC).poly(dG-dC) modified by the O-acetyl derivative of the carcinogen 4-hydroxyaminoquinoline 1-oxide. Nucleic Acids Res. 1984 Oct 25;12(20):7915–7927. doi: 10.1093/nar/12.20.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Galiègue S., Demeunynck M., Lhomme M. F., Lhomme J., Loucheux-Lefebvre M. H. Identification of 4-acetoxyaminoquinoline from the hydrolysis of 1-acetoxy-4-acetoxyimino-1,4-dihydroquinoline, in vitro and in vivo properties. Chem Biol Interact. 1983 Jan;43(1):87–98. doi: 10.1016/0009-2797(83)90106-0. [DOI] [PubMed] [Google Scholar]
- Bailleul B., Galiègue S., Loucheux-Lefebvre M. H. Adducts from the reaction of O,O'-diacetyl or O-acetyl derivatives of the carcinogen 4-hydroxyaminoquinoline 1-oxide with purine nucleosides. Cancer Res. 1981 Nov;41(11 Pt 1):4559–4565. [PubMed] [Google Scholar]
- Basu A. K., Loechler E. L., Leadon S. A., Essigmann J. M. Genetic effects of thymine glycol: site-specific mutagenesis and molecular modeling studies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7677–7681. doi: 10.1073/pnas.86.20.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. DNA binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli. A correlation between the conformation of the premutagenic lesion and the mutation specificity. J Mol Biol. 1985 Jun 5;183(3):341–351. doi: 10.1016/0022-2836(85)90005-1. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Decuyper-Debergh D., Piette J., Van de Vorst A. Singlet oxygen-induced mutations in M13 lacZ phage DNA. EMBO J. 1987 Oct;6(10):3155–3161. doi: 10.1002/j.1460-2075.1987.tb02626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fronza G., Tornaletti S., Menichini P., Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H., Abbondandolo A., Pedrini A. M. Extent of helix perturbation associated with DNA modification by the o-acetyl derivative of the carcinogen 4-hydroxyaminoquinoline-1-oxide. Biochim Biophys Acta. 1990 Nov 30;1087(3):330–335. doi: 10.1016/0167-4781(90)90007-o. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Ginot Y. M., Perly B., Vigny P., Loucheux-Lefebvre M. H. N2-guanyl and N6-adenyl arylation of chicken erythrocyte DNA by the ultimate carcinogen of 4-nitroquinoline 1-oxide. Cancer Res. 1986 Apr;46(4 Pt 1):1858–1863. [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H. Adducts from in vivo action of the carcinogen 4-hydroxyaminoquinoline 1-oxide in rats and from in vitro reaction of 4-acetoxyaminoquinoline 1-oxide with DNA and polynucleotides. Cancer Res. 1985 Feb;45(2):520–525. [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H. Guanyl-C8-arylamination of DNA by the ultimate carcinogen of 4-nitroquinoline-1-oxide: a spectrophotometric titration. Anal Biochem. 1984 May 1;138(2):454–457. doi: 10.1016/0003-2697(84)90839-x. [DOI] [PubMed] [Google Scholar]
- Galiègue-Zouitina S., Daubersies P., Loucheux-Lefebvre M. H., Bailleul B. Mutagenicity of N2 guanylarylation is SOS functions dependent and reminiscent of the high mutagenic property of 4NQO. Carcinogenesis. 1989 Oct;10(10):1961–1966. doi: 10.1093/carcin/10.10.1961. [DOI] [PubMed] [Google Scholar]
- Galiègue S., Lecocq G., Loucheux-Lefebvre M. H. In vitro DNA reaction with a carcinogen: the O,O'-diacetyl-4-hydroxyaminoquinoline 1-oxide changes of stability of modified DNA. Biochim Biophys Acta. 1980 Oct 17;609(3):383–391. doi: 10.1016/0005-2787(80)90112-4. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Lee M. S., King C. M. Comparison of mutagenesis induced in single- and double-stranded M13 viral DNA by treatment with N-hydroxy-2-aminofluorene. Carcinogenesis. 1988 Aug;9(8):1337–1345. doi: 10.1093/carcin/9.8.1337. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F. A transversion mutation hypothesis for chemical carcinogenesis by N2-substitution of guanine in DNA. Chem Biol Interact. 1980 Sep;31(3):255–263. doi: 10.1016/0009-2797(80)90014-9. [DOI] [PubMed] [Google Scholar]
- Kasinova G. V., Gracheva L. M. Izuchenie molekuliarnoi prirody priamykh mutatsii, indutsirovannykh 4-nitrokhinolin-N-oxide i 3-metil-4-nitrokhinolin-N-oksidom, v gene ADE2 Saccharomyces cerevisiae. Genetika. 1985 Jun;21(6):914–918. [PubMed] [Google Scholar]
- Kohda K., Tada M., Kasai H., Nishimura S., Kawazoe Y. Formation of 8-hydroxyguanine residues in cellular DNA exposed to the carcinogen 4-nitroquinoline 1-oxide. Biochem Biophys Res Commun. 1986 Sep 14;139(2):626–632. doi: 10.1016/s0006-291x(86)80036-5. [DOI] [PubMed] [Google Scholar]
- Kouchakdjian M., Bodepudi V., Shibutani S., Eisenberg M., Johnson F., Grollman A. P., Patel D. J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991 Feb 5;30(5):1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K. E., Villarejo M. R., Fowler A. V., Zamenhof P. J., Zabin I. Molecular basis of beta-galactosidase alpha-complementation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1254–1257. doi: 10.1073/pnas.72.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Apurinic sites as mutagenic intermediates. Cell. 1985 Mar;40(3):483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Loechler E. L. Adduct-induced base-shifts: a mechanism by which the adducts of bulky carcinogens might induce mutations. Biopolymers. 1989 May;28(5):909–927. doi: 10.1002/bip.360280502. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgerink J. T., Retèl J., Loman H. Effects of adduct formation on the biological activity of single- and double-stranded øX174 DNA, modified by N-acetoxy-N-acetyl-2-aminofluorene. Biochim Biophys Acta. 1984 Feb 24;781(1-2):81–91. doi: 10.1016/0167-4781(84)90126-x. [DOI] [PubMed] [Google Scholar]
- Menichini P., Fronza G., Tornaletti S., Galiègue-Zouitina S., Bailleul B., Loucheux-Lefebvre M. H., Abbondandolo A., Pedrini A. M. In vitro DNA modification by the ultimate carcinogen of 4-nitroquinoline-1-oxide: influence of superhelicity. Carcinogenesis. 1989 Sep;10(9):1589–1593. doi: 10.1093/carcin/10.9.1589. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moriya M., Ou C., Bodepudi V., Johnson F., Takeshita M., Grollman A. P. Site-specific mutagenesis using a gapped duplex vector: a study of translesion synthesis past 8-oxodeoxyguanosine in E. coli. Mutat Res. 1991 May;254(3):281–288. doi: 10.1016/0921-8777(91)90067-y. [DOI] [PubMed] [Google Scholar]
- Panigrahi G. B., Walker I. G. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3'-5' exonuclease action of T4 DNA polymerase. Biochemistry. 1990 Feb 27;29(8):2122–2126. doi: 10.1021/bi00460a023. [DOI] [PubMed] [Google Scholar]
- Prakash L., Stewart J. W., Sherman F. Specific induction of transitions and transversions of G-C base pairs by 4-nitroquinoline-1-oxide in iso-1-cytochrome c mutants of yeast. J Mol Biol. 1974 May 5;85(1):51–65. doi: 10.1016/0022-2836(74)90128-4. [DOI] [PubMed] [Google Scholar]
- Sambamurti K., Callahan J., Luo X., Perkins C. P., Jacobsen J. S., Humayun M. Z. Mechanisms of mutagenesis by a bulky DNA lesion at the guanine N7 position. Genetics. 1988 Dec;120(4):863–873. doi: 10.1093/genetics/120.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Kohda K. H., Kawazoe Y. Biomimetic preparation and structure determination of QGI, one of the quinoline-DNA base adducts formed in cells treated with 4-nitroquinoline 1-oxide. Gan. 1984 Nov;75(11):976–985. [PubMed] [Google Scholar]
- Thomas D. C., Husain I., Chaney S. G., Panigrahi G. B., Walker I. G. Sequence effect on incision by (A)BC excinuclease of 4NQO adducts and UV photoproducts. Nucleic Acids Res. 1991 Jan 25;19(2):365–370. doi: 10.1093/nar/19.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. Carcinogens leave fingerprints. Nature. 1992 Jan 16;355(6357):209–210. doi: 10.1038/355209a0. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]


