Abstract
Over the years, plague has caused a large number of deaths worldwide and subsequently changed history, not the least during the period of the Black Death. Of the three plague pandemics, the third is believed to have originated in China. Using the spatial and temporal human plague records in China from 1850 to 1964, we investigated the association of human plague intensity (plague cases per year) with proxy data on climate condition (specifically an index for dryness/wetness). Our modeling analysis demonstrates that the responses of plague intensity to dry/wet conditions were different in northern and southern China. In northern China, plague intensity generally increased when wetness increased, for both the current and the previous year, except for low intensity during extremely wet conditions in the current year (reflecting a dome-shaped response to current-year dryness/wetness). In southern China, plague intensity generally decreased when wetness increased, except for high intensity during extremely wet conditions of the current year. These opposite effects are likely related to the different climates and rodent communities in the two parts of China: In northern China (arid climate), rodents are expected to respond positively to high precipitation, whereas in southern China (humid climate), high precipitation is likely to have a negative effect. Our results suggest that associations between human plague intensity and precipitation are nonlinear: positive in dry conditions, but negative in wet conditions.
Keywords: climate variations, Yersinia pestis, generalized additive modeling
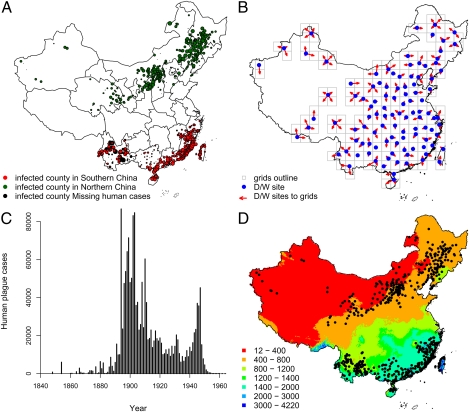
From 1954 to 1997, human plague was reported in 38 countries (1), making the case of plague a reemerging threat to human health (1–3). Historically, there have been three pandemics, which collectively resulted in the death of ≈200 million people (4). The first pandemic, named the “Justinianic Plague,” occurred in AD 541 in the Egyptian port of Pelusium on the eastern edge of the Nile Delta. It spread quickly to the Middle East, and to Mediterranean Europe, where it remained virulent for more than two centuries (5). The second pandemic, the “Black Death,” was responsible for ≈25 million deaths in Europe from 1347 to 1665 (6, 7). The third pandemic resulted in ≈2.2 million deaths (8), originating in Yunnan Province of southwest China in 1772, spreading beyond China's borders after it reached the southeast coast. This plague spread from Yunnan Province in 1867 to Beihai on the coastline. It was then observed in Taiwan Province in 1869 and Hainan Island in 1882. In February 1894, plague spread to Guangzhou Province (Canton) and resulted in the death of ≈70,000 people (9). In the same year, plague erupted in Hong Kong and spread throughout the world via marine shipping vessels. Plague appeared in both northern and southern parts of China. In northeast China, plague killed tens of thousands of people between the years of 1910 and 1920 (see Fig. 1 for spatial and temporal distribution of human plague cases during the third pandemic in China).
Fig. 1.
Human plague data of the third pandemic and dryness/wetness (D/W) index in China during AD 1772–1964. (A) Locations with reported plague occurrence. Red and green circles show plague-infected counties in southern and northern China, respectively. The radii of the circles scale with the amount of human plague cases. Black circles indicate infected counties with missing values for the amount of human cases (mainly in Yunnan Province in early periods, see Materials and Methods). The plot shows serious plague outbreaks in Southeast coastal China, Inner Mongolia, and Northeast China. (B) Locations of climate stations with D/W data (blue points). Arrows indicate which climate station was used as reference for each 200 × 200 km quadrate used as basis for plague analysis. (C) Time series of human plague cases from AD 1850–1964. (D) Annual mean precipitation pattern and political central locations of plague-infected counties in China. Precipitation is <800 mm in northern China, >1,400 mm in southeastern China and mainly 800–1,400 mm in southwestern China Gridded precipitation data were downloaded from WorldClim database (http://www.worldclim.org). Northern and southern China refer to two regions within China, geographically divided by the Huai River-Qinling Mountains line, which approximates the 800-mm isohyet (Fig. 1D).
In 1894, during the Hong Kong epidemic, Alexandre Yersin first isolated the bacterium Yersinia pestis, the pathogen causing plague (4, 9, 10). Plague is generally vectored by fleas (11), although Wu demonstrated airborne spread of plague, and that coughing patients might propel Y. pestis-laden droplets (9, 12). Depending on the route of infection (13), the three plague forms are as follows: (i) Bubonic plague, which is the most common form, resulting from a bite from a Y. pestis-positive flea after which the plague bacillus enters the body and travels through the lymphatic system to the nearest lymph node, (ii) Septicaemic plague, occurring when infection spreads directly through the bloodstream without evidence of a “bubo,” and (iii) Pneumonic plague, occurring as a result of inhalation of aerosolized Y. pestis-laden droplets that can be transmitted from human to human.
Plague exists in natural foci involving transmission between rodent hosts and flea vectors (11). Both reservoir and vector animals differ geographically, especially between northern and southern China. Specifically, 10 types of natural plague foci can be identified based on several criteria, such as natural geographic landscape, host species, flea species, and Y. pestis strain (8). Humans are highly susceptible to plague infection (2) and, in the natural foci, occasional human epidemics arise from bubonic plague persisting in rodent populations (14).
Plague outbreaks, both among rodents and humans, have been linked to climate variability. Stenseth et al. (15) demonstrated that plague intensity among great gerbils (Rhombomys opimus) in central Asia was highest in years with wet summers and warm springs; a 1 °C increase in spring would lead to a 50% increase in plague prevalence among gerbils. Climate-driven synchrony of dynamics of gerbil abundances across geographical areas is likely a condition for large-scale plague outbreaks (16). In North America, the rate of plague transmission between black-tailed prairie dog (Cynomys ludovicianus) colonies increases with increasing precipitation, whereas the rate of infection from unknown sources decreases in response to hot weather (17).
In New Mexico, human plague cases occurred more frequently after winter and spring periods with above-average precipitation (18). Time-lagged winter-spring precipitation was also positively associated with the frequency of human plague cases in the southwestern United States (19). Plague outbreaks have further been linked to large-scale climate fluctuations, specifically, Pacific Decadal Oscillation and El Ninño Southern Oscillation explain much of the plague variability in the western United States (20). The trophic cascade hypothesis was proposed to explain the positive effect of precipitation on human plague (18, 21). According to this hypothesis, increase of rainfall often leads to an increase of food resources and then to increased rodent and flea populations. The trophic cascade hypothesis has been tested in studies of C. ludovicianus rodent–host populations of plague in the southwestern United States; the climatic predictors of plague occurrence in the rodents are quite similar to those of human plague cases in the same area. This correspondence provides support for a (temperature-modulated) trophic cascade model for plague (22). In contrast to the studies from North America, however, in Vietnam most human plague cases have been found to occur in dry seasons (23); the risk of plague increased during dry seasons when rainfall was <10 mm (24), showing that the trophic cascade hypothesis may not be generally valid.
The relationship between human plague and climate variation is still not fully understood, and few studies cover both temperate and tropical (subtropical) zones. To shed new light on the general association between climate and plague, there is therefore a need for studies based on long-term and larger spatial scale data. Using China's human plague data, collected over a period of >100 y, with 1.6 million confirmed infected cases, we here provide insight on the relationship between plague intensity and the level of precipitation in different parts of China. Our analysis reveals different responses of human plague to climate variations in southern and northern China, explainable through the contrasting climate in these two regions.
Results
We used generalized additive models to explore the association between climate and human plague intensity (see Fig. 1 for data coverage). A dryness/wetness (D/W) index from 120 stations across China was used as climate proxy for historical precipitation, and we quantified its effects on plague both in the current and the following year. Besides the D/W proxy, the regional model detailed below also accounts for temporal and spatial correlation and plague spread across areas by including, as predictors, plague intensity in the same area (200 × 200 km grid) and in surrounding areas in the previous year (Materials and Methods, model A).
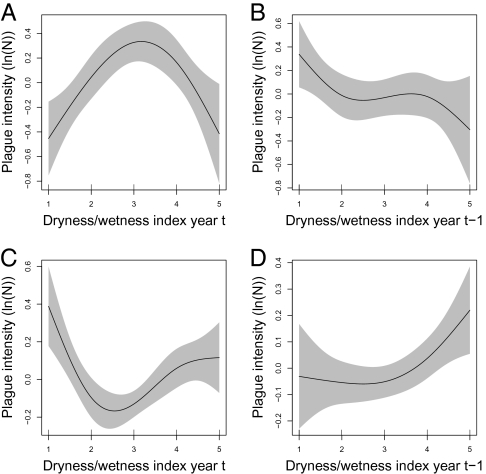
Analyzing data from northern and southern China separately, we found that human plague intensity in northern China exhibits a dome-shaped association with dryness/wetness of the current year (F2.5, 432.6 = 7.1; P < 0.001; Fig. 2A). Both extremely wet (D/W index = 1) and extremely dry (D/W index = 5) conditions are associated with reduced plague intensity compared with normal years (D/W index = 3). Further, plague intensity in northern China was found to be progressively lower with an increase in dryness from the preceding year (F2.6, 432.6 = 2.7; P < 0.05; Fig. 2B). In contrast, human plague intensity in southern China exhibits a U-shaped association with dryness/wetness of the current year, with extremely wet and extremely dry conditions being associated with increased plague intensity compared with normal years (F2.9, 580.7 = 6.3; P < 0.001; Fig. 2C). Plague intensity in southern China was found to be progressively higher with an increase in dryness from the preceding year (F1.9, 580.7 = 3.0; P < 0.05; Fig. 2D). In both northern and southern China, plague intensity in a given area is positively associated with the plague intensity in the area in the preceding year (SI Appendix, Fig. S1 C and F). In southern China, there is also a positive association with plague intensity in surrounding areas in the preceding year, whereas in northern China, this association is strongly nonlinear but generally negative (SI Appendix, Fig. S3 C and F).
Fig. 2.
Partial effects on the intensity of human plague cases of the D/W index in the current year (A) and previous year (B) in northern China and of the D/W index in the current year (C) and previous year (D) in southern China. D/W = 1, very wet; 5, very dry. Regional models for northern and southern China were fitted separately and also accounted for effects of year, location (within each region), and previous year's plague intensity in the same and in adjacent quadrates (SI Appendix, Figs. S1 and S3).
Second, we analyzed data from across all of China in one statistical model (Materials and Methods, model B) to explore in more detail how effects of dryness/wetness depend on location and, thus, local climate conditions. China's climate is characterized by extreme dryness in the north and extreme wetness in the south. The results corroborate the general finding that (extreme) dryness tends to inhibit plague in northern parts and favor plague in southern parts of China.
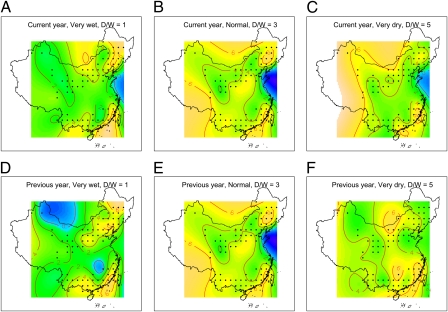
Fig. 3 shows the spatial response pattern of human plague intensity to extremely wet (D/W index = 1), normal (D/W index = 3), and extremely dry (D/W index = 5) climatic conditions of the current year (Fig. 3 A–C) and the previous year (Fig. 3 D–F). Patterns were estimated by a generalized additive model that allowed for spatially variable and nonlinear effects of climate. The results suggest considerable heterogeneities in the effects of climate within northern and southern China, and that the general effects described above are both caused by changes in plague intensity in the core areas where plague occurs and by changes in the extent of these areas.
Fig. 3.
Predicted plague intensity of human plague cases from model B covering both northern and southern China under very wet, normal, and very dry conditions in the current year (A–C) and previous year (D–F). The color gradient represents predicted log-transformed plague intensity ranging from yellow (strong) to blue (weak). Points show the center grids of plague-infected area. Contour lines represent log- transformed predicted plague intensity equal to 0, 2, 4, and 6.
Discussion
Our results demonstrate that the response of human plague to dryness/wetness index was nonlinear at a regional scale. In general, drier conditions were associated with decreased plague intensity in northern China and with increased plague intensity in southern China. Extreme wetness had, however, opposite short-term effects, leading to a dome-shaped effect of the current-year dryness/wetness index in the north and a U-shaped effect in the south. The contrasting effects found for northern and southern China suggest that the effects of precipitation on human plague intensity may differ between climatic zones.
Increased plague intensity after wet years, and reduced plague intensity during and after dry years in northern China is consistent with the trophic cascade hypothesis (18, 21). Abundant precipitation has been demonstrated to promote population growth of small rodents through increased food availability in many continents (25–27). Fluctuations in abundance of a wildlife reservoir may explain the temporal variation in human cases of a zoonosis because high abundance may lead to more contact between humans and animals, but also to outbreaks of disease within the reservoir population (28). In the semiarid grasslands of Inner Mongolia, China, preliminary studies have suggested that high rainfall facilitates increased population levels of Mongolia gerbils (Meriones unguiculatus) by promoting seed production and growth of grass (29, 30). Ma et al. (31) reported that precipitation promoted vegetation growth in the grasslands of Inner Mongolia, where community biomass production has also been shown to be positively correlated to January–July precipitation (31, 32). Our results for northern China are thus explainable through vegetation-mediated effects of precipitation on rodent populations and are in line with a recent study showing that human plague cases were positively associated with wet conditions in Inner Mongolia, northern China, whereas no significant relationship was detected in Fujian Province. southeast China (33). However, in arid north China, although wet condition is good for rodents too heavy rain may kill rodents directly by flooding their burrows (rodents carrying plague pathogens only live in field). This phenomenon may help to explain the bell-shaped responses of plague intensity to precipitation in the current year in northern China.
The traditional trophic cascade hypothesis fails to explain the increased plague intensity recorded during, and after, dry years in southern China. The plague foci here are dominated by Rattus tanezumi, Apodemus chevrieri, and Eothenomys miletus (8). Rattus norvegicus may also play a significant role in plague transmissions. R. tanezumi and R. norvegicus live both in human houses and in fields. Previous studies showed that a field population of R. norvegicus was significantly and negatively associated with precipitation during April to August in croplands of the Dongting Lake regions, Hunan Province, southern China (34). A population of Rattus losea inhabiting rice fields was found to be significantly and negatively associated with precipitation in Guangdong Province. also southern China (35). Heavy rain also caused high mortality of the Yangtze voles (Microtus fortis) living in close proximity to Dongting Lake's beaches (36, 37), and its population size was found to be negatively correlated with water levels of Dongting Lake (38). Precipitation thus tends to have negative effects on rodent plague hosts in southern China.
Heavy precipitation may also inhibit population growth of fleas in the region, as shown by a study from Vietnam, adjacent to southern China, demonstrating that a flea index decreased exponentially with monthly average rainfall (24). The flea Xenopsylla cheopis on Rattus rattus increased threefold monthly during the dry seasons from 1966 to 1968 in Vietnam (39). Experimental work has shown that relative humidity significantly affects the survival of fleas (40). Climate conditions were found to influence human plague cases in Vietnam by regulating the density of the flea population and by regulating the efficiency of X. cheopis in transmitting the plague bacterium (23).
Heavy rain may also increase contact with human populations by driving the rodents into human habitats. Indeed, Madsen and Shine (41) found that rats living in soil crevices on the Adelaide River floodplains, in Australia's wet-dry tropic region were forced to higher ground during wet season flooding. In wet south China, although wet conditions are not good for rodents in the field, heavy rain may push rodents into living houses (the rodents carrying plague pathogens move frequently between field and house), which may increase risk of plague infection to people. This behavioral response may help to explain the U-shaped response of plague intensity to precipitation in the current year in southern China.
The large-scale pattern in the effect of precipitation on plague in China coincides with the pattern in climate. For the regions with human plague occurrences, the annual precipitation is <800 mm in northern China, 800–1,400 mm in southwestern China, and ≈1,400–3,000 mm in southeastern China (Fig. 1D). The difference in precipitation between northern and southern China may explain why associations between human plague and precipitation tend to be positive in drier regions, but negative in wetter regions. It is notable that the most apparent effects consistent with this pattern are found in the case of a one-year time lag, suggesting lagged effects of precipitation on vegetation and thereby rodent populations. In comparison, the immediate effects of dryness/wetness are more complex and nonlinear; immediate effects of extreme dryness, but not of extreme wetness (compared with normal conditions), conform to the pattern above. We believe these differences are linked to droughts and floods affecting vegetation, vectors, and hosts through several mechanisms with different time lags. In the short term, several mechanisms are likely to operate at the same time, whereas in the long term, effects through vegetation and rodent population growth may dominate.
Not only climate and rodent communities differed between northern and southern China. In particular, the occurrence of the pneumonic type of plague appeared to differ between northern and southern China, implying potentially different mechanisms linking climate to plague in the different regions. Pneumonic plague is transmitted through inhalation of aerosolized infection-laden droplets from human to human and is less common but more virulent than bubonic plague. From incomplete human plague records from 1947 to 1962, 267 pneumonic cases of 4,123 plague cases were recorded, with conspicuous geographic differences in frequency (42). In the marmot foci of northern China, >70% of the 221 cases recorded were pneumonic plague, in contrast to <5% in southern China (SI Appendix, Fig. S7). Pneumonic plague is reportedly more likely observed in cold, humid environments and overcrowded living conditions (2). Based on surveillance and control of pneumonic plague in China (mainly in northern China) during the third pandemic, Wu believed that humans were more susceptible to pneumonic plague in cold and dry weather, a climate that encouraged small, shared living spaces, often resulting in living conditions that did not allow for adequate ventilation (9). In addition, relative humidity is believed to affect droplet persistence (12).
It is notable that the human plague cases are clustered in two fairly tight bands in the middle of northern China and along the coast of southern China, with no reports of human plague cases in the area between. The two clustered areas closely match the distribution areas of natural plague foci (8; also see SI Appendix, Fig. S8); there are no natural plague foci in the central region of China because of extensive land cultivation and crop plantations.
We found that human plague of the previous year showed a significant positive effect on plague intensity of the current year (SI Appendix, Figs. S1C and S3C), revealing the positive-regulation nature of infectious diseases. However, we found that surrounding spatial transmission showed an overall positive, nonlinear effect on plague intensity in southern China (SI Appendix, Fig. S3F), but an overall negative nonlinear effect in northern China (SI Appendix, Fig. S1F). The reason for this contrast is unclear but may be related to differences in outbreak and transmission patterns. In the north, plague pandemics occurred scattered, noncontinuously in space, whereas in the south, plague pandemics occurred in large regions, continuously. This difference may result in the opposite associations in space.
An important finding of this study revealed by the regional models is the nonlinear association between plague levels in China and climate. This result is at least partly explainable through nonlinear dryness/wetness effects on rodent population abundance. Several studies have demonstrated reduced survival, reproduction, and/or population size of rodents when precipitation is either higher or lower than optimal (27, 43). Higher precipitation increases abundance by bottom-up regulation of primary production up to a threshold, above which precipitation becomes detrimental (e.g., through drowning of rodents or spoiling of their food storage; ref. 27). The large-scale patterns in the effect of climate on human plague occurrences can thus generally be understood in terms of climate effects on rodents. Other factors, such as precipitation effects on rodent behavior, flea survival, human migration, and bacteria survival in droplets, complicate the effects of climate variation, especially when examining its short-term effects. Untangling the separate effects of these factors on human plague occurrences should be investigated in further studies.
Materials and Methods
Historical Plague Data.
Human plague is well documented in China's historical, medical, and chorographical records. From 1963 to 1980, with the support of the Chinese government, the locations (villages) and number of human plague cases (both number infected and deaths from) during AD 1644–1964 in China were investigated and recorded. These investigations involved the analysis of data retrieved from historical records, visits with plague survivors, and the surveying of graveyards. This investigation and analysis of data were compiled in a report (42) that indicated that in the period from 1772 to 1850, human plague was reported intermittently in Yunnan Province. After 1850, the data are sufficient for conducting modeling analysis; therefore, data from 1850 to 1964 was used for statistical analysis in this study (Fig. 1C).
For the spatial analysis, we identified the coordinates by using the high-resolution digital aerial map under Krasovsky 1940 Albers Coordinated System. We divided China into 200 × 200 km quadrates according to the highest resolution of climate data during this period (Fig. 1B). We defined the plague intensity as the total number of human plague cases per quadrate per year.
Climate Proxy Data.
Spatial-temporal dryness/wetness (D/W) index (a proxy of summer precipitation) data were derived from the ref. 43. The book was comprised of information from >2,200 local annals and other historical records. There are 120 stations with historical record of drought and flood. The D/W index is classified into five categories from 1 to 5, representing extremely wet (category 1) to extremely dry conditions (category 5). The scaled D/W index shows approximately (inversely) linear relation with precipitation (SI Appendix, Fig. S9). We used the D/W index from 67 stations to evaluate the wet or dry conditions of each quadrate, selecting the nearest station for each quadrate (lowest Euclidean distance to the center of the quadrate) (Fig. 1B and SI Appendix, Fig. S10). Quadrates with no meteorological station within a 200-km range were not included in the statistical analysis. We used only quadrates having records of human plague cases. There was significant autocorrelation in the D/W series (SI Appendix, Table S2), but this autocorrelation did not seem to bias results (see below). For detailed information about D/W data, see SI Appendix.
Statistical Modeling.
Generalized additive models (44) with quasi-Poisson distribution family (link function was logarithm) were used to analyze the gridded plague data in China. All analysis was carried out in the R environment (version 2.5.1) via the mgcv library (version 1.3–29) (45, 46). The optimal roughness of the smooth terms was determined by minimizing the generalized cross validation value (GCV) (47). The GCV of a model is a proxy for the model's out-of-sample predictive mean squared error (48) and was also used to compare alternative model formulations. A model with lower GCV has more explanatory power and was hence preferred to ones with higher GCV. Model selection started with a formulation that contained all covariates under scrutiny, and then we performed a backward selection. We explored effects of the D/W index on human plague intensity at both a regional scale (analyzing data for northern China and southern China separately) and at a whole-China scale; in both models, gridded (quadrate) data were analyzed, but in the former models, climate effects were assumed a priori to be identical across each modeled area, whereas in the latter model, spatial patterns in the effects of climate were derived from the data.
Model A.
The regional models were built to investigate the effects of D/W index on plague occurrences separately in northern and southern China, respectively (also see SI Appendix). The initial model was as follows:
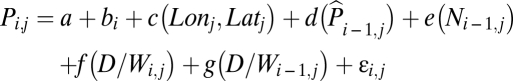 |
Here, Pi,j is the natural logarithm of the number of plague cases in quadrate j and in the year i. Parameter a is the overall intercept, bi is a smooth (natural cubic spline) function of year, c(Lonj, Latj) is a 2D smooth function modeling the spatial effects [thin plate spline with maximally 24 degrees of freedom (df), i.e., 25 knots], 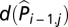 is a smooth function of log transformed observed plague occurrence at the same quadrate the previous year (added 1 to avoid taking the logarithm of zeros; same below), e(Ni-1,j) is a smooth function of log transformed total plague intensity in the eight adjacent quadrates the previous year, f(D/Wi,j) is a smooth function of D/W of the current year, g(D/Wi-1,j) is a smooth function of D/W of last year, and εi,j are uncorrelated random errors of zero mean and finite variance. The d, e, f and g smooth functions were natural cubic spline functions with maximally 4, 4, 3, and 3 df, respectively. Note that the term
is a smooth function of log transformed observed plague occurrence at the same quadrate the previous year (added 1 to avoid taking the logarithm of zeros; same below), e(Ni-1,j) is a smooth function of log transformed total plague intensity in the eight adjacent quadrates the previous year, f(D/Wi,j) is a smooth function of D/W of the current year, g(D/Wi-1,j) is a smooth function of D/W of last year, and εi,j are uncorrelated random errors of zero mean and finite variance. The d, e, f and g smooth functions were natural cubic spline functions with maximally 4, 4, 3, and 3 df, respectively. Note that the term 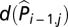 was included to account for serial correlation. Alternatively, one can replace
was included to account for serial correlation. Alternatively, one can replace 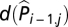 with
with 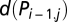 , resulting in a so-called parameter-driven model (48), which is a more elegant approach but makes the model estimation more difficult because
, resulting in a so-called parameter-driven model (48), which is a more elegant approach but makes the model estimation more difficult because 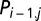 are unobservable. Hence, we preferred the observation-driven model incorporating the term
are unobservable. Hence, we preferred the observation-driven model incorporating the term 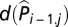 .
.
Model B.
The spatial model for whole-China was built to estimate the spatial patterns in the effects of D/W on the (natural logarithm of the) intensity of plague occurrences:
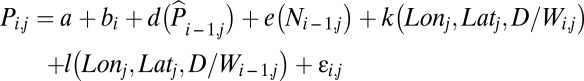 |
The previous descriptions of the above terms still apply but in this case, k(Lonj,Latj,D/Wi,j) and l(Lonj,Latj,D/Wi-1,j) are tensor-product anisotropic smooth functions constructed as the sum whose summands are products of an unidimensional smooth function of the current year's (for the k function) and the previous year's (for the l function) D/W (natural cubic spline function with maximally 4 df) with a 2D smooth function of longitude and latitude (thin-plate regression spline with maximally 39 df).
Model diagnostics (SI Appendix, Figs. S2 and S4) for models A for northern China and southern China did not reveal residual temporal correlations and little or no residual spatial correlations. The residuals from plague models without climate did not show positive temporal autocorrelation (SI Appendix, Fig. S11), indicating that the modeled effects of D/W were not caused by low-frequency trends in both climate and plague. The residuals of model B are approximately white (uncorrelated over space), but there are some slight temporal autocorrelations (SI Appendix, Fig. S6). As a conservative way to evaluate the out-of-sample predictive power of the models, we conducted genuine cross-validation, leaving out data for whole years at a time (SI Appendix, Table S1). Although mean leave-one-year-out cross-validatory sum of squared errors (CV) of model A could have been reduced slightly by choosing somewhat different restrictions on the df, the results give no indication of any dramatic effects of overfitting. It should be noted, however, that the effect of D/Wi-1 for northern China did not reach statistical significance in some of the alternative formulations with different restrictions on the df for spatial and autoregressive terms. Mean CV of model B was lower than the sample size-weighted mean of CV of model A fitted to northern and southern China separately, showing that the relatively complex spatial patterns in the effects of D/W depicted by the model are indeed supported by the data.
In this study, we only considered the effects of D/W index of the current year and the previous year. We did so because time lags of climate effects on plague have generally been found to be one year at most (15, 19) and longer time lags may not be biologically plausible. Nevertheless, as an additional test of the robustness of the D/W effects, we tested for significant effects of D/W at other time lags, expecting to find none, given that the plague-D/W association is nonspurious. Indeed, these additional analyses indicate that other time lags (−5 to 5 y) have no extra effects on plague intensity after the autocorrelation effects of D/W of the current and previous years are removed (SI Appendix, Tables S3 and S4).
Supplementary Material
Acknowledgments
We thank the following people for their great contributions in compiling the historical plague data in China: Naiwu Chen, Jiefan Zhang, Guangming Wang, Chengzhong Yan, Yongling Zhao, Zongxiao Mao, Chongxi Lei, Kechang Gong, Yan Yang, Zizhong Yu, Gengxing Wang, Yuanmin Chen, Shengchang Tong, Di Zhang, Wu Ma, Zuyin Pan, Zhenhua Liu, Biaocheng Zeng, Bohong Pan, Jiabao Huang, Xiyi Fu, Hua Zhang, Daxi Lin, Renguang Hu, Rongxuan Shen, Liusheng Liu, Guanwen Wei, Wuyi Yang, Wenyuan Wong, Ligong Zhou, Aihua Huang, Zhenpan Hong, Hongqing Lu, Jingya Xiao, and Tianmin Sun; the two anonymous reviewers for their valuable comments and suggestions in improving the manuscript; and Laura Johnson Hill for improving the English of this manuscript. This work was supported by National Basic Research Program Grant 2007CB109101 of the Ministry of Science and Technology (MOST) China, Chinese Academy of Sciences Grant XDA05080701, and Special Infectious Disease Program of MOST Grant 2008ZX10004-010, and support from the Centre for Ecological and Evolutionary Synthesis of the University of Oslo. The work of K.-S.C. was partially supported by National Science Foundation Grant DMS-0934617. The data collection and sorting spanned many years, from 1963 to 1980 (see ref. 42).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019486108/-/DCSupplemental.
References
- 1.World Health Organization Plague Manual: Epidemiology Distribution, Surveillance and Control. Wkly Epidemiol Rec. 1999;74:447. [PubMed] [Google Scholar]
- 2.Stenseth NC, et al. Plague: Past, present, and future. PLoS Med. 2008;5:e3. doi: 10.1371/journal.pmed.0050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Plague in China. 2009. http://www.who.int/csr/don/2009_08_11/en/index.html. Accessed August 11, 2009.
- 4.Perry RD, Fetherston JD. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little LK. Plague and the End of Antiquity: The Pandemic of 541-750. New York: Cambridge Univ Press; 2007. pp. 3–32. [Google Scholar]
- 6.Haensch S, et al. Distinct clones of Yersinia pestis caused the black death. PLoS Pathog. 2010;6:e1001134. doi: 10.1371/journal.ppat.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn GC. Encyclopedia of Plague and Pestilence: From Ancient Times to the Present. New York: Facts On File; 2008. pp. 31–33. [Google Scholar]
- 8.Liu Y, Tan J. The Atlas of Plague and Its Environment in the People's Republic of China. Beijing: Science Press; 2000. pp. I–IV. [Google Scholar]
- 9.Wu L, Chen YH, Pollitzer R, Wu CY. Plague: A Manual for Medical and Public Health Workers. Shanghai: Weishengshu Natl Quarantine Serv Shanghai Station; 1936. pp. 1–55. [Google Scholar]
- 10.Clinical, Characters P The plague at Hong-Kong. Brit Med J. 1894;2:423–427. [Google Scholar]
- 11.Gage KL, Kosoy MY. Natural history of plague: Perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 12.Erickson DL, Hinnebusch BJ. Pneumonic Plague. In: Anderson B, Friedman H, Bendinelli M, editors. Microorganisms and Bioterrorism. New York: Springer; 2006. pp. 155–179. [Google Scholar]
- 13.World Health Organization Plague overview. 2005. http://www.who.int/mediacentre/factsheets/fs267/en/. Accessed February, 2005.
- 14.Keeling MJ, Gilligan CA. Metapopulation dynamics of bubonic plague. Nature. 2000;407:903–906. doi: 10.1038/35038073. [DOI] [PubMed] [Google Scholar]
- 15.Stenseth NC, et al. Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA. 2006;103:13110–13115. doi: 10.1073/pnas.0602447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kausrud KL, et al. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc Biol Sci. 2007;274:1963–1969. doi: 10.1098/rspb.2007.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snäll T, O'Hara RB, Ray C, Collinge SK. Climate-driven spatial dynamics of plague among prairie dog colonies. Am Nat. 2008;171:238–248. doi: 10.1086/525051. [DOI] [PubMed] [Google Scholar]
- 18.Parmenter RR, Yadav EP, Parmenter CA, Ettestad P, Gage KL. Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hyg. 1999;61:814–821. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- 19.Enscore RE, et al. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960-1997. Am J Trop Med Hyg. 2002;66:186–196. doi: 10.4269/ajtmh.2002.66.186. [DOI] [PubMed] [Google Scholar]
- 20.Ben Ari T, et al. Human plague in the USA: The importance of regional and local climate. Biol Lett. 2008;4:737–740. doi: 10.1098/rsbl.2008.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben Ari T, et al. Interannual variability of human plague occurrence in the Western United States explained by tropical and North Pacific Ocean climate variability. Am J Trop Med Hyg. 2010;83:624–632. doi: 10.4269/ajtmh.2010.09-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collinge SK, et al. Testing the generality of a trophic-cascade model for plague. EcoHealth. 2005;2:102–112. [Google Scholar]
- 23.Cavanaugh DC, Marshall JD., Jr The influence of climate on the seasonal prevalence of plague in the Republic of Vietnam. J Wildl Dis. 1972;8:85–94. doi: 10.7589/0090-3558-8.1.85. [DOI] [PubMed] [Google Scholar]
- 24.Pham HV, Dang DT, Tran Minh NN, Nguyen ND, Nguyen TV. Correlates of environmental factors and human plague: An ecological study in Vietnam. Int J Epidemiol. 2009;38:1634–1641. doi: 10.1093/ije/dyp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton GR. Population dynamics of an outbreak of house mouse (Mus domesticus) in the mallee wheatlands of Australia – hypothesis of plague formation. J Zool (Lond) 1989;219:495–515. [Google Scholar]
- 26.Pech RP, et al. Models for predicting plagues of house mice (Mus domesticus) in Australia. In: Ecologically-based RM, editor. Singleton G, Hinds L, Leirs H, Zhang ZB. Canberra, Australia: Aust Centre for Intl Agric Res; 1999. pp. 81–113. [Google Scholar]
- 27.Brown JH, Ernest SKM. Rain and rodents: Complex dynamics of desert consumers. Bioscience. 2002;52:979–987. [Google Scholar]
- 28.Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector Borne Zoonotic Dis. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. [DOI] [PubMed] [Google Scholar]
- 29.Li ZL, Zhang WR. Analysis on the relation between population of Meriones unguiculatus and factors of meterological phenomena. Acta Theriologica Sinica. 1993;13:131–135. [Google Scholar]
- 30.Xia W, Liao C, Zhong W, Sun C, Tian Y. Population dynamics and regulation of Mongolia gerbils (Meriones unguiculatus) In north Yinshan mountain, Inner Mongolia of China. Acta Theriologica Sinica. 1982;2:51–71. [Google Scholar]
- 31.Ma W, Yang Y, He J, Zen H, Fang J. Relationship between biological mass and environmental factors in Inner Mongolia of China. Science in China. 2008;38:84–92. [Google Scholar]
- 32.Bai YF, Han XG, Wu JG, Chen ZZ, Li LH. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Wang W, Yang L, Tan J. Coupling analysis of climate change with human plague prevalence. Chin J Zoonoses. 2005;21:887–891. [Google Scholar]
- 34.Chen A. The ecological characteristics and integrative management of agricultural rodent pests in southern China. In: Wang ZW, Zhang ZB, editors. Theory and Practice of Rodent Pest Management. Beijing: Science Press; 1996. pp. 247–312. (Chinese) [Google Scholar]
- 35.Chang H, Zhang G. Studies on the short-term forecasting models of population abundances of Rattus losea. Zhongshan University Journal Series. 1995;1:75–80. [Google Scholar]
- 36.Li B, et al. Forecast of population occurrences of Microtus fortis in the Dongting Lake region. Plant Protection. 2007;33:134–136. [Google Scholar]
- 37.Zhang ZB, Xu L, Guo C, Wang Y, Guo YW. Effect of ENSO-driven precipitation on population irruptions of the Yangtze vole Microtus fortis calamorum in the Dongting Lake region of China. Integr Zool. 2010;5:176–184. doi: 10.1111/j.1749-4877.2010.00199.x. [DOI] [PubMed] [Google Scholar]
- 38.Ye R, Zeng C, Hu R. Occurrence and control of Microtus fortis in Dongting Lake Area. Crop Res. 2006;2:151–153. [Google Scholar]
- 39.Olson WP. Rat-flea indices, rainfall, and plague outbreaks in Vietnam, with emphasis on the Pleiku area. Am J Trop Med Hyg. 1969;18:621–628. doi: 10.4269/ajtmh.1969.18.621. [DOI] [PubMed] [Google Scholar]
- 40.Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NI. Time of survival under starvation in two flea species (Siphonaptera: Pulicidae) at different air temperatures and relative humidities. J Vector Ecol. 2002;27:70–81. [PubMed] [Google Scholar]
- 41.Madsen T, Shine R. Rainfall and rats: Climatically-driven dynamics of a tropical rodent population. Aust J Ecol. 1999;24:80–89. [Google Scholar]
- 42.Institute of Epidemiology and Microbiology, Chinese Academy of Medical Sciences . The Epidemic History of Plague in China (Zhong Guo Shu Yi Liu Xing Shi) Beijing: People's Medical Publishing House; 1980. (in Chinese) [Google Scholar]
- 43.Central Meteorological Bureau . Yearly Charts of Dryness/Wetness in China for the Last 500-Year Period. Beijing: Cartographic Publishing House; 1981. [Google Scholar]
- 44.Hastie T, Tibshirani R. Generalized Additive Models. London: Chapman and Hall; 1990. [DOI] [PubMed] [Google Scholar]
- 45.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 46.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation Stat Comput; 2006. [Google Scholar]
- 47.Stige LC, Ottersen G, Brander K, Chan KS, Stenseth NC. Cod and climate: Effect of the North Atlantic Oscillation on recruitment in the North Atlantic. Mar Ecol Prog Ser. 2006;325:227–241. [Google Scholar]
- 48.Cox DR. Statistical analysis of time-series: Some recent developments. Scand J Stat. 1981;8:93–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.