Abstract
Nerve growth factor (NGF) was originally discovered as a neurotrophic factor essential for the survival of sensory and sympathetic neurons during development. However in the adult, NGF has been found to play an important role in nociceptor sensitization following tissue injury. Here we outline mechanisms by which NGF activation of its cognate receptor, tropomyosin-related kinase A receptor, regulates a host of ion channels, receptors, and signaling molecules to enhance acute and chronic pain. Further, we document that peripherally restricted antagonism of NGF-tropomyosin-related kinase A receptor signaling is effective for controlling human pain while appearing to maintain normal nociceptor function. Understanding whether there are any unexpected adverse events as well as how humans may change their behavior and use of the injured/degenerating tissue following significant pain relief without sedation will be required to fully appreciate the patient populations that may benefit from these therapies targeting NGF.
The Problem of Pain and Its Management
Pain is essential for the protective sensibility that enables the avoidance of tissue injury and promotes healing after injury. However, many types of chronic pain become more of a burden than benefit as they have a significant, negative impact on functional status and quality of life. Persistent chronic inflammatory, neuropathic, or cancer pain presents a major health challenge throughout the world.1,2 However, management of chronic pain is often ineffective or incomplete3,4 as current therapies are far from ideal, due in part to a high incidence of dose-limiting side effects4,5. Indeed, there are very few current treatments that effectively control chronic pain without unwanted side effects and/or abuse liability.
International guidelines recommend a multimodal combination of pharmacologic and non-pharmacologic modalities as the most effective strategy to manage the pain and disability associated with chronic pain, where the goal of treatment should be to effectively reduce pain while improving function and reducing suffering.6 Acetaminophen (paracetamol), non-steroidal anti-inflammatory drugs such as ibuprofen and cyclooxygenase-2 inhibitors, and opioids such as tramadol or morphine are the gold standard analgesic drugs in clinical practice. However, concerns regarding the cardiovascular risks of cyclooxygenase-2 inhibitors and the gastrointestinal and renal side effects of non-steroidal anti-inflammatory drugs may limit the use of these medications.7 Where more conservative methods have failed, opioids, appropriately dosed and monitored, are associated with a lower incidence of organ toxicity and fewer potentially life-threatening complications than non-steroidal anti-inflammatory drugs.6,8–10 However, there are a broad spectrum of opioid-mediated side effects and liabilities including loss of effectiveness, constipation (the most common long-term side effect causing non-compliance), drug diversion, respiratory depression and accidental death from overdose.
The effective management of chronic pain can improve patients’ quality of life, functional status, and reduce health care costs.4,11 However, despite significant advances in our understanding of the pathophysiology of chronic pain,12 its management continues to challenge physicians.3 The development of new agents to manage chronic pain, but without significant cardiovascular, gastrointestinal, and central nervous system side-effects, remains a significant, unmet clinical need.
In the present article we present evidence for a new approach to the management of chronic pain that targets the effects elicited by nerve growth factor (NGF). The major objective of this article is to review the science behind targeting NGF or its cognate receptor tropomyosin-related kinase A receptor (TrkA) for the relief of pain, to outline the preclinical and clinical data suggesting that these therapies may be efficacious for relieving several types of chronic pain, and to discuss potential side effects of these therapies. For more detailed and exhaustive scientific discussion of NGF and its receptors, there are several excellent reviews.13–16
NGF belongs to a family of neurotrophins
NGF belongs to a family of molecules known as neurotrophins, which are approximately 12.5 kD proteins that form tightly bound homodimers. The neurotrophin family of target-derived proteins regulates the survival, development, and function of subsets of sensory and sympathetic neurons.17,18 Other mammalian members of the neurotrophin family are brain derived neurotrophic factor (BDNF), neurotrophin-3 and neurotrophin-4/5. The specificity of action of these molecules is a result of their binding specificity to a family of receptors called tropomyosin-related kinase (Trk) receptors19. TrkA preferentially binds NGF, TrkB binds both BDNF and neurotrophin-4/5, and TrkC binds neurotrophin-3. Neurotrophins also signal via a second receptor called the p75 receptor which binds all neurotrophins, i.e., there is little specificity exerted via the p75 receptor. Trk receptors are often referred to as high affinity receptors in contrast to the low affinity p75 receptor. However, the difference between trk and p75 receptors is not one of affinity but rather kinetics.
NGF binds to TrkA, whereupon the NGF-TrkA complex is internalized and transported from peripheral terminals to sensory cell bodies in the dorsal root ganglion (DRG).20–22 Evidence from several sources suggests that NGF itself cannot initiate signaling in the cell soma, and that instead the NGF-TrkA complex activates transcription factors that control downstream gene expression.21,23 Interactions between p75 and TrkA receptors in determining the response to NGF have been reported24,25. Furthermore, there is evidence that NGF and BDNF can sensitize the discharge of sensory neurons through p75 receptors26,27. However, because this review is directed towards the effects of NGF in enhancing acute and chronic pain in the adult, and Trk antagonists also produce significant relief of chronic pain, in this review we focus on the NGF-TrkA system.
The NGF-TrkA Nociceptor Axis: From Development to Adulthood
The role of NGF in neuronal development has been known since its discovery nearly 60 years ago.28 NGF plays a critical role in the development of the peripheral nervous system by promoting growth and survival of some neural crest- derived cells in developing embryos, in particular sensory and sympathetic neurons.28,29 An important documentation of these relationships is the fact that selective mutations in NGF or TrkA genes cause congenital insensitivity to pain in humans and loss of pain behaviors in genetically altered mice.30–34 For example, congenital insensitivity to pain with anhidrosis, a human condition in which patients generally have normal proprioception and normal sensation to innocuous pressure but abnormal sensation to thermal stimuli, is caused by a mutation in the TrkA gene35, which results in a structural neuropathy affecting unmyelinated peripheral nerve fibers. Indeed, genetically modified animals lacking the NGF or TrkA gene are born with virtually no small caliber primary sensory neurons, and are correspondingly profoundly unresponsive to noxious stimuli.19,32,33
Studies of NGF deprivation during critical periods of growth support the results of these genetic manipulation experiments. One method of producing long-term NGF deprivation is by immunizing animals to induce autoimmunity against NGF. Such studies reported that NGF is involved in maintenance of sympathetic neurons and the regulation of the substance P (SP) content of embryonic and neonatal sensory neurons.36,37 Immunizing pregnant rats against NGF causes depletion of SP in DRG neurons in those animals exposed in utero or as newborns,38,39 although the regenerative capacity of DRG neurons following axotomy in NGF-immunized animals was unimpaired.37 Anti-NGF antibody administered during early postnatal development in rats has revealed that DRG neurons lose the requirement for NGF for survival shortly after birth, but NGF still has an influence on the phenotype of nociceptors for another 10 days. This was shown by demonstrating that withdrawal of NGF during a critical period led to a developmental switch of high- threshold mechanoreceptors to sensitive mechanoreceptors which normally are relatively rare.40 Importantly, this phenotypic switch of nociceptors occurs in the absence of cell death despite the loss of NGF.41
Collectively, immunologic and genetic studies of NGF deprivation during development and maturation demonstrate that NGF has three separate roles one for survival and development of sensory and sympathetic neurons, the second in maintaining the peptidergic phenotype of primary afferent neurons in the early post-natal period, and the third being a key upstream modulator of the expression and sensitization of a variety of neurotransmitter, receptor and ion channels expressed by adult nociceptors. However, whether adult sensory neurons require NGF for maintenance of their phenotype, if so, how much NGF remains to be determined.
NGF-TrkA Signaling, Nociceptors, and Pain in the Adult
A Role for NGF in Nociception in the Adult
A role for NGF has been demonstrated in both acute, transient nociceptive responses, as well as in longer-term, chronic pain.42–45 As early as 1977, a report that NGF exerts effects on mast cells suggested that the physiologic effects of NGF were not limited to neuronal development and maturation.42 Interestingly, the involvement of NGF in nociception, and the ability of NGF to sensitize nociceptors, only occurs after sensory fibers have lost their dependence on NGF for survival.46 As we discuss below, the NGF-TrkA axis appears to play a pivotal role in the early, intermediate, and long-term generation and maintenance of several types of acute and chronic pain.
An important point in assessing the involvement of the NGF/TrkA pathway in driving a particular chronic pain state is the issue of the specific populations of primary afferent sensory nerve fibers that innervate the injured/diseased tissue. Four broad subtypes of primary sensory neurons have been characterized within the DRG, of which three broad categories are known to be important in nociceptive transmission in the normal animal: thin myelinated Aδ-fibers, peptidergic unmyelinated (C-) fibers, and non-peptidergic unmyelinated (C-) fibers.47 Peptidergic C-fibers and the majority of Aδ-fibers express TrkA, corresponding to approximately 40% of adult DRG cells,48 and are responsive to NGF.47,48 These TrkA-positive fibers innervate skin, viscera, muscle, and bone.49–52 In contrast, non-peptidergic C-fibers (which express c-RET or the binding site for the lectin Griffonia simplicifolia IB4) lack TrkA or p75 and are therefore unresponsive to NGF (TrkA-negative); these fibers innervate skin, but not the skeleton.52–54 These data suggest that a key factor to consider when assessing the analgesic efficacy of targeting NGF-TrkA signaling in an acute or chronic pain state is the fraction of NGF-responsive (TrkA+) nociceptors that innervate the tissue from which the pain is arising, as this innervation and thus the analgesic efficacy of targeting NGF-TrkA signaling may vary considerably from tissue to tissue.
Direct Actions of NGF
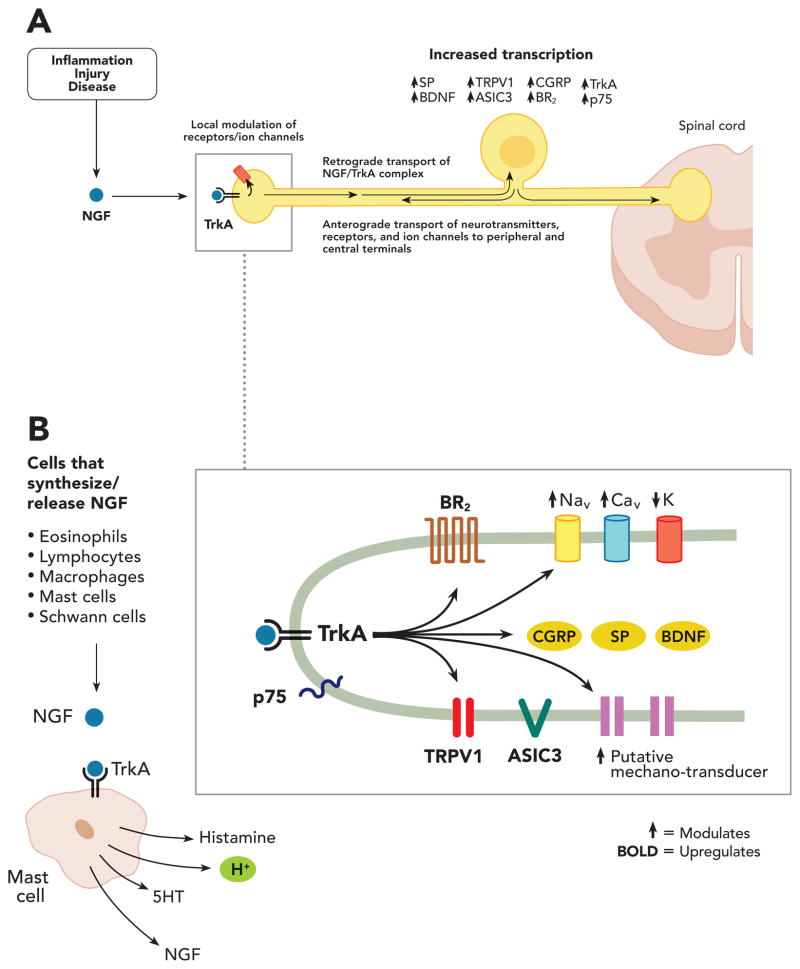
The pivotal role of NGF in inflammatory pain is exemplified by the expression and/or release of NGF by certain inflammatory cells, including eosinophils, lymphocytes, macrophages55,56 and mast cells,57 as a consequence of injury (fig. 1). Moreover, NGF is up-regulated in experimental models of inflammation, including those induced by carrageenan, formalin, and complete Freund’s adjuvant,45,58–60 as well as in models of autoimmune arthritis,61 and ultraviolet B radiation-induced acute inflammation.62 Cutaneous administration of NGF to rodents63 and to humans64 causes hyperalgesia within 1 or 3 hours, respectively, suggesting that NGF leads to a relatively rapid sensitization of cutaneous nociceptors. These rapid effects in the rat are thought to be mediated primarily through NGF binding with TrkA expressed on mast cells, causing degranulation and release of a variety of algogenic mediators, such as histamine, prostaglandin E2, serotonin, hydrogen ions, and bradykinin, as well as additional NGF (fig 1b), although the contribution of mast cells is not as clear in humans. NGF can also be produced by non-inflammatory cells, such as keratinocytes65 and endothelial cells,66 in addition to other inflammatory cells, such as fibroblasts67 and T cells in various in vitro culture models.68
Fig. 1.
Schematic showing the neurotransmitters, receptors and ion channels that are modulated and up-regulated by NGF binding to TrkA+ primary afferent sensory nerve fibers. (A) Tropomyosin-related kinase A receptor (TrkA)+ primary afferent have their cell body in the dorsal root ganglia (DRG) and transmit sensory information from the periphery to the spinal cord and brain. During inflammation, injury or certain diseases, inflammatory/immune/Schwann cells release nerve growth factor (NGF) that binds to TrkA, which in turns directly activates and/or sensitizes nociceptors. NGF and its cognate receptor TrkA are retrogradely transported to the DRG, resulting in increased synthesis of neuropeptides (e.g.: substance P (SP), brain-derived neurotrophic factor (BDNF)), receptors, ion channels, and anterograde transport of certain neurotransmitters, receptors and ion channels from the DRG to the periphery tissue and spinal cord. (B) NGF is released during inflammatory injury, principally from mast cells, but also from other recruited cells. Binding of NGF to TrkA on mast cells causes release of inflammatory mediators, such as histamine, serotonin (5HT), and protons (H+) as well as NGF. Binding of NGF to TrkA on the peptidergic (TrkA+) fiber terminal activates intracellular signaling pathways (represented by arrows), which results in either increased expression (bold) or modulation (↑ or ↓) at the membrane surface of a number of receptors, including, bradykinin (BK) receptors (B2R), ion channels, including transient receptor potential vanilloid 1 (TRPV1), acid-sensing ion channels (ASIC) 2/3, voltage-gated sodium (Nav) or calcium (Cav) ion channels, delayed rectifier potassium (K+) currents and putative mechanotransducers. These rapid changes (taking from minutes to hours) in the afferent terminal modify the sensory fiber’s response to sensory stimuli, and the propagation of sensory impulses to the dorsal horn. CGRP = calcitonin gene-related peptide
The NGF-induced release of inflammatory mediators from mast cells contributes to the sensitization of polymodal nociceptors. In addition, NGF also binds TrkA receptors expressed on the peptidergic fiber terminal itself (fig. 1), leading to sensitization of primary afferent nociceptors to thermal and chemical stimuli in vitro and in vivo.69,70 This NGF-TrkA activation of intracellular signaling cascades in the primary afferent neurons results in sensitization or increased expression of a number of receptors and channels at the membrane surface, including transient receptor potential vanilloid 1 (TRPV1), acid-sensing ion channels 2 and 3, endothelin receptors, bradykinin receptors, voltage-gated sodium, and calcium channels, delayed rectifier potassium currents and putative mechanotransducers,59,71–73 that contribute to immediate hypersensitivity after inflammation (fig. 1b).
An important mechanism seen within minutes to hours of NGF-TrkA binding is the sensitization of the heat-sensitive ion channel, TRPV1,69,74 expressed by small diameter peptidergic fibers. Acute sensitization of TRPV1 by NGF may involve direct phosphorylation, at least partly due to TrkA-mediated activation of p38 mitogen-activated protein kinases,75 or phosphoinositol kinase 3 and disinhibition following hydrolysis of phosphatidylinositol-4,5-bisphosphate.76,77 Ultimately, sensitization of TRPV1 lowers the temperature threshold of sensory neurons to noxious heat.75–77 Interestingly, however, this does not happen at the level of individual TRPV1 channels recorded in dissociated DRG cells—the inward current response to noxious heat increases as TRPV1 channels are translocated from the interior of the cell to the plasma membrane,78,79 but the temperature threshold does not change.74 Thus any change in temperature threshold of a thermal nociceptor due to NGF-induced sensitization of TRPV1 receptors results from a greater depolarization causing the fiber to reach firing threshold at a lower temperature.
Retrograde Transport of NGF-TrkA Drives Transcriptional Changes in Nociceptors
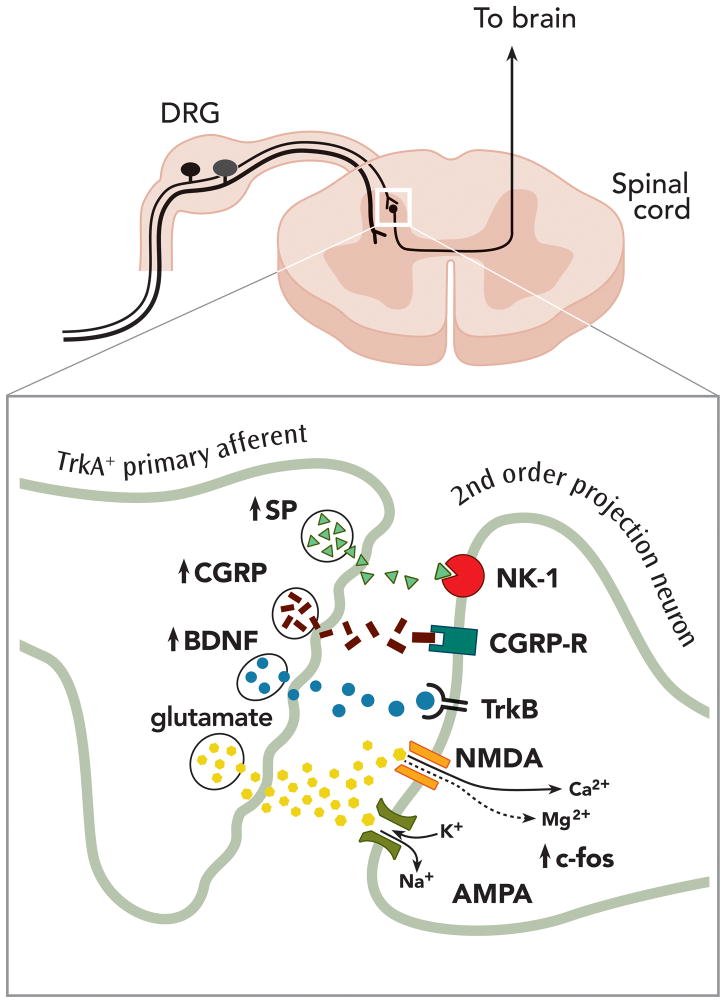
Following the period of immediate hypersensitivity with NGF release after tissue injury, early transcriptional changes occur in the sensory signaling pathway. As NGF principally signals via retrograde transport of the internalized NGF-TrkA complex, there is a delay (from hours to days) before some of NGF’s contribution to hypersensitivity is seen. After retrograde transport to the DRG, the signal from the NGF-TrkA complex can produce changes in sensory phenotype through the switching on (and off) of gene promoters (fig. 2), which leads to increased synthesis of peptides (e.g., SP, calcitonin gene-related peptide [CGRP], and BDNF), and of nociceptor-specific ion channels (NaV1.8, CaV 3.2, 3.3) at the DRG.80–83 For example, exposure of TrkA-positive sensory neurons to NGF elevates expression of the nociceptive acid-sensing ion channel-3 via control of the promoter region of its gene.81 NGF-induced altered gene expression can also lead to a change in phenotype, whereby a population of sensory neurons switches from non-peptidergic to peptidergic and becomes more responsive to NGF.84 Peripheral and dorsal horn terminals of peptidergic fibers express elevated levels of peptides (SP, CGRP and BDNF) as a result of these proteins being packaged and transported retrogradely and anterogradely from the soma (fig. 2).58,83 Indeed, systemic administration of anti-NGF neutralizing antibodies prevents the inflammation-induced up-regulation of neuropeptides (SP, CGRP) and the increased expression of the immediate early gene c-Fos in dorsal horn neurons, without modifying swelling and erythema.60 The peptides, SP and CGRP, may contribute, on subsequent stimulation of the peptidergic primary afferent neurons, to an exaggerated inflammatory response.58,85 In addition, SP itself has been reported to cause local expression of NGF in keratinocytes.86
Fig. 2.
Changes at the dorsal horn synapse following activation of a TrkA+ sensory nerve fiber. Longer-term (days) post-translational effects of nerve growth factor (NGF)-Tropomyosin-related kinase A receptor (TrkA) binding and transport to the dorsal root ganglia (DRG), include an increase (shown as ↑) in the concentration of peptides (e.g., substance P [SP], calcitonin gene-related peptide [CGRP], and brain-derived neurotrophic factor [BDNF]) in dorsal horn terminals of peptidergic (TrkA+) primary afferent neurons. Release of these peptides, in addition to glutamate acting on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, on subsequent stimulation of peptidergic (TrkA+) primary afferent neurons, and binding to their respective receptors (SP to NK-1; CGRP to CGRP-R, BDNF to TrkB) may cause strong depolarization of the post-synaptic second order projection neuron, changes in transcriptional activity in the second order projection neuron (e.g., increased expression of c-fos), and ultimately removal of the magnesium (Mg2+) block of the glutamatergic N-Methyl-D-Aspartate (NMDA) receptor. BDNF acts specifically as a central modulator, via binding to post-synaptic TrkB receptors, whereupon the BDNF-TrkB complex switches on intracellular protein kinases leading to phosphorylation of NMDA receptors and facilitated opening. This increases the probability of central sensitization and facilitated transmission through the dorsal horn synapse and via third-order neurons to the sensory cortex in the brain.
NGF, BDNF, and Central Sensitization
A delayed phase of the inflammatory response to NGF (7 hours to 4 days after NGF-TrkA binding in rodents), involves an indirect effect of NGF on synaptic transmission between nociceptors and second-order cells in laminae I and II of the spinal cord via its effect on the release of peptides such as BDNF (fig. 2).87 Evidence from 1994 suggested a role for the glutamatergic N-Methyl-D-Aspartate channel, as NGF-induced behavioral hypersensitivity was selectively blocked by the non-competitive N-Methyl-D-Aspartate antagonist, MK-801.45 The N-Methyl-D-Aspartate channel plays a fundamental role in the development of wind-up and central sensitization, mechanisms that are thought to contribute to the development of facilitated sensory signals following injury.88,89 One potential mechanism that is thought to contribute to the development of central sensitization in the dorsal horn is the NGF-dependent upregulation of BDNF in peptidergic nociceptors.90,91 Furthermore, BDNF is transported not only retrogradely to peripheral terminals, but also anterogradely from the DRG to terminals in the dorsal horn (see fig. 2).91–93 BDNF is constitutively expressed in small-and medium-sized DRG neurons, and only released with strong pre-synaptic stimulation.94 Upon release, BDNF acts as a central modulator via post-synaptic TrkB the cognate receptor for BDNF.95,96 BDNF-TrkB binding on second-order cells can activate intracellular protein kinases, which can lead to phosphorylation of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. This phosphorylation has been shown to contribute to central sensitization at the dorsal horn synapse, particularly in combination with upregulated peptides (SP and CGRP) acting on post-synaptic receptors (fig. 2). BDNF upregulation after peripheral inflammation is NGF dependent, as upregulation is inhibited with administration of anti-NGF antibody.97 Behavioral observations indicate that antagonism of central BDNF attenuates the second (delayed) phase of hyperalgesia induced by formalin and the thermal hyperalgesia induced by carageenan in an NGF-dependent manner, demonstrating a role for BDNF in hypersensitivity and pain.95 Collectively, the data suggest that BDNF-dependent activation of TrkB signaling is required for the development of the central sensitization process that underlies the development of persistent heat and mechanical hypersensitivity in the setting of tissue inflammation or injury.98
These preclinical data point to a fundamental difference between the role of NGF as a factor during growth and differentiation, and its role in the adult sensory system, when NGF-TrkA becomes a major player in the modulation and sensitization of a significant population of nociceptors which are involved in driving chronic pain. As NGF plays a prominent role not only in acute nociception, but in mechanisms behind chronic hypersensitivity, there is a clear scientific rationale for interrupting NGF-TrkA signaling as a target for pain relief therapeutics.
NGF/TrkA induced sprouting and neuroma formation
One intriguing but largely unexplored mechanism by which NGF may also generate and maintain hypersensitivity is by inducing aberrant sprouting and/or neuroma formation in response to tissue and/or nerve injury.99–101 In previous studies in a rat model of neuroma, an NGF-sequestering fusion protein reduced both neuroma formation and the spontaneous, ectopic discharge that is a defining characteristic of painful neuromas.100 Other evidence suggests that local administration of NGF to normal peripheral nerves can also induce nerve sprouting of peptidergic (TrkA+) nociceptors.101
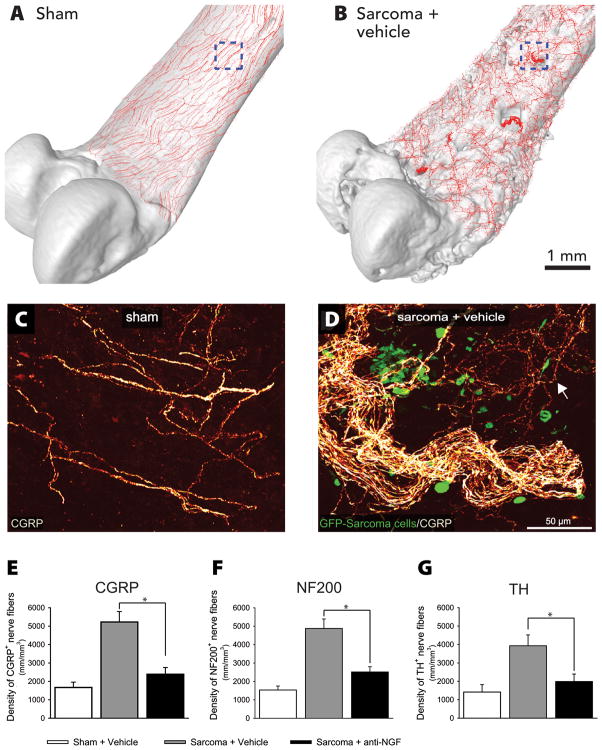
NGF activation of TrkA+ has also been demonstrated to induce a remarkable reorganization of sensory and sympathetic nerve fibers. In a mouse model of bone cancer it was shown that when osteosarcoma cells induce a tumor within bone, there is a remarkable sprouting and formation of neuroma-like structures by TrkA+ sensory and sympathetic nerve fibers in the periosteum (fig. 3).102 This sprouting appears to occur within a week that tumor and tumor-associated stromal cells release NGF (fig. 3). Within this week interval these sensory and sympathetic nerve fibers appear to grow more than a millimeter in length and achieve a density never observed in normal bone (fig. 3). Interestingly, sustained administration of an anti-NGF sequestering therapy largely blocked the pathological sprouting of sensory and sympathetic nerve fibers, the formation of neuroma-like structures, and significantly inhibited the generation and maintenance of cancer pain in this model (fig. 3).102
Fig. 3.
Nerve growth factor (NGF) induces sprouting and neuroma formation by sensory and sympathetic nerve fibers in a model of skeletal pain. Confocal images of periosteum of bone were acquired from whole mount preparations, tiled and overlaid (to scale) on a three-dimensional micro-computed tomography rendering of a sham femur (A) or sarcoma + vehicle femur (B), respectively, using Amira software. Note that the tumor-injected femur (B) has significant cortical bone deterioration and a pathological reorganization of calcitonin gene-related peptide (CGRP) nerve fibers (in red) compared to the sham bone (A). The boxed areas in (A) and (B) correspond to the confocal images in (C) and (D), respectively. High power confocal images of non-decalcified whole mount preparations of the femoral periosteum from sham + vehicle (C) or sarcoma + vehicle mice (D) showing CGRP+ nerve fibers and green fluorescent protein (GFP)+ sarcoma cancer cells (green). When GFP+ tumor cells invade the periosteum, they induce ectopic sprouting of CGRP+ sensory fibers (D, arrow) and the formation of neuroma-like structures. Administration of NGF sequestering therapy (10 mg/kg; intraperitoneal, given at days 6, 12, and 18 post cell injection) reduces sarcoma-induced nerve sprouting of CGRP+ (E), 200kd neurofilament (NF200)+ (F), and tyrosine hydroxylase (TH)+ (G) nerve fibers at day 20 post-cancer cell injection. Nerve fiber density was determined by measuring the total length of nerve fibers per unit volume in the periosteum. *p<0.05. Bars represent the mean ± SEM. Reproduced and modified with permission from Mantyh et al. 2010.102
A major issue in interpreting this remarkable and pathological nerve sprouting is the source of the NGF driving this growth? Recent studies using canine prostate cells injected into the mouse bone shed light on the possible source of NGF as these canine prostate cells themselves do not express NGF.103 Following their injection into bone, sclerotic bone lesions similar to that found in human prostate cancer patients are observed and TrkA+ sensory and sympathetic nerve fibers innervating the prostate tumor-bearing bone marrow also undergo a truly remarkable and pathological sprouting.104 As these prostate cells do not express detectable levels of messenger RNA coding for NGF103, these studies suggest that the source of NGF is not the tumor cells themselves but rather NGF released by tumor-associated stromal, inflammatory and immune cells68,105,106 which frequently account for 10–80% of the cells comprising the tumor mass. These data demonstrate that even in the adult bone marrow, NGF released by these inflammatory, immune and stromal cells can induce a 10–70 fold increase in density of TrkA+ sensory nerve fibers in the bone marrow. It should be noted that the phenotype of these newly sprouted nerve fibers may be quite different from nerve fibers that innervate the normal bone and as such these newly sprouted nerve fibers may provide an anatomical substrate which drives skeletal pain. In support of this hypothesis, preventive treatment with an antibody that sequesters NGF, administered when prostate tumor-induced pain and bone remodeling is first observed, blocks the ectopic sprouting and significantly attenuates the development and severity of cancer pain.104
Sprouting of presumptive TrkA+ nerve fibers has also been observed in non-malignant skeletal pain states in both human and animals. For example, prior studies have reported that in humans with chronic discogenic pain there is growth of CGRP+ nerve fibers into normally aneural and avascular areas of the intervertebral disc107. Other studies have demonstrated significant sprouting of CGRP+ nerve fibers following bone fracture in rat and in the arthritic joints of humans and animals108–111. These reports suggest that following injury or disease of the skeleton, significant sprouting of TrkA+ nerve fibers can occur, and it appears that endogenous stromal, inflammatory and immune cells are a major source of NGF68,105,106.
These data on the ectopic sprouting of TrkA+ sensory and sympathetic nerve fibers are interesting as they indicate how pre-emptive treatment with therapies that block NGF activation of TrkA may reduce the attendant pain, but also may block the pathologic remodeling of sensory and sympathetic nerve fibers that are themselves a major driver of chronic hypersensitivity. This might be relevant in situations where one can predict that tissue/nerve injury is about to occur, such as before amputation, orthopedic surgery, or where disease progression is highly likely, such as in osteoarthritis, pancreatic cancer, or tumor metastasis to bone.
NGF-TrkA Interactions and Chronic Pain—Preclinical Evidence
Anti-NGF Reduces Pain in Animal Models
A number of strategies have been developed to investigate the role of endogenous NGF in chronic pain. Most commonly, anti-NGF antibodies or a TrkA-IgG fusion protein to sequester NGF have been developed to block the biological activity of NGF. Alternatively, it is possible to prevent NGF binding and activation of TrkA, for example with anti-TrkA antibody or a small molecular inhibitor of TrkA, although NGF activity via p75 will remain intact. These approaches have provided further evidence for the role of NGF in acute and chronic hypersensitivity in adult animals after inflammatory injury.
The systemic administration of anti-NGF antibody has been shown to prevent the acute thermal45,60 and mechanical hyperalgesia induced by complete Freund’s adjuvant,60 while administration of a TrkA-IgG fusion protein minimized behavioral symptoms of hyperalgesia induced by carrageenan112,113 or ultraviolet B radiation.62 Furthermore, although not considered in detail here, it is important to point out that in models of visceral inflammatory pain, hyperalgesia is markedly reduced by pre-treatment with an NGF-neutralizing antibody or TrkA-IgG fusion molecule, for example in acetic acid-induced gastric inflammation,114 trinitrobenzene sulfonic acid-induced colonic hypersensitivity,115 and turpentine or acrolein-induced cystitis.116,117 Furthermore, in a model of colitis, trinitrobenzene sulfonic acid-induced colonic hypersensitivity was also reversed by administering an anti-NGF antibody.115
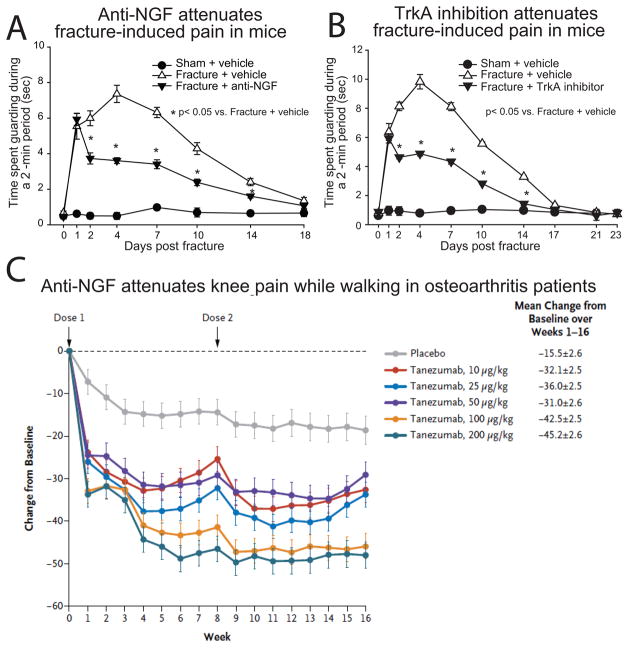
Antibodies to NGF reversed the established hyperalgesia in a rodent model of autoimmune arthritis,61 suggesting that NGF is involved in prolonged hyperalgesia. Furthermore, the NGF-neutralizing antibody was at least as effective as indomethacin,61 used clinically for relieving arthritis pain. A role for NGF in maintenance of hypersensitivity in chronic injury has also been demonstrated using a model of bone cancer,103,118 and a model of closed femur fracture119,120 (fig. 4). Indeed, anti-NGF produces a profound reduction in both ongoing and movement-evoked bone cancer pain-related behaviors that is greater than that achieved with acute administration of morphine.103,118
Fig. 4.
Therapies that sequester nerve growth factor (NGF) or inhibit tropomyosin-related kinase A receptor (TrkA) demonstrate significant analgesic efficacy in mouse and a human model of non-malignant skeletal pain. In a mouse model of bone fracture, pain-related behaviors (the time spent guarding of the fractured limb over a 2-minute observation) were significantly reduced by (A) anti-NGF therapy (10 mg/kg, i.p., administered at day 1, day 6 and day 11 post-fracture) and the pan-Trk antagonist ARRY-470 (30 mg/kg, p.o., administered twice daily beginning on day 1 post fracture). Note that anti-NGF therapy (A) and the pan-Trk inhibitor (B) both reduced non-malignant fracture pain-related behaviors by approximately 50%. (C) Anti-NGF therapy reduced walking pain in human patients with moderate to severe osteoarthritis pain. The patient’s assessment of knee pain while walking in response to therapy were obtained at baseline and at the indicated times with the use of a visual-analogue scale that ranged from 0 to 100. In the case of knee pain, a decrease in the score indicates improvement (i.e., less pain). Changes are reported as least-squares means ±SE. P<0.001 for the comparisons of all doses of anti-NGF (tanezumab) with placebo in the assessment of knee pain, except for the comparison of 10 μg of tanezumab per kilogram of body weight with placebo in the patient’s global assessment, for which P = 0.001. Reproduced with permission from Koewler et al. 2007,120 Ghilardi et al., 2011,147 and Lane et al. 2010140.
Early preclinical experiments modeling long-term NGF deprivation by active immunization of adult animals to auto-produce antibodies against NGF demonstrated a reduction in the number of peripheral DRG fibers compared with untreated controls.121,122 This reduction was selective for unmyelinated C-fibers and was associated with diminished responsiveness to nociceptive stimuli.122 However, in later studies that utilized passive immunization, in which antibodies raised against NGF or TrkA were injected into mature animals, normal nociceptive function remained intact with minimal loss of functional sympathetic or sensory neurons.118,119 Significantly, such anti-NGF antibody treatment reduces pain due to fracture or tumor growth in bone by about 50%,118–120 despite no reduction in the number of peripheral sensory or sympathetic nerve fibers innervating the skin or bone.102,118
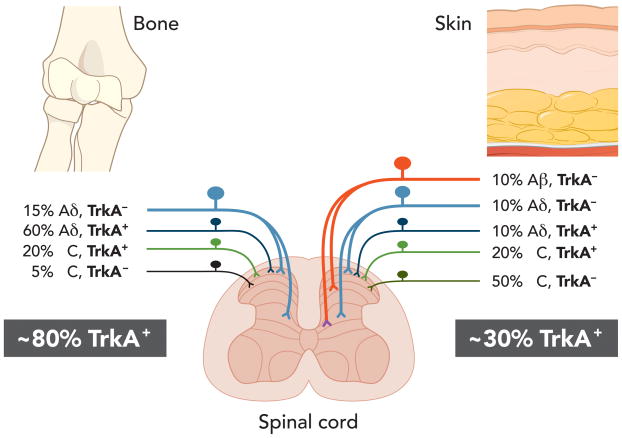
One rather unique aspect of the sensory innervation of bone and joint, which may partially explain why anti-NGF therapy is effective in relieving both malignant and non-malignant skeletal pain, is that more than 50% of nerve fibers innervating bone are CGRP+ fibers52, nearly all of which co-express TrkA (fig. 5).123 Accordingly, few unmyelinated non-peptidergic (IB4+/RET+) nerve fibers are present in bone,52–54 and therefore therapies that target NGF or TrkA may be particularly efficacious in relieving bone pain where the tissues are innervated by nociceptors that express TrkA and respond to NGF.
Fig. 5.
There are differences in the percentages of tropomyosin-related kinase A receptor (TrkA)+ sensory nerve fibers that innervate the bone vs skin. The skin is innervated by thickly myelinated A-beta fibers (TrkA−), thinly myelinated A delta fibers (both TkA− and TrkA+), unmyelinated peptide-rich C fibers (TrkA+) and unmyelinated peptide-poor C-fibers (TrkA−). In contrast, the bone appears to be predominantly innervated by thinly myelinated A-delta fibers (TrkA− but mostly TrkA+) and peptide-rich C-fibers (mostly TrkA+ and a small proportion TrkA−). The percentages and types of sensory nerve fibers innervating the skin 51,54,148,149 and bone52,123,150,151 were estimated from previous studies. As greater than 80% of all sensory nerve fibers that innervate the bone are TrkA+ whereas only 30% of the sensory nerve fibers that innervate skin are TrkA+, these data might help explain why blocking nerve growth factor or TrkA is highly efficacious in attenuating skeletal pain. Modified from. Castaneda-Hernandez et al. 2011.123
Importantly, preventing NGF-TrkA signaling does not appear to compromise normal physiologic responses to injury, which are critical for effective healing. For example, NGF blockade does not affect the normal inflammatory response (erythema, heat, and swelling).60,113 Furthermore, at least cursory examination of anti-NGF therapy reveals no modification of the biomechanical properties of the femur or histomorphometric indices of bone healing120 and load bearing remains intact although more extensive and exhaustive studies on bone healing are clearly needed. In contrast, in some but not all studies in mouse, rat and humans using various models of bone injury, non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors have been shown to inhibit effective bridging of the fracture site, resulting in delayed bone healing and decreased bone strength.124,125 Also, in a model of bone cancer, neurochemical markers associated with peripheral and central sensitization, such as c-Fos, are reduced on administration of anti-NGF antibody;118 although tumor growth, bone destruction and markers of sensory or sympathetic innervation in the skin are unaffected.
Collectively, the preclinical data suggest that reducing or preventing the NGF production that is associated with some types of injury, through the sequestering of NGF or the inhibition of NGF-TrkA signaling, is effective for reducing hypersensitivity in animal models. Importantly, the studies discussed suggest that, at least at the time points examined, this approach does not obviously compromise normal nociceptor function or cause the loss of sympathetic or sensory nerve fiber innervation of the skin or bone.
NGF-TrkA Interactions and Pain—Human Studies
In humans, as in animal models, subcutaneous NGF evokes long-lasting mechanical hyperalgesia.126–128 Furthermore, NGF is locally upregulated in humans presenting with chronic pain, such as arthritis, migraine/headache, fibromyalgia, or peripheral nerve injury.129–132 These observations suggest that in humans, as in preclinical animal models, the ongoing production of NGF may be involved in chronic pain and changes in sensitization. Indeed, there are at least three major pharmacologic strategies under development that target NGF-TrkA signaling for the treatment of chronic pain and that have produced effective reduction in hypersensitivity in preclinical models. These are: sequestration of NGF or inhibiting its binding to TrkA;61,133 antagonizing TrkA so as to block NGF from binding to TrkA;134–136 and blocking TrkA kinase activity.137 Among the first such molecules to be investigated pre-clinically were a TrkA-IgG fusion protein,138 as well as MNAC13,134 and PD90780,136 which act by inhibiting the binding of NGF to TrkA and ALE0540135, which appears to act by modulating the interaction of NGF with p75 and indirectly affecting TrkA activation. While several of these molecules showed efficacy in reducing nociceptive behaviors, they were not advanced into clinical trials due to specificity or immunologic response issues. For instance, ALE0540 does not appear to have sufficient selectivity over other tested receptors in vitro, MNAC13 is a mouse monoclonal antibody unsuitable for use in humans and TrkA-IgG contains the extracellular domain of a normal human receptor (TrkA) and therefore is likely to have significant consequences if immunogenicity does develop. This potentially would be similar to the problems seen in rare patients treated with recombinant analogues of erythropoetin when they became autoimmune to their endogenous erythropoietin.139 In contrast, a number of humanized anti-NGF monoclonal antibodies—RN624 (tanezumab), JNJ-42160443, REGN475, PG110, Alpha-D11, AMG-403, which exert their analgesic effect by sequestering endogenous NGF — are currently being investigated in clinical trials in patients with various types of chronic non-cancer pain133,140. The outcomes of these clinical trials will provide key information on the efficacy of anti-NGF antibody therapy for the relief of pain in patients with different forms of chronic pain. Importantly, in studies published to date, and in line with preclinical studies, anti-NGF therapy appears to be anti-hyperalgesic (i.e. normalizing a decreased nociceptive threshold) as opposed to analgesic (i.e. increasing normal and sensitized nociceptive threshold). Long-term studies are required to enable a comparison of the safety profile of anti-NGF antibody therapy with those of currently used analgesic agents for chronic non-cancer pain, where adverse side effects include gastrointestinal problems and potential cardiovascular risks. In addition, the safety profile of anti-NGF therapies must be investigated in a range of patients with different types of chronic pain.
The Potential for NGF-TrkA Therapeutics
Ultimately, the utility of NGF antagonism for pain relief in humans will depend on the contribution of the various NGF signaling pathways to the specific chronic pain condition. It is likely that not all types of pain are effectively reversed by antagonizing NGF-TrkA signaling. This therapeutic approach clearly relies on NGF being an important driver of the increased pain sensitivity; if other factors are responsible for driving the hyperalgesic state, inhibition of NGF may not be effective. For example, target- derived NGF is lost under conditions such as diabetes where peripheral fibers suffer damage, a condition often accompanied by pain. Here, NGF might be expected to improve regeneration141,142 thereby reducing pain. However, this approach was abandoned in patients with diabetic peripheral neuropathy due to dose-limiting painful side effects.143
NGF may be primarily involved in the initiation of changes that lead to chronic pain, and may not itself have a prominent role in maintenance of hypersensitivity. Therefore, the stage at which NGF is important in the development of ongoing hypersensitivity needs to be defined. Moreover, the extent to which signaling pathways are interlinked may limit their use clinically as well as in the interpretation of preclinical results. For example, anti-TrkA antibodies should suppress TrkA signaling, but they may also affect p75 signaling since there is speculation that the two pathways interact.144 Furthermore, specific nociceptor innervation of each tissue may influence the efficacy of NGF-TrkA blocking strategies. Preclinical investigators who have focused on skeletal pain have proposed that anti-NGF treatment may be particularly effective in pain that originates in bone,102–104,118,119,145 around 80% of the myelinated and unmyelinated nerve fibers that innervate the bone are responsive to NGF.123
In order to optimize the therapeutic potential of NGF inhibitors, further research is needed to establish which types of human chronic pain are driven by, and more importantly, maintained by NGF. It is also important to understand when in the disease process NGF antagonism is most effective. For example, the pain that immediately follows bone fracture (from seconds to minutes later) is not inhibited by treatment with anti-NGF antibody in preclinical studies, whereas 24 hours following fracture anti-NGF therapy reduced bone fracture pain by >50% (fig. 4).119,120 This may indicate that initial nociceptive signals are driven by activation of, for example, mechanotransducers, independent of NGF, whereas secondary nociception that occurs hours to days after fracture may be increased by the release of NGF contributing to activation and sensitization of nociceptors.120 Further study is also required to evaluate the putative effects of anti-NGF on other disease processes, such as weight loss in autoimmune arthritis61 and bone loss in the chronic pain condition known as complex regional pain syndrome I.146
In addition to defining the analgesic efficacy of blocking the NGF-TrkA axis, key safety issues which need to be addressed with any therapy targeting NGF or TrkA include effects on normal autonomic and sensory neuron structure and function; physiological responses to injury, wound healing and endocrine function; ability to cross placental or blood–brain-barrier in the normal or injured state, and thus any influence on central nervous system neurons such as the basal forebrain cholinergic neurons that are sensitive to NGF. Additionally, given that bone pain may be a major target for NGF-TrkA therapies, understanding how these therapies effect individuals that have advanced bone degeneration will be critical. Indeed, recent human clinical trials in elderly humans with osteoarthritis have been halted due to the need for earlier than expected joint replacement in a small subset of patients.140 Whether this earlier than expected joint replacement in patients being treated with anti-NGF is simply due to greater use of the diseased joint or to unforeseen adverse events on the biomechanical properties of bone itself remains a critical but as yet unanswered question. These data emphasize the need to clearly understand not only the analgesic efficacy of TrkA-NGF blocking therapies and any unexpected effects but how patients with chronic pain change their behavior and use of the injured/degenerating tissue following administration of a therapy that provides significant pain relief without sedation.
Conclusion
This review provides an overview of the mechanisms by which NGF drives acute and chronic pain in the adult, and outlines how NGF has a distinct role in the adult as compared with the developing nervous system. To date, therapies that target NGF-TrkA-signaling have shown significant analgesic efficacy in both animals and humans in several difficult-to-treat chronic pain states. In choosing which chronic pain states to target with NGF-TrkA therapies, a key issue to consider is the fraction of NGF-responsive (TrkA+) nociceptors that innervate the tissue from which the pain is arising, as this innervation varies considerably from tissue to tissue. If successful, therapies that target NGF-TrkA signaling represent a new class of analgesic therapy that have the potential to profoundly change the therapeutic landscape of how we treat several types of chronic pain.
Acknowledgments
Editorial support was provided by Karen Burrows, MPhil (Senior Medical Writer), and Aideen Young, PhD (Senior Medical Writer), of UBC Scientific Solutions, Ltd., Horsham, UK, and funded by Pfizer Inc, New York, NY.
Footnotes
Conflict of interest: Karen Burrow and Aideen Young wrote the first rough draft of the manuscript in September 2009. Patrick Mantyh (PM), Martin Koltzenburg (MK), Lorne Mendell (LM), David Shelton (DS) and Leslie Tive (LT) then extensively edited and rewrote the manuscript in 20 separate drafts during the next 12 months. PM served as the lead author in rewriting, deleting, and adding new material and sections to the manuscript. PM drew the rough outlines of the figures which were then redrawn in professional format by Annemarie Johnson at Wake Forest University. PM, MK and LM are responsible for the views and opinions of this manuscript and did not receive any fees eror compensation for the writing of this review.
Author disclosure information: Dr. Mantyh is supported by the National Institutes of Health grant (NS23970, Bethesda, MD) and by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Grants (O4380-I and A6707-R, Washington, DC). Dr. Mantyh received anti-nerve growth factor as a research tool and gift from Rinat Laboratories, Pfizer Inc (South San Francisco, CA) and has served as a consultant and has had research grants from Abbott (Abbott Park, IL), Adolor (Exton, PA), Array Biopharma (Boulder, CO), Johnson and Johnson (New Brunswick, NJ), Pfizer (New York, NY), Plexxikon (Berkeley, CA) and Roche (South San Francisco, CA).
Dr. Koltzenburg is currently supported by the Brain Research Trust (London WC1A 2AJ, United Kingdom), the Department of Health’s Comprehensive Biomedical Research Centre at University College London Hospitals National Health Service Foundation Trust (London W1T 7NF, United Kingdom) and the Medical Research Council (London, WC2B 4AN, United Kingdom). In the past 3 years he has served as consultant for AstraZeneca (Sodertalje, Sweden), Endo (Chadds Ford, PA), Johnson and Johnson (New Brunswick, NJ), Grunenthal (Aachen, Germany), Merck (Whitehouse Station, NJ), Novartis Pharmaceuticals UK (Horsham, United Kingdom) Organon (Oss, The Netherlands), Quintiles (Durham, NC), Pfizer (New York, NY), QRX Pharma (Bedminster, NJ) and Schering-Plough (Kenilworth, NJ).
Dr. Mendell is supported by the NIH (grant number 5RO1 NS 16996, Bethesda, MD), the Christopher and Dana Reeve Foundation (LMC- 2010, Short Hills, NJ), and the William Heiser Foundation (Wantagh, NY). His neurotrophin research has been aided by gifts of brain-derived nerve growth factor and neurotrophin-3 from Regeneron Pharmaceuticals Inc (Tarrytown, NY), and nerve growth factor and neurotrophin-4/5 from Genentech, Inc (South San Francisco, CA) for whom he has also consulted. He has also consulted for Pfizer, Inc (New York, NY).
Dr. Tive is an employee of Pfizer Inc.
Dr. Shelton is an employee of Rinat Laboratories, Pfizer Inc.
References
- 1.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–12. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 2.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Fishman SM, Teichera D. Challenges and choices in drug therapy for chronic pain. Cleve Clin J Med. 2003;70:119–38. doi: 10.3949/ccjm.70.2.119. [DOI] [PubMed] [Google Scholar]
- 4.Katz WA, Barkin RL. Dilemmas in chronic/persistent pain management. Dis Mon. 2010;56:233–50. doi: 10.1016/j.disamonth.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–20. [PubMed] [Google Scholar]
- 6.Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, Yagi T, Arai T, Sano M, Fukuda K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton Neurosci. 2010;156:27–35. doi: 10.1016/j.autneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Whelton A. Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther. 2000;7:63–74. doi: 10.1097/00045391-200007020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, Hauselmann HJ, Herrero-Beaumont G, Jordan K, Kaklamanis P, Leeb B, Lequesne M, Lohmander S, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Swoboda B, Varatojo R, Verbruggen G, Zimmermann-Gorska I, Dougados M. EULAR evidence based recommendations for the management of hip osteoarthritis: Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2005;64:669–81. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang GY, Yi CG, Li X, Liang ZQ, Wang RX, Liu DE, Zhang LM, Meng CY, Guo SZ. Proliferation hemangiomas formation through dual mechanism of vascular endothelial growth factor mediated endothelial progenitor cells proliferation and mobilization through matrix metalloproteinases 9. Med Hypotheses. 2008;70:815–8. doi: 10.1016/j.mehy.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippe PM, Brock C, David J, Crossno R, Gitlow S. The First National Pain Medicine Summit--final summary report. Pain Med. 2010;11:1447–68. doi: 10.1111/j.1526-4637.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 12.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–57. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 14.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45:252–61. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 16.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–55. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 17.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 19.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–9. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 20.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: Evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 21.Woolf CJ. Phenotypic modification of primary sensory neurons: The role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–8. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- 22.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–70. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 23.Kendall G, Brar-Rai A, Ensor E, Winter J, Wood JN, Latchman DS. Nerve growth factor induces the Oct-2 transcription factor in sensory neurons with the kinetics of an immediate-early gene. J Neurosci Res. 1995;40:169–76. doi: 10.1002/jnr.490400205. [DOI] [PubMed] [Google Scholar]
- 24.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–83. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- 25.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7:26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–92. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol. 2008;586:3113–27. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi-Montalcini R, Hamburger V. Selective growth-stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exptl Zool. 1951;116:321–62. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay RM. Role of neurotrophins and trk receptors in the development and maintenance of sensory neurons: An overview. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–73. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 30.Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13:799–805. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–51. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- 32.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–11. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 33.Brown JW, Podosin R. A syndrome of the neural crest. Arch Neurol. 1966;15:294–301. doi: 10.1001/archneur.1966.00470150072012. [DOI] [PubMed] [Google Scholar]
- 34.Amann R, Schuligoi R, Herzeg G, Donnerer J. Intraplantar injection of nerve growth factor into the rat hind paw: Local edema and effects on thermal nociceptive threshold. Pain. 1996;64:323–9. doi: 10.1016/0304-3959(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 35.Indo Y. Genetics of congenital insensitivity to pain with anhidrosis (CIPA) or hereditary sensory and autonomic neuropathy type IV: Clinical, biological and molecular aspects of mutations in TRKA(NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Clin Auton Res. 2002;12 (Suppl 1):I20–32. doi: 10.1007/s102860200016. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz JP, Pearson J, Johnson EM. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982;244:378–81. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- 37.Rich KM, Yip HK, Osborne PA, Schmidt RE, Johnson EM., Jr Role of nerve growth factor in the adult dorsal root ganglia neuron and its response to injury. J Comp Neurol. 1984;230:110–8. doi: 10.1002/cne.902300110. [DOI] [PubMed] [Google Scholar]
- 38.Johnson EM, Jr, Osborne PA, Taniuchi M. Destruction of sympathetic and sensory neurons in the developing rat by a monoclonal antibody against the nerve growth factor (NGF) receptor. Brain Res. 1989;478:166–70. doi: 10.1016/0006-8993(89)91491-1. [DOI] [PubMed] [Google Scholar]
- 39.Ross M, Lofstrandh S, Gorin PD, Johnson EM, Schwartz JP. Use of an experimental autoimmune model to define nerve growth factor dependency of peripheral and central substance P-containing neurons in the rat. J Neurosci. 1981;1:1304–11. doi: 10.1523/JNEUROSCI.01-11-01304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter AM, Lewin GR, Kremer NE, Mendell LM. Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature. 1991;350:500–2. doi: 10.1038/350500a0. [DOI] [PubMed] [Google Scholar]
- 41.Lewin GR, Ritter AM, Mendell LM. On the role of nerve growth factor in the development of myelinated nociceptors. J Neurosci. 1992;12:1896–905. doi: 10.1523/JNEUROSCI.12-05-01896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aloe L, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–66. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- 43.Taiwo YO, Levine JD, Burch RM, Woo JE, Mobley WC. Hyperalgesia induced in the rat by the amino-terminal octapeptide of nerve growth factor. Proc Natl Acad Sci USA. 1991;88:5144–8. doi: 10.1073/pnas.88.12.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Della Seta D, de Acetis L, Aloe L, Alleva E. NGF effects on hot plate behaviors in mice. Pharmacol Biochem Behav. 1994;49:701–5. doi: 10.1016/0091-3057(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 45.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–12. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92:3148–52. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- 47.Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- 48.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–94. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aoki Y, Ohtori S, Takahashi K, Ino H, Takahashi Y, Chiba T, Moriya H. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine (Phila Pa 1976) 2004;29:1077–81. doi: 10.1097/00007632-200405150-00005. [DOI] [PubMed] [Google Scholar]
- 50.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 51.Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res. 2001;41:355–63. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 52.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: Therapeutic opportunity for treating skeletal pain. Bone. 2010;46:306–13. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: Differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- 54.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–9. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- 56.Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc Natl Acad Sci USA. 1987;84:8735–9. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739–43. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donnerer J, Schuligoi R, Stein C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth factor in vivo. Neuroscience. 1992;49:693–98. doi: 10.1016/0306-4522(92)90237-v. [DOI] [PubMed] [Google Scholar]
- 59.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–40. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 60.Woolf CJ, Safieh-Carabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–31. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 61.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116:8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Bishop T, Hewson DW, Yip PK, Fahey MS, Dawbarn D, Young AR, McMahon SB. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 63.Andreev NY, Dmitrieva N, Koltzenburg M, McMahon SB. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995;63:109–15. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- 64.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–5. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 65.Tron VA, Coughlin MD, Jang DE, Stanisz J, Sauder DN. Expression and modulation of nerve growth factor in murine keratinocytes (PAM 212) J Clin Invest. 1990;85:1085–9. doi: 10.1172/JCI114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foster PA, Costa SK, Poston R, Hoult JR, Brain SD. Endothelial cells play an essential role in the thermal hyperalgesia induced by nerve growth factor. FASEB J. 2003;17:1703–5. doi: 10.1096/fj.02-1000fje. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, Kitamura Y. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174:7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci USA. 1993;90:10984–8. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–62. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 70.Bennett DL, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998;10:1282–91. doi: 10.1046/j.1460-9568.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 71.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–48. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rueff A, Dawson AJ, Mendell LM. Characteristics of nerve growth factor induced hyperalgesia in adult rats: Dependence on enhanced bradykinin-1 receptor activity but not neurokinin-1 receptor activation. Pain. 1996;66:359–72. doi: 10.1016/0304-3959(96)03060-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galoyan SM, Petruska JC, Mendell LM. Mechanisms of sensitization of the response of single dorsal root ganglion cells from adult rat to noxious heat. Eur J Neurosci. 2003;18:535–41. doi: 10.1046/j.1460-9568.2003.02775.x. [DOI] [PubMed] [Google Scholar]
- 75.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 76.Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–46. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–62. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–23. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–22. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12:3077–80. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 81.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–70. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, Black JA. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 83.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–4. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 84.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci USA. 1999;96:7723–30. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garry MG, Hargreaves KM. Enhanced release of immunoreactive CGRP and substance P from spinal dorsal horn slices occurs during carrageenan inflammation. Brain Res. 1992;582:139–42. doi: 10.1016/0006-8993(92)90328-7. [DOI] [PubMed] [Google Scholar]
- 86.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40:251–63. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18:2467–76. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- 88.Woolf CJ. Windup and central sensitization are not equivalent [editorial] Pain. 1996;66:105–8. [PubMed] [Google Scholar]
- 89.Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA. 1999;96:7680–6. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–42. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- 91.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–90. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–53. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
- 93.Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–83. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–77. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–48. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci USA. 1999;96:7714–8. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23:4117–26. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, Ratnam J, Zou B, England PM, Basbaum AI. TrkB signaling is required for both the induction and maintenance of tissue and nerve injury-induced persistent pain. J Neurosci. 2009;29:5508–15. doi: 10.1523/JNEUROSCI.4288-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diamond J, Foerster A, Holmes M, Coughlin M. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J Neurosci. 1992;12:1467–76. doi: 10.1523/JNEUROSCI.12-04-01467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kryger GS, Kryger Z, Zhang F, Shelton DL, Lineaweaver WC, Buncke HJ. Nerve growth factor inhibition prevents traumatic neuroma formation in the rat. J Hand Surg Am. 2001;26:635–44. doi: 10.1053/jhsu.2001.26035. [DOI] [PubMed] [Google Scholar]
- 101.Ruiz G, Ceballos D, Banos JE. Behavioral and histological effects of endoneurial administration of nerve growth factor: Possible implications in neuropathic pain. Brain Res. 2004;1011:1–6. doi: 10.1016/j.brainres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–98. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, Rosol TJ, Boustany L, Shelton DL, Mantyh PW. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–35. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 104.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30:14649–56. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Brain Res Mol Brain Res. 2001;97:177–85. doi: 10.1016/s0169-328x(01)00314-x. [DOI] [PubMed] [Google Scholar]
- 106.Artico M, Bronzetti E, Felici LM, Alicino V, Ionta B, Bronzetti B, Magliulo G, Grande C, Zamai L, Pasquantonio G, De Vincentiis M. Neurotrophins and their receptors in human lingual tonsil: An immunohistochemical analysis. Oncol Rep. 2008;20:1201–6. [PubMed] [Google Scholar]
- 107.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–92. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 108.Wu Z, Nagata K, Iijima T. Involvement of sensory nerves and immune cells in osteophyte formation in the ankle joint of adjuvant arthritic rats. Histochem Cell Biol. 2002;118:213–20. doi: 10.1007/s00418-002-0443-x. [DOI] [PubMed] [Google Scholar]
- 109.Buma P, Verschuren C, Versleyen D, Van der Kraan P, Oestreicher AB. Calcitonin gene-related peptide, substance P and GAP-43/B-50 immunoreactivity in the normal and arthrotic knee joint of the mouse. Histochemistry. 1992;98:327–39. doi: 10.1007/BF00270017. [DOI] [PubMed] [Google Scholar]
- 110.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66:1423–8. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70:523–9. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 112.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 113.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–80. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 114.Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Nerve growth factor and gastric hyperalgesia in the rat. Neurogastroenterol Motil. 2003;15:355–61. doi: 10.1046/j.1365-2982.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 115.Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105:489–97. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- 116.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–22. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–99. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 118.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, Kubota K, Kuskowski MA, Boustany L, Shelton DL, Mantyh PW. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115:128–41. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 119.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR, Lewis JL, Kuskowski MA, Mantyh PW. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133:183–96. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 120.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL, Mantyh PW. Effects of a Monoclonal Antibody Raised Against Nerve Growth Factor on Skeletal Pain and Bone Healing Following Fracture of the C57BL/6J Mouse Femur. J Bone Miner Res. 2007;22:1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 121.Gorin PD, Johnson EM. Experimental autoimmune model of nerve growth factor deprivation: Effects on developing peripheral sympathetic and sensory neurons. Proc Natl Acad Sci USA. 1979;76:5382–6. doi: 10.1073/pnas.76.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gorin PD, Johnson EM., Jr Effects of long-term nerve growth factor deprivation on the nervous system of the adult rat: An experimental autoimmune approach. Brain Res. 1980;198:27–42. doi: 10.1016/0006-8993(80)90341-8. [DOI] [PubMed] [Google Scholar]
- 123.Castaneda-Corral G, Jimenez Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarksa MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express TrkA. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 125.Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, Cullinane D, Einhorn TA. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 126.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2009;148:407–13. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 127.Andersen H, Arendt-Nielsen L, Svensson P, Danneskiold-Samsoe B, Graven-Nielsen T. Spatial and temporal aspects of muscle hyperalgesia induced by nerve growth factor in humans. Exp Brain Res. 2008;191:371–82. doi: 10.1007/s00221-008-1531-5. [DOI] [PubMed] [Google Scholar]
- 128.Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–7. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 129.Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, Acciarresi M, Pini LA, Calabresi P. Increased levels of neurotrophins are not specific for chronic migraine: Evidence from primary fibromyalgia syndrome. J Pain. 2007;8:737–45. doi: 10.1016/j.jpain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Anand P, Terenghi G, Birch R, Wellmer A, Cedarbaum JM, Lindsay RM, Williams-Chestnut RE, Sinicropi DV. Endogenous NGF and CNTF levels in human peripheral nerve injury. Neuroreport. 1997;8:1935–8. doi: 10.1097/00001756-199705260-00028. [DOI] [PubMed] [Google Scholar]
- 131.Iannone F, De Bari C, Dell’Accio F, Covelli M, Patella V, Lo Bianco G, Lapadula G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2002;41:1413–8. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 132.Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992;35:351–5. doi: 10.1002/art.1780350315. [DOI] [PubMed] [Google Scholar]
- 133.Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: What is the therapeutic potential? BioDrugs. 2008;22:349–59. doi: 10.2165/0063030-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 134.Ugolini G, Marinelli S, Covaceuszach S, Cattaneo A, Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:2985–90. doi: 10.1073/pnas.0611253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Owolabi JB, Rizkalla G, Tehim A, Ross GM, Riopelle RJ, Kamboj R, Ossipov M, Bian D, Wegert S, Porreca F, Lee DK. Characterization of antiallodynic actions of ALE-0540, a novel nerve growth factor receptor antagonist, in the rat. J Pharmacol Exp Ther. 1999;289:1271–6. [PubMed] [Google Scholar]
- 136.Colquhoun A, Lawrance GM, Shamovsky IL, Riopelle RJ, Ross GM. Differential activity of the nerve growth factor (NGF) antagonist PD90780 [7-(benzolylamino)-4,9-dihydro-4-methyl-9-oxo-pyrazolo[5,1-b]quinazoline-2 -carboxylic acid] suggests altered NGF-p75NTR interactions in the presence of TrkA. J Pharmacol Exp Ther. 2004;310:505–11. doi: 10.1124/jpet.104.066225. [DOI] [PubMed] [Google Scholar]
- 137.Winston JH, Toma H, Shenoy M, He ZJ, Zou L, Xiao SY, Micci MA, Pasricha PJ. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain. 2003;4:329–37. doi: 10.1016/s1526-5900(03)00636-9. [DOI] [PubMed] [Google Scholar]
- 138.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: Molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–91. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Casadevall N. Pure red cell aplasia and anti-erythropoietin antibodies in patients treated with epoetin. Nephrol Dial Transplant. 2003;18(Suppl 8):viii37–41. doi: 10.1093/ndt/gfg1091. [DOI] [PubMed] [Google Scholar]
- 140.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Anand P, Terenghi G, Warner G, Kopelman P, Williams-Chestnut RE, Sinicropi DV. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2:703–7. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 142.Colangelo AM, Bianco MR, Vitagliano L, Cavaliere C, Cirillo G, De Gioia L, Diana D, Colombo D, Redaelli C, Zaccaro L, Morelli G, Papa M, Sarmientos P, Alberghina L, Martegani E. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci. 2008;28:2698–709. doi: 10.1523/JNEUROSCI.5201-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: What went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 144.Mendell LM. Does NGF binding to p75 and trkA receptors activate independent signalling pathways to sensitize nociceptors? J Physiol. 2002;544:333. doi: 10.1113/jphysiol.2002.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, Dawbarn D, Vincent TL, Inglis JJ. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–92. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 146.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138:47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Bouhana KS, Trollinger D, Winkler J, Lee P, Andrews SW, Kuskowski MA, Mantyh PW. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone. 2011;48:389–98. doi: 10.1016/j.bone.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. TrkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–6. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- 149.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–91. doi: 10.1016/j.pain.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 150.Sugiura A, Ohtori S, Yamashita M, Inoue G, Yamauchi K, Koshi T, Suzuki M, Norimoto M, Orita S, Eguchi Y, Takahashi Y, Watanabe TS, Ochiai N, Takaso M, Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976) 2008;33:2047–51. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- 151.Nakajima T, Ohtori S, Yamamoto S, Takahashi K, Harada Y. Differences in innervation and innervated neurons between hip and inguinal skin. Clin Orthop Relat Res. 2008;466:2527–32. doi: 10.1007/s11999-008-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]