Abstract
Rheumatoid arthritis (RA) is a debilitating, chronic, persistent inflammatory disease that is characterised by painful and swollen joints. The aetiology of RA is unknown, however whereas past research has concentrated on the role of immune or inflammatory infiltrating cells in inflammation, it is becoming clear that stromal cells play a critical part in regulating the quality and duration of an inflammatory response. In this review we assess the role of fibroblasts within the inflamed synovium in modulating immune responses; in particular we examine the role of stromal cells in the switch from resolving to persistent inflammation as is found in the rheumatoid synovium.
Keywords: Rheumatoid arthritis, Cytokines, Chemokines, Synovitis, Inflammation
1. Introduction
Inflammation is a complex process in which leukocytes are recruited to tissue in response to injury or invading pathogens. In many cases the inflammatory response is terminated when the causative agent is eliminated. In situations where this is not the case, the response becomes persistent, often leading to sustained tissue damage.
Previously, the main function attributed to resident stromal cells in the inflammatory response was the provision of extracellular matrix to aid tissue repair; stromal cells were not thought to be major contributors to the immune system [1]. However, increasing evidence has shown that fibroblasts are actively involved in regulating the immune response, and in particular the switch from acute to chronic inflammation [2-4].
Homeostasis of the immune system is vital to prevent leukocytes accumulating in the wrong place at the wrong time, thus potentially exacerbating the inflammatory response. Most organs lack significant leukocyte traffic in a non-inflamed state, however, during inflammation, various subsets of leukocytes are recruited leading to accumulation in inflamed tissue. Once the inflammation has resolved and the infiltrating immune cells are no longer required, loss of survival signals initiates leukocyte apoptosis, whilst changes in chemokine and adhesion molecule profiles enables the migration of leukocytes out of the inflamed tissue into draining lymph nodes, thereby terminating the inflammatory response.
A characteristic of chronic inflammation is the persistence of such redundant cells, which may in turn lead to further leukocyte accumulation and the destruction of associated healthy tissues. A good example of a disordered cellular environment that leads to chronic inflammation and tissue degradation is found in the joints of patients with rheumatoid arthritis.
2. Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic, persistent inflammatory disease, the characteristic clinical feature of which is persistent joint inflammation. The disease is characterised by inflammation of the synovial membranes of diarthrodial joints in a symmetrical distribution. This results in erosion of articular cartilage and marginal bone, with subsequent joint destruction [5].
RA is a complex, multi-factorial disease with a mild to moderate genetic association. Annually RA affects 10–20/100,000 males and 20–40/100,000 females, its prevalence varying from approximately 0.5–1% across different populations [6]. Genetic linkage studies have shown a strong association between destructive forms of RA and certain MHC class II alleles. A particular sequence of amino acids that lies inside the highly polymorphic region lining the peptide-binding groove of HLA DR4 is encoded by each of these alleles. This amino-acid sequence is known as the “shared epitope” [7]. However the coincidence rate for identical twins is at best 20% [6], making the interpretation of the shared epitope difficult.
2.1. The rheumatoid synovium
The synovium is a soft tissue, which in healthy people lines the non-cartilaginous surfaces of diarthrodial joints and tendon sheaths [8,9]. It is composed of a thin cellular layer and a specialised matrix known as the intima and the subintima, respectively. In early inflammatory arthritis, tissue oedema is prominent, new blood vessels are formed (angiogenesis) and synovial-lining hyperplasia can occur. The inflammatory infiltrate is usually quite mild and consists of scattered neutrophils and lymphocytes (Fig. 1) [10].
Fig. 1.

Synovial changes in the rheumatoid synovium. In the normal joint, the bones move past each other at the cartilage–cartilage interface, lubricated by the synovial fluid. The capsule is composed of connective tissue and synovial membrane. The synovial membrane covers most of the intraarticular surface. The synovial membrane has two layers, known as the intima and the sub-intima. The intima is usually three cells thick and is in direct contact with the intimal fibroblasts, fat cells and mononuclear cells. In a joint with RA, the synovial membrane is swollen, hyperplasic and has villi projecting into the joint cavity (pannus). The pannus is able to migrate under the cartilage and into the bone and causing damage to the bone that can be seen in X-rays. Extensive angiogenesis creates an infiltration of leukocytes that cause oedema.
As the disease enters its chronic phase, the synovial membrane shows multiple redundant folds and villi. The subintima contains a heavy chronic inflammatory infiltrate, which is composed mainly of mononuclear cells, including T-cells, B cells, macrophages and dendritic cells [11,12]. There are three characteristic types of infiltrate: diffuse, where there is a lack of leukocyte organisation (~50% of synovia); perivascular cuffs: where leukocytes surround the endothelium (~20%); and ectopic lymphoid structures where the leukocytes are organised in such a way as to resemble germinal centres (~20%) [13].
3. The stromal cell contribution to rheumatoid arthritis
3.1. The role of fibroblasts in chronic rheumatoid arthritis
Inflammatory responses occur within tissue microenvironments with contributions from both haematopoietic (such as lymphocytes) and stromal cells (such as fibroblasts). Fibroblast-like cells constitute a large population of cells that can be extracted from synovial tissue. There is growing evidence that activated synovial fibroblasts contribute to the pathogenesis of rheumatoid arthritis. As well as responding to the factors produced by lymphocytes and macrophages that are present in the joint (such as IFN-γ, TNFα and IL-17), they actively produce and respond to many factors that they themselves make, forming an autocrine loop [14,15]. This results in changes in the expression of regulatory genes, signalling molecules, adhesion molecules and over-expression of matrix degrading enzymes (such as matrix-metalloproteases (MMPs)) that contribute to tissue destruction in the joint [16]. There is accumulating evidence showing clear phenotypic differences between fibroblasts of normal and pathological tissue, thus suggesting a role for fibroblasts in determining the pattern of organ involvement in a particular disease. Fibroblasts therefore, along with macrophages help define the stromal microenvironment that is unique to each tissue [17,18].
Studies in which the NF-κB family of transcription factors have been knocked out in mice have shown the importance of this family of transcription factors in regulating the stromal cell contribution to inflammation. Mice deficient in RelB, for example, show multi-organ inflammation, which fails to respond to adoptive transfer of haemopoietic cells, thereby indicating the importance of stromal elements in these pathological processes. Fibroblasts from RelB deficient mice over-produce a variety of chemokines and cytokines in response to stimulation [19,20]; reciprocal studies using human synovial fibroblasts have shown that many pro-inflammatory cytokines are dependent on NF-κB for their production [21-23].
It has been speculated that synovial fibroblasts share a similar phenotype to mesenchymal stem cells. This would potentially allow the synovial fibroblast to differentiate into a range of cell types which share a common mesenchymal origin, such as osteoblasts, chondroblasts, myocytes and adipocytes, all of which are constituent cell types of the joint [24-26]. This mesenchymal phenotype would allow the synovial fibroblasts to divide, thereby contributing to the synovial hyperplasia that is a feature of rheumatoid arthritis.
Further studies have shown that rheumatoid synovial fibroblasts maintain their phenotype over many passages in culture ex vivo even in the absence of pro-inflammatory cytokines. This has been most elegantly demonstrated using a SCID mouse model of RA. Isolated RA synovial fibroblasts were implanted into normal cartilage and transplanted into SCID mice. RA synovial fibroblasts attached to and invaded deep into the cartilage, whereas fibroblasts from skin or osteoarthritis synovial fibroblasts did not show such invasive behaviour. The invasive behaviour shown by the rheumatoid fibroblasts in this model was without cytokine or chemokine influence, implying a stable, imprinted alteration in cell function [27]. This and other behaviour has been suggested to indicate a functional similarity of rheumatoid fibroblasts to invasive tumour cells.
For example, in culture, RA synovial fibroblasts can grow in an anchorage-independent manner and lose contact inhibition (i.e. normal fibroblasts grow until confluence and then stop). Molecular mechanisms responsible for this altered phenotype have been suggested to include somatic mutations in genes such as the p53 tumour suppressor gene. Although controversial, these mutations have been identified in synovial tissue and synovial cells. It is possible that these changes in p53 are not primary (i.e. causal), but secondary to prolonged exposure to hypoxic conditions (i.e. consequential) [28].
In support of the link between hypoxia, cancer and chronic inflammation it is now clear that hypoxia leads to the activation of chemokine receptors such as CXCR4 that lead to changes in the migratory properties of monocytes in inflamed tissues [29] as well as a more motile phenotype in metastatic cancers [30]. The molecular basis for this has been elucidated and shown to be due to the ability of the hypoxia inducible factor (HIF), regulated by the tumour suppressor gene (von Hippel–Lindau pVHL), to regulate CXCR4 expression as well as a range of other proteins including erythropoietin (Epo) and vascular endothelial growth factor (VEGF). Therefore the link between hpyoxia and tumour formation and hypoxia and chronic inflammation may reside at the level of environmental stresses such as oxidative stress on tumour supressor genes and their subsequent effects on growth factors.
4. The switch from acute to chronic persistent inflammation
4.1. Chronic inflammation
Chronic, persistent inflammation is a complex pathophysiological process which is characterised both pathologically, in that the predominant cell types are lymphocytes and macrophages, and temporally, in that the inflammatory response lasts for weeks to years as opposed to days, as seen in most cases of acute inflammation.
During the initial phases of an inflammatory response, large numbers of leukocytes are recruited to the injured site. They are recruited in response to endothelial changes such as adhesion molecule up-regulation and chemokine-mediated attraction. Normally inflammation resolves, and naturally occurring anti-inflammatory mediators gradually replace pro-inflammatory mediators. Examples of naturally occurring anti-inflammatory mediators include annexin 1 which acts via paracrine and autocrine routes to down-regulate the process of leukocyte extravasation into tissues [31]. Lipoxins are another example of endogenously produced mediators that are involved in inhibiting neutrophil chemotaxis, adhesion and transmigration, induced by mediators such as leukotrienes (products of arachidonic acid) [32]. While lipoxins inhibit attraction of neutrophils, they are potent chemoattractants for monocytes. This is important for the resolution of inflammation as macrophages remove apoptotic neutrophils from the inflammatory environment by phagocytosis. Once inflammatory cells are no longer required, those cells that are in the tissues either exit out of the tissues (via draining lymphatics) or they die through the loss of survival signals and initiation of apoptosis. However in RA, stromal elements within the microenvironment continue to provide survival signals and express lower levels of naturally occurring anti-inflammatory agents such as annexin-1, resulting in the inappropriate accumulation and survival of leukocytes (Fig. 2).
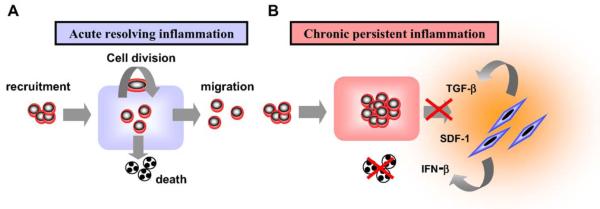
Fig. 2.
Tissue homeostasis depends upon a balance between cell recruitment, division, emigration and death. During a normal inflammatory response tissue homeostasis is returned as cells emigrate out of the inflamed tissue or die by apoptosis (a). However in chronic, persistent inflammation, inappropriate signals from the stroma cause the accumulation/retention and prevent the onset of apoptosis of inflammatory leukocytes.
4.2. Persistence of the chronic inflammatory infiltrate
In acute resolving inflammation, the processes of cell recruitment, proliferation, emigration and death are carefully balanced, resulting in tissue homeostasis (Fig. 2). However, if one or more of these processes become imbalanced, persistence of the infiltrate may occur, contributing to tissue hyperplasia and the destruction of associated healthy tissues, a feature that is characteristic of persistent chronic inflammation in the synovium. Examples of disordered cellular environments which may lead to tissue hyperplasia, and/or degradation can also be found in-patients autoimmune thyroid disease and Sjögrens syndrome. The stromal microenvironment plays an important role in these processes, by producing cytokines and chemokines such as IFNβ and SDF-1α (CXCL12), which promote the survival and retention respectively of cells in the rheumatoid joint.
Synovial fibroblasts have been shown to be potent producers of cytokines, adhesion molecules and chemokines, such as IL-6, IFN-β, MCP-1 (CCL2), VCAM (CD 106) that attract and retain large numbers of leukocytes in the inflamed synovium [33-35]. These two properties of synovial fibroblasts from RA patients may sustain chronic inflammation and prevent the resumption of normal tissue homeostasis within the rheumatoid synovium. Recent experimental data has suggested that stromal cells, such as fibroblasts might be involved in the switch from acute to chronic inflammation (Switch 1) and the switch to resolution or persistent inflammation (Switch 2) (Fig. 3). Synovial fibroblasts secrete high levels of IL-6 along with IL-8 (CXCL8), a potent chemoattractant for neutrophils [36]. In response to pro-inflammatory stimuli, neutrophils shed IL-6 receptors, which in combination with IL-6 bind to the gp-130 transducing molecule of the IL-6 receptor complex. This autocrine stimulation of stromal cells by IL-6 causes them to release MCP-1 (CCL2) a chemokine that attracts monocytes to the site of inflammation. In this way, stromal cells are important in organising the quality and quantity as well as the sequential recruitment of leukocyte subsets during an inflammatory response [37,38].
Fig. 3.

Switches from acute to chronic, persistent inflammation. Switch 1 to chronic inflammation. During acute inflammation, neutrophils are recruited by IL-8 (CXCL8) released by stromal cells. Pro-inflammatory stimuli cause neutrophils to shed their IL-6 receptor, that in combination with gp-130 expressed on the surface of stromal cells causes the release of chemokines such as MCP-1 (CCL2) which attracts mononuclear cells such as monocytes and lymphocytes leading to a chronic inflammatory response. Switch 2 to chronic persistent inflammation. Normally chronic inflammation resolves itself, however in some cases it fails to resolve and instead leads to chronic, persistent inflammation that be characterised by the formation of ectopic germinal centre-like structures within the inflamed tissue.
Rheumatoid synovial fibroblasts support the survival of T cells within the rheumatoid synovium by producing molecules that prevent T cells from entering apoptosis. Initial investigations showed that fibroblast conditioned media could sustain T cells in vitro without causing T cells to proliferate [39]; subsequently rheumatoid synovial fibroblasts were shown to secrete interferon beta (IFN-β). IFN-β increases levels of Bcl-XL, an anti-apoptotic protein within T cells, but does not affect levels of Bcl-2 [40]. It is by this mechanism that the rheumatoid synovium is thought to inappropriately promote the survival of T cells that under normal conditions should die by apoptosis. Synovial fibroblasts also support the survival of B cells by expressing VCAM (CD106) on their surface by a similar mechanism of up-regulating Bcl-XL [41].
Chronic persistent inflammation has strong links with the physiological process of wound repair. It has been suggested that chronic, persistent inflammation may occur as a result of failure of wound healing [42]. Both processes are characterised by the formation of new tissue, such as endothelium, smooth muscle and fibroblasts. Interestingly these new cells are not necessarily derived from adjacent tissue, but from blood borne precursors called fibrocytes. Fibrocytes defined by markers of both haemopoietic (CD45) and stromal lineages (smooth muscle actin and vimentin) circulate within the blood and can home to areas of inflammation, where they differentiate into a variety of different stromal cell types [43-45]. Further credence to the idea that chronic, persistent inflammation is a failure of wound healing comes from the recent finding that synovial fibroblasts can support the expansion of inflammatory T cells by expressing HLA-DR and thrombospondin, that is mainly expressed in chronically inflamed and injured tissues [46,47].
5. Chemokines are actively involved in cross-talk between stromal cells and infiltrating leukocytes
For the immune system to be able to successfully mount a response against foreign pathogens and maintain tolerance it needs a system to organise the recruitment and positioning of specific populations of cells, both temporally and spatially. Specific microenvironments release chemokines that in combination with chemokine receptors recruit specific populations of cells to specific sites. Cell recruitment relies on the co-ordinated action of cell activation, adhesion, chemoattraction and transmigration across the endothelium. Chemokines are a super-family of small, secreted proteins (8–10 kDa) that share the ability to attract leukocytes. Presently 50 chemokines have been identified, classified into four structural groups based on the positioning of conserved cysteine residues, resulting in CXC, CC, XC and CX3C motifs [48]. Chemokines induce cell migration and activation by binding to specific G protein coupled cell surface receptors on target cells [49,50].
5.1. Homeostatic and inflammatory chemokines
Chemokines can be classified functionally as either inflammatory (inducible) or homeostatic (constitutive) [51]. Homeostatic chemokines are constitutively expressed in discrete microenvironments, both within lymphoid tissues and in non-lymphoid tissues such as skin and mucosa, where they are involved in basal cell trafficking and positioning of cells during haematopoiesis, antigen sampling in secondary lymphoid tissue and immune surveillance. For example the chemokine receptor CCR7 binds the ligands SLC (CCL21) and ELC (CCL19) which are critical in the physiological circulation of naive T cells, B cells and dendritic cells through secondary lymphoid organs [52]. Inflammatory chemokines are expressed in most tissues by different types of resident cells and by infiltrating leukocytes in response to inflammatory cytokines and other pro-inflammatory stimuli.
Surprisingly, in chronic inflammatory diseases such as RA and Hashimoto’s disease, in addition to inflammatory chemokines such as IP-10 (CXCL10), RANTES (CCL5) and IL-8 (CXCL8), homeostatic chemokines are inappropriately expressed leading to the formation of ectopic lymphoid structures [53]. The presence of pro-inflammatory cytokines such as TNFα and lymphotoxin in areas of chronic inflammation mirrors the involvement of these cytokines in the development and organisation of secondary lymphoid organs, so that in chronic inflammation, ontogeny appears to be recapitulated just as has been observed in cancer [54,55].
In rheumatoid arthritis, TGF-β derived from stromal cells within the synovial microenvironment has been shown to maintain inappropriately high levels of CXCR4 on synovial T cells. This along with synovial endothelial cells producing SDF-1 (the ligand for CXCR4) implicates these chemokines in the retention of T cells within the joint and thus suggests a role for the stromal microenvironment in the pathology of this chronic persistent disease [56,57]. Data from our own laboratory has indicated how the stromal microenvironment up-regulates the chemokine receptor profile expression on T cells including CCR5, CCR7, CXCR3 and CXCR4. In this way the stromal microenvironment retains T cells within the synovium.
6. Conclusions
The persistent accumulation of leukocytes within inflamed tissues results in an inflammatory milieu that encourages disordered interactions between leukocytes and stromal cells. Chemokines are important molecules, vital for the spatial and temporal positioning of cells. When their production or the expression of their receptors become dysregulated, cells no longer have the appropriate stimulus to enter or leave sites of inflammation. Recent evidence has shown that chemokines derived from synovial fibroblasts and the endothelium lead to the selective recruitment of leukocyte subsets. Under normal conditions, fibroblasts in the synovium have little contact with immune cells. In RA, however, inappropriate synovial fibroblast–T cell interactions have the ability to influence both the survival and accumulation of lymphocytes in the synovium [58]. In this review we have illustrated that fibroblasts are not just structural cells but play an important part in regulating the intensity and persistence of the inflammatory response.
Many inflammatory diseases are site specific, with well-defined tissue tropism. It remains unclear why multiple sclerosis lesions are confined to the brain, or why psoriatic lesions occur only in the skin. The molecular basis for site specific inflammatory responses remains obscure, but there is now accumulating evidence that distinct stromal cells such as fibroblasts are responsible for directing the site-specificity of inflammation. The stromal microenvironment may imprint a “post-code” on inflammatory leukocytes that governs their subsequent return to that specific location [59]. Evidence from our own laboratory has shown that there are clear differences in the cytokines and chemokines produced, along with distinct gene profiles of fibroblasts from different sites [60]. In this way stromal cells might direct the location of an inflammatory response, in addition to perpetuating inflammation in persistent inflammatory diseases.
References
- [1].Brewer DB. The fibroblast. Proc R Soc Med. 1967;60:778–81. [PMC free article] [PubMed] [Google Scholar]
- [2].Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- [3].Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- [4].Wahl SM, Malone DG, Wilder RL. Spontaneous production of fibroblast-activating factor(s) by synovial inflammatory cells. A potential mechanism for enhanced tissue destruction. J Exp Med. 1985;161:210–22. doi: 10.1084/jem.161.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- [6].Nepom GT, Nepom B. Genetics of the major histocompatibility complex in rheumatoid arthritis. In: Dieppe PA, Klippel JH, editors. Rheumatology. Mosby; 2002. [Google Scholar]
- [7].Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- [8].Tak PP. Examination of the synovium and synovial fluid. In: Firestein GS, Panayi GSWFA, editors. Rheumatoid arthritis New frontiers in pathogenesis and treatment. Oxford University Press; 2000. pp. 55–67. [Google Scholar]
- [9].Edwards JC. The synovium. In: Dieppe PA, Klippel JH, editors. Rheumatology. Mosby; 1998. [Google Scholar]
- [10].Firestein G. Rheumatology. Mosby International; 1998. Rheumatoid arthritis and pannus; pp. 1–24. [Google Scholar]
- [11].Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–90. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- [12].Janossy G, Panayi G, Duke O, Bofill M, Poulter LW, Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981;2:839–42. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- [13].Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- [14].Firestein GS, Alvaro-Gracia JM, Maki R, Alvaro-Garcia JM. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–53. published erratum appears in J Immunol 1990;145:1037. [PubMed] [Google Scholar]
- [15].Saxne T, Palladino MA, Jr, Heinegard D, Talal N, Wollheim FA. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31:1041–5. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- [16].Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik B, Skowronski J, Chwiecko J. Serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in different histological variants of rheumatoid synovitis. Rheumatology (Oxford) 2002;41:78–87. doi: 10.1093/rheumatology/41.1.78. [DOI] [PubMed] [Google Scholar]
- [17].Brouty-Boye D, Pottin-Clemenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–9. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [18].Pap T, Muller-Ladner U, Gay RE, Gay S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2:361–7. doi: 10.1186/ar113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–40. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- [20].Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–87. [PMC free article] [PubMed] [Google Scholar]
- [21].Bondeson J, Brennan F, Foxwell B, Feldmann M. Effective adenoviral transfer of IkappaBalpha into human fibroblasts and chondrosarcoma cells reveals that the induction of matrix metalloproteinases and proinflammatory cytokines is nuclear factor- kappaB dependent. J Rheumatol. 2000;27:2078–89. [PubMed] [Google Scholar]
- [22].Foxwell B, Browne K, Bondeson J, Clarke C, de Martin R, Brennan F, et al. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–5. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bondeson J, Foxwell B, Brennan F, Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-kappaB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci USA. 1999;96:5668–73. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gulko PS, Seki T, Winchester R. The role of fibroblasts- like synoviocytes in rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid arthritis New frontiers in pathogenesis and treatment. Oxford University Press; 2000. pp. 113–36. [Google Scholar]
- [25].De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [26].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [27].Muller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607–15. [PMC free article] [PubMed] [Google Scholar]
- [28].Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- [29].Schioppa T, Unanchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bernards R. Cues for migration. Nature. 2003;425:247–8. doi: 10.1038/425247a. [DOI] [PubMed] [Google Scholar]
- [31].Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brady HR, Lamas S, Papayianni A, Takata S, Matsubara M, Marsden PA. Lipoxygenase product formation and cell adhesion during neutrophil-glomerular endothelial cell interaction. Am J Physiol. 1995;268:F1–12. doi: 10.1152/ajprenal.1995.268.1.F1. [DOI] [PubMed] [Google Scholar]
- [33].Bombara MP, Webb DL, Conrad P, Marlor CW, Sarr T, Ranges GE, et al. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J Leukoc Biol. 1993;54:399–406. doi: 10.1002/jlb.54.5.399. [DOI] [PubMed] [Google Scholar]
- [34].Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22. [PMC free article] [PubMed] [Google Scholar]
- [35].Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–74. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Georganas C, Liu H, Perlman H, Hoffmann A, Thimmapaya B, Pope RM. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c- Jun. J Immunol. 2000;165:7199–206. doi: 10.4049/jimmunol.165.12.7199. [DOI] [PubMed] [Google Scholar]
- [37].Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- [38].Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- [39].Salmon M, Scheel-Toellner D, Huissoon AP, Pilling D, Shamsadeen N, Hyde H, et al. Inhibition of T cell apoptosis in the rheumatoid synovium. J Clin Invest. 1997;99:439–46. doi: 10.1172/JCI119178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pilling D, Akbar AN, Girdlestone J, Orteu CH, Borthwick NJ, Amft N, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- [41].Hayashida K, Shimaoka Y, Ochi T, Lipsky PE. Rheumatoid arthritis synovial stromal cells inhibit apoptosis and up-regulate Bcl-xL expression by B cells in a CD49/CD29-CD106-dependent mechanism. J Immunol. 2000;164:1110–6. doi: 10.4049/jimmunol.164.2.1110. [DOI] [PubMed] [Google Scholar]
- [42].Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–9. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- [44].Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- [45].Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [46].Vallejo AN, Yang H, Klimiuk PA, Weyand CM, Goronzy JJ. Synoviocyte-mediated expansion of inflammatory T cells in rheumatoid synovitis is dependent on CD47-thrombospondin 1 interaction. J Immunol. 2003;171:1732–40. doi: 10.4049/jimmunol.171.4.1732. [DOI] [PubMed] [Google Scholar]
- [47].Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–84. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- [49].Ahuja SK, Gao JL, Murphy PM. Chemokine receptors and molecular mimicry. Immunol Today. 1994;15:281–7. doi: 10.1016/0167-5699(94)90008-6. [DOI] [PubMed] [Google Scholar]
- [50].Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–8. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- [51].Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- [52].Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–50. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–8. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nishikawa SI, Hashi H, Honda K, Fraser S, Yoshida H. Inflammation, a prototype for organogenesis of the lymphopoietic/hematopoietic system. Curr Opin Immunol. 2000;12:342–5. doi: 10.1016/s0952-7915(00)00097-2. [DOI] [PubMed] [Google Scholar]
- [55].Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F, et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423–9. doi: 10.4049/jimmunol.165.6.3423. [DOI] [PubMed] [Google Scholar]
- [57].Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170:2147–52. doi: 10.4049/jimmunol.170.4.2147. [DOI] [PubMed] [Google Scholar]
- [58].McInnes IB, Leung BP, Liew FY. Cell–cell interactions in synovitis. Interactions between T lymphocytes and synovial cells. Arthritis Res. 2000;2:374–8. doi: 10.1186/ar115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- [60].Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, et al. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003;90:688–97. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]