Abstract
Co-chaperonin GroES from Escherichia coli works with chaperonin GroEL to mediate the folding reactions of various proteins. However, under specific conditions, i.e. the completely disordered state in guanidine hydrochloride, this molecular chaperone forms amyloid fibrils similar to those observed in various neurodegenerative diseases. Thus, this is a good model system to understand the amyloid fibril formation mechanism of intrinsically disordered proteins. Here, we identified a critical intermediate of GroES in the early stages of this fibril formation using NMR and mass spectroscopy measurements. A covalent rearrangement of the polypeptide bond at Asn45-Gly46 and/or Asn51-Gly52 that eventually yield β-aspartic acids via deamidation of asparagine was observed to precede fibril formation. Mutation of these asparagines to alanines resulted in delayed nucleus formation. Our results indicate that peptide bond rearrangement at Asn-Gly enhances the formation of GroES amyloid fibrils. The finding provides a novel insight into the structural process of amyloid fibril formation from a disordered state, which may be applicable to intrinsically disordered proteins in general.
Keywords: Amyloid, Chaperone Chaperonin, NMR, Protein Conformation, Protein Folding, Asn-Gly Rearrangement, Intrinsically Disordered Protein
Introduction
Intrinsically disordered proteins are commonly defined as proteins that do not adopt a well defined structure in solution (1), and they can fold into ordered structures only upon binding to their cellular targets (2). They are abundant in eukaryotic proteins and play a significant role in biological functions associated with signaling and regulation events (3). Some intrinsically disordered proteins, exemplified by α-synuclein (4–6) and poly(Q) (7–9) proteins, are capable of forming insoluble aggregates referred to as amyloid fibrils. Understanding the detailed mechanisms in which intrinsically disordered proteins self-assemble into amyloid fibrils is a very important issue because they associate closely with amyloid-related degenerative diseases (10).
The prevalent model to explain the mechanisms of amyloid fibril formation in vitro is nucleation-dependent fibril formation (11). Fibril formation kinetics consists of two phases, that is, nucleation and extension, as traced with Thioflavin-T-binding fluorescence or turbidity of incubated samples. Nucleus formation requires a series of associations between protein monomers that are thermodynamically unfavorable, and this step represents the rate-limiting step in amyloid fibril formation. Once the nucleus has been formed, the further addition of monomers becomes thermodynamically favorable, resulting in a rapid extension of amyloid fibrils (12, 13). It has generally been assumed until recently that the cytotoxicity in amyloid-related degenerative diseases was due to mature amyloid fibrils, but now attention has shifted to various intermediate species formed during nucleation, such as an oligomeric but soluble state (14–16) or even some monomeric states (17, 18). Therefore, details regarding the structural characteristics of these species that appear in the early stage of the fibril formation are very important.
Here, we set out to investigate the structural changes that occur during the amyloid fibril formation of molecular chaperone GroES, a member of hsp10 from Escherichia coli. Native GroES forms heptameric oligomers of subunits abundant in β-strands (Fig. 1A) and acts as the co-chaperonin of GroEL that mediates various protein folding reactions in vivo and in vitro. We have previously found that GroES may form typical amyloid fibrils from the guanidine hydrochloride (Gdn-HCl)2 unfolded state (19) and determined the core sequence of the mature GroES amyloid fibrils that are resistant to protease (Fig. 1B) (20). Our findings proved that even a molecular chaperone may form amyloid fibrils under certain conditions, supporting the recent finding that amyloid fibrils are an inherently common structure of many proteins under certain conditions in vitro (21–23). As typical amyloid fibrils of GroES are formed from an extensively disordered (unfolded) state (19), this system is a good model for elucidating the common mechanism of amyloid fibril formation of intrinsically disordered proteins.
FIGURE 1.
Structural characteristics of GroES. A, top view of the molecular structure of GroES heptamer, drawn using MOLMOL (60) with coordinates from PDB file 1AON. One subunit is drawn as a space-filling model. B, amino acid sequence of GroES. Thick arrows above the sequence indicate regions corresponding to β-strands seen in the native x-ray structure. The core sequence of the amyloid fibril of GroES determined previously (20) is boxed and shaded in gray.
In this study, we found very early, initial conformational changes in unfolded GroES by using solution NMR spectroscopy, a powerful approach to obtain insights into soluble species formed during fibril formation at an atomic level. We first assigned the 1H-15N resonances of monomeric unfolded GroES in Gdn-HCl and next detected structural changes within a specific region during fibril formation. Interestingly, this region was adjacent to the fibril core sequence that we determined previously (20). It was also found that formation of this intermediate hinged on an Asn-Gly rearrangement reaction, which yielded β-aspartic acid as a consequence of deamidation of the asparagine. The relevance of this reaction was further examined by site-directed mutagenesis where the asparagine residue in the Asn-Gly sequence was substituted with an alanine, which showed that the period required for nucleus formation was prolonged. These results indicated that the contiguous Asn-Gly sequence before the fibril core region leads to the formation of GroES amyloid fibril. Considering the minimal criterion that is required for this reaction to occur, our findings provide insight into the mechanism of fibril formation related to amyloid-related degenerative diseases as well as the structural characteristics and aggregation propensities of the intrinsically disordered proteins in general.
EXPERIMENTAL PROCEDURES
Preparation of Wild-type and Mutant GroES Proteins
Genes encoding the GroES mutants, N45A, N51A, and N45A/N51A, were constructed by using the QuikChange site-directed mutagenesis kit (Stratagene) with pETES (24) as a template. The successful construction of each mutant was confirmed by DNA sequence analysis of the entire GroES coding region. Both wild-type and mutant proteins were expressed in E. coli BL21(DE3) (Novagen) and purified as described previously (24). Chlorella medium (Chlorella Industry) uniformly labeled with stable isotope (15N or 15N and 13C) was used for the expression of wild-type GroES, and M9 minimal medium supplemented with [15N]NH4Cl (Shoko) was used for the expression of N51A and N45A/N51A for NMR measurements. The cells were resuspended in 10-fold ice-cold buffer A (50 mm Tris-HCl (pH 8.0), 1 mm EDTA, 1 mm DTT, 0.1 mm PMSF) and lysed by sonication on ice. The crude extracts were cleared by centrifugation and nucleic acids were removed by the addition of 2% streptomycin. After addition of 55% ammonium sulfate and centrifugation, precipitates were resuspended in buffer A and heated at 80 °C for 20 min. After the heat treatment, the mixture was quickly cooled in ice-cold water for 20 min. To remove heat-denatured proteins, the heated fraction was centrifuged at 13,000 × g for 40 min at 4 °C. The supernatant was applied to a Q-Sepharose anion-exchange column (φ2.7 cm × 18 cm) equilibrated in buffer B (50 mm Tris-HCl (pH 8.0), 1 mm EDTA, 1 mm DTT). Proteins were eluted by a 0–0.5 m NaCl gradient (total 1000 ml), and eluted GroES fractions were dialyzed against Milli-Q water. The GroES thus obtained was filtered through a 0.22-μm cellulose-acetate membrane and stocked at 4 °C after lyophilization. The purity of GroES was checked by SDS-PAGE. The concentration of GroES protein was determined by using either an extinction coefficient at 280 nm, E1 cm0.1% = 0.143 (25) or a protein dye reagent (Protein Assay kit; Bio-Rad Laboratories) using bovine serum albumin (Sigma) as a standard.
NMR Measurement
Lyophilized GroES that are labeled with single 15N or double 15N-13C stable isotopes were dissolved in 10 mm sodium phosphate buffer (pH 6.5), 1.6 m Gdn-HCl, 90% (v/v) H2O/10% D2O, and NMR measurements were performed at 1 mm (10 mg/ml) GroES concentration and 25 °C. Incubation for fibril formation was done in a Shigemi tube (Shigemi) without agitation at 25 °C. Samples were recorded using a Varian Unity Inova 500 spectrometer operating at a 1H resonance frequency of 500 MHz. All NMR data were processed with NMRPipe (26) and analyzed in NMRView (27) and Sparky (28). Proton chemical shifts were referenced to 4,4-dimethyl-4-silapentane-1-sulfonate as 0.00 ppm. The assignments of the resonances in 1H-15N HSQC spectrum of GroES WT were carried out by using 15N-edited TOCSY-HSQC, CBCANH, CBCA(CO)NH, HN(CA)CO, and HNCO. The 1H-15N HSQC peaks of Gly46 and Gly52 of the intermediate species that appeared after a 28-day incubation in Gdn-HCl were assigned by performing time-lapse measurements of 15N-labeled GroES N51A and N45A/N51A mutants. The secondary structure propensity (SSP) program has been developed by Marsh et al. and gives a measure of the secondary structure populated (29). The SSP score of GroES was calculated using the Cα and Cβ chemical shifts of GroES sample against those of random coil. The relative peak intensity between spectra is given as δI = I/I0, where I and I0 represent the peak intensities at 28 days and 2 days, respectively.
In 15N backbone relaxation experiments, 1H-15N NOE, longitudinal relaxation (R1), and transverse relaxation (R2) measurements of GroES were recorded. 1H-15N NOE values were measured by recording spectra with or without a 1H saturation period of 3 s. The R1 experiments were collected using the following relaxation delay times: 0.01, 0.05, 0.17, 0.49, and 1.80 s. The R2 experiments were collected using the following relaxation delay times: 0.01, 0.03, 0.05, 0.09, and 0.21 s. The relaxation rates were extracted by fitting the peak intensities to a decaying exponential I(t) = I0 exp(−Rt), where t is the spectrum time parameter and I(t) is peak intensity in each t. The relaxation rates and error estimate were calculated by the best fit time constant T (rate constant R = 1/T) for well resolved peaks in Sparky using the fitting function (supplemental Fig. S1).
Amyloid Fibril Formation and Thioflavin-T Binding Assay
Experiments of amyloid fibril formation were performed with 1 mg/ml GroES dissolved in 10 mm sodium phosphate (pH 7.4) containing 1.6 m Gdn-HCl with linear agitation (90 min−1) at 37 °C in glass test tubes. Fluorescence of Thioflavin-T was measured using a Jasco FP-6300 spectrofluorometer at 25 °C. At appropriate times, aliquots of GroES samples were withdrawn and mixed thoroughly with a staining solution of 25 μm Thioflavin-T, 5 mm sodium phosphate (pH 7.4), 150 mm NaCl (final GroES: 7.5 μg/ml). Fluorescence intensities were monitored at 480 nm with excitation at 440 nm.
Separation of Intermediate Species
Aliquots of samples during fibril formation were withdrawn and ultracentrifuged (150,000 × g, 1 h, 4 °C), and the supernatants were subjected to a desalting PD Spin Trap G-25 (GE Healthcare) to remove Gdn-HCl. Mature amyloid fibrils obtained as a precipitate after ultracentrifugation of samples were denatured and dissolved in 7.5 m Gdn-HCl for 24 h, then Gdn-HCl was removed by a PD Spin Trap G-25 column. These samples were subjected to 10% native-PAGE, and protein bands were stained with Coomassie Brilliant Blue R-250.
Protease Digestion and MALDI-TOF Mass Spectroscopy
In-gel digestion was performed by the method described by Shevchenko et al. (30). Gel slices containing about 5 μg of sample (GroES in various intermediate forms) were destained and dried in a vacuum centrifuge and then digested by adding 10 ng/μl lysyl endopeptidase (enzyme:substrate = 1:17–50 in molar ratio) in 25 mm NH4HCO3 at 37 °C for 16 h. To extract the digests, extraction buffer (50% acetonitrile/5% TFA) was added to the mixture, and the supernatant was collected, followed by further drying of gel pieces by addition of 100% acetonitrile, and the extract was dried completely in a vacuum centrifuge. The dried samples were dissolved in 33% acetonitrile/0.07% TFA and mixed with an equal amount of the matrix solution, 33% acetonitrile/0.07% TFA saturated with α-cyano-4-hydroxy-cinnamic acid. Resultant samples were spotted onto a target plate (MTP 384, Bruker Daltonics) and dried. Measurements were performed on an Autoflex (Bruker Daltonics) in reflection mode with positive ion detection. The mass spectra were calibrated by Peptide Calibration Standard (1000–4000 Da, Bruker Daltonics).
RESULTS
Backbone Assignments of GroES in a Disordered State
As reported previously, we found that GroES formed typical amyloid fibrils from a disordered state formed in 1.6 m Gdn-HCl, where GroES heptamer totally unfolded to a monomeric state (19, 24). To investigate further mechanisms of GroES amyloid fibril formation at atomic resolution, we prepared singly (15N) and doubly (15N-13C)-labeled GroES and performed a series of solution NMR experiments. As shown in Fig. 2A, the 1H-15N resonances of GroES in HSQC measurements were typical of those of unfolded proteins, showing a limited resonance dispersion of ∼1 ppm in the 1H dimension, but retaining relatively good dispersion covering ∼24 ppm in the 15NH dimension. The assignment of the backbone resonances of GroES to their respective locations within its sequence was carried out by triple-resonance measurements including CBCANH and CBCA(CO)NH, as well as HN(CA)CO and HNCO to overcome ambiguities that arise in the standard experiments. The dispersions in Cα and Cβ chemical shifts were poor in the unfolded state, whereas the dispersions of 13CO resonances were much greater, as in the case of 15NH chemical shifts, reflecting the sensitivity of these nuclei to the nature of the neighboring amino acid in the primary sequence (31). Although Asn2 and Val10 were not identified in the HSQC spectrum due to the narrow chemical shift dispersion, in total, 92 resonances out of 97 residues (excluding the N-terminal Met, Pro5, and Pro56) in the sequence have been assigned.
FIGURE 2.
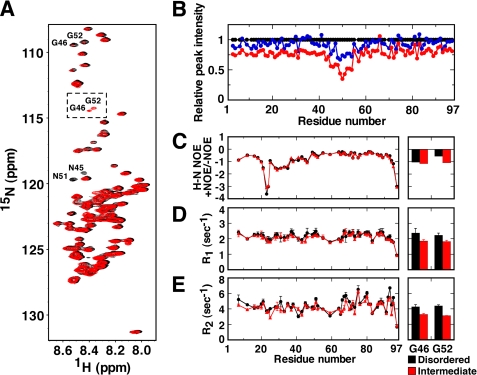
NMR peak assignments of GroES in the unfolded state. A, two-dimensional 1H-15N HSQC spectrum of GroES. Assignments are indicated in the figure. Measurement conditions are: 10 mg/ml GroES, 10 mm sodium phosphate buffer, 1.6 m Gdn-HCl, 10% (v/v) D2O (pH 6.5) at 25 °C. B, SSP of GroES. Chemical shifts of Cα and Cβ of GroES were used to calculate the residue-specific SSP scores. A SSP score of 1 or −1 at a given residue position reflects a fully formed α- or β-structure, respectively, and 0 reflects a random coil.
Chemical shifts of Cα and Cβ are often used to calculate the secondary structure propensities of unfolded proteins. Although GroES is totally unfolded in 1.6 m Gdn-HCl, the facts that (i) GroES is still capable of forming amyloid fibrils and that (ii) native GroES heptamer is rich in β-strands, promoted us to examine whether residual β-structure remained in some regions of the sequence, through which GroES may be prone to aggregate. In this study, we used the SSP score (29) to calculate the propensity of each amino acid residue of GroES to assume a certain secondary structure. In Fig. 2B, although a very slight propensity was observed in certain sequence segments (8.3% of β-structure in average for residues 1–80 and 8.2% of α-structure in average for residues 81–97), the overall SSP profile showed that at atomic resolution the conformation of GroES was close to a random coil, in agreement with previous small angle x-ray scattering measurements (24).
Detection of Structural Changes from the Disordered State
To determine the existence of intermediate species during GroES amyloid fibril formation, we performed time-lapse 1H-15N HSQC measurements of unfolded GroES at 25 °C. Very interestingly, several noticeable changes were observed (Fig. 3). Overlays of sample spectra measured at 2 days and 28 days revealed a significant decrease in the peaks corresponding to Gly46 and Gly52. In addition, the peak intensities of the adjacent Asn45 and Asn51 residues were also decreased greatly. Correspondingly, two new peaks were detected in the 28-days spectra (Fig. 3A). Identification of these two new peaks was performed by three-dimensional TOCSY and time-lapse measurement analysis for wild-type GroES, GroES N51A, and N45A/N51A mutants (see below), and it was shown that they corresponded to Gly46 and Gly52. Slight chemical shift perturbations were also observed for Gly44, Glu50, Lys55, and Leu57. Samples incubated for more than 28 days showed very low peak intensities due to amyloid fibril formation (data not shown). We analyzed the relative peak intensities of each peak using the data of Fig. 3A (Fig. 3B). Although the peak intensities of all of the amino acid residues in general gradually decreased to ∼76% at 28 days, the peak intensities within the region Val43-Leu57 decreased remarkably (to 30–50%). Because after these changes formation of amyloid fibrils was confirmed by Thioflavin-T binding assay in separate experiments, these data strongly suggested the appearance of soluble intermediate species formed during nucleus formation. These residues were adjacent to the fibril core region (Asp58-Lys74) previously identified (20) in GroES amyloid fibrils.
FIGURE 3.
Changes in NMR spectra of GroES after prolonged incubation at 25 °C. A, 1H-15N HSQC spectra after a 2-day (black) and 28-day (red) incubation in 1.6 m Gdn-HCl without agitation at 25 °C. Notable changes: peak intensities of Asn45, Gly46, Asn51, and Gly52 decreased significantly, and the peaks of Gly46 and Gly52 were detected partially at new positions (dotted square), indicating that intermediate species were formed. B, relative peak intensity of resonances at 2 days (black circles), 14 days (blue circles), and 28 days (red circles), given as δI = I/I0, where I represents the peak intensities at 14 or 28 days and I0 is equal to the intensity at 2 days. C–E, 15N relaxation parameters of GroES. The 1H-15N NOE (C), relaxation data R1 (D), and R2 (E) of disordered species (black circles) and intermediate species (red triangles) are shown in each panel. The 15N relaxation parameters of Gly46 and Gly52, detected at new positions in the intermediate species, are also plotted (red columns) along with those in the disordered species (black columns). The data of the disordered and intermediate species were recorded between day 0 and day 4, and between day 21 and 25, respectively.
We next performed 15N relaxation experiments to explore the dynamics of the Gdn-HCl denatured and the intermediate species. NOE values are very sensitive to motions in the picosecond time scale, whereas R1 (longitudinal relaxation rates) and R2 (transverse relaxation rates) values are sensitive to motions in picosecond to nanosecond time scales and microsecond to millisecond time scales, respectively (32–34). As seen in the left panels of Fig. 3, C–E, each amino acid residue displayed different values in these measurements, but no significant changes of the overall dynamics between the initial form and the intermediate species ensemble were seen. The average values of 1H-15N NOE, R1, and R2 of the unfolded species at 2 days were −0.8, 2.2 s−1, and 4.5 s−1, and those of the intermediate species ensemble at 28 days were −0.8, 2.1 s−1, and 4.2 s−1, respectively, and the lack of large differences in these values indicated that the soluble intermediate species were in a monomeric state. Also, the NOE values were negative across the entire primary sequence, demonstrating a highly dynamic motion throughout the sequence in the presence of Gdn-HCl. This character was especially pronounced in the mobile loop region (around position 22). A highly dynamic motion in the C terminus was also observed in the R1 and R2 measurements.
When we looked at the specific residues of Gly46 and Gly52 in the intermediate species (peaks in the dotted square in Fig. 3A), it was revealed that all values of NOE, R1, and R2 of the intermediate were slightly but significantly smaller than those of the disordered structure (the right panels of Fig. 3, C–E). This finding suggested that flexibilities of Gly46 and Gly52 in the intermediate species were increased compared with the initial state.
Separation and Mass Analysis of the Intermediate: Implication of Covalent Structural Changes in Asn-Gly Sequence
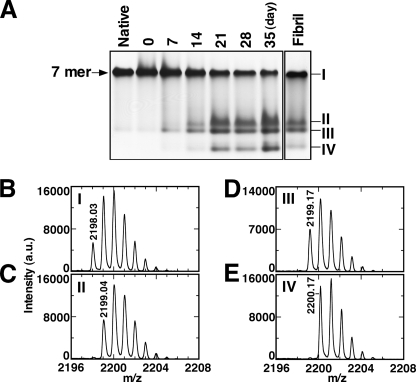
It is evident that the intermediate species observed during the nucleus formation process were formed from a Gdn-HCl disordered state, as described above. This result raised an interesting question: What causes the structural changes in the region of Val43-Leu57 adjacent to the fibril core sequence? To address the question, a series of biochemical experiments were performed. GroES is capable of refolding reversibly from 1.6 m Gdn-HCl as described previously (35), which is detectable by native-PAGE (band I in Fig. 4A). In GroES samples incubated for a prolonged interval in Gdn-HCl (7–35 days), however, new species (bands II, III, and IV in Fig. 4A) were detected that could not properly refold to heptamer. The time scale of the formation of these nonheptameric species also corresponded to the time required for the formation of intermediates observed in the NMR measurements (Fig. 3). When mature amyloid fibrils of GroES were dissolved by 7.5 m Gdn-HCl and then Gdn-HCl was removed to allow refolding, we found a fraction of GroES formed intermediate species, which could not form the heptameric native state (Fig. 4A, Fibril). These results indicated the intermediate species could be detected by both NMR and native-PAGE.
FIGURE 4.
Separation of the intermediate species that are formed during GroES amyloid fibril formation. A, native-PAGE analysis of GroES native heptamer, GroES incubated in 1.6 m Gdn-HCl for 0–35 days, and mature GroES amyloid fibrils resolubilized in 7.5 m Gdn-HCl and refolded. Preparation of the incubated GroES in Gdn-HCl is as follows. Aliquots were taken during amyloid fibril formation (10 mg/ml GroES in 10 mm sodium phosphate buffer (pH 6.5), 1.6 m Gdn-HCl incubated without agitation at 25 °C in a Shigemi tube) and ultracentrifuged, and Gdn-HCl was removed from the supernatant using a PD Spin Trap G-25. After the removal of Gdn-HCl, refolding was allowed to occur. Mature amyloid fibrils were solubilized in 7.5 m Gdn-HCl, then Gdn-HCl was removed in similar fashion. Five micrograms of each sample were loaded onto each lane. In-gel digestion with lysyl endopeptidase and extraction of peptides from the relevant bands were performed followed by MALDI-TOF analysis. B–E, mass spectra of the target peptide Ser35-Lys55 (STRGEVLAVGNGRILENGEVK (M+H)+ is 2198.18) containing two Asn-Gly sequences shown for native heptamer (B) and intermediates I–IV marked in the native-PAGE gels (C–E), respectively. The parent ion mass value of the each spectrum is also shown in each panel.
The intermediate species observed in the native-PAGE gels were further analyzed by MALDI-TOF mass spectroscopy after in-gel digestion by lysyl endopeptidase. Measurements of the mass spectrum focused on a peptide corresponding to residues 35–55 (STRGEVLAVGNGARILGENGGEVK (M+H)+ = 2198.18), a region that includes the site of the initial structural changes identified in Fig. 3. A peak of this peptide derived from native heptameric GroES (band I in Fig. 4A) displayed a parent ion mass of 2198.03, together with several isotopic peaks (Fig. 4B). Interestingly, for the corresponding peptide obtained from the intermediate species of bands II and III in Fig. 4A, a 1-Da increase in mass was observed, where the parent ion mass peaks were 2199.04 and 2199.17, respectively (Fig. 4, C and D). For the corresponding peptide from band IV in Fig. 4A, a 2-Da increase in mass (a parent ion mass = 2200.17) was observed (Fig. 4E). Taken together with our results showing significant NMR peak changes for Asn45, Gly46, Asn51, and Gly52 (Fig. 3A), our results are consistent with a covalent Asn-Gly rearrangement reaction, which yields β-aspartic acid due to deamidation of the asparagine. Rearrangement of the α-peptide bond at Asn-Gly to a β-peptide bond renders the entire protein incapable of refolding to native heptamer (Fig. 4A). Thus, this structural change, that presumably occurs within the sequences Asn45-Gly46 and Asn51-Gly52, results in formation of intermediate species, which then trigger or accelerate fibril nucleus formation. Once the fibril nucleus forms, fibril extension reaction follows by incorporating various forms of intact GroES monomer.
Amyloid Fibril Formation by GroES Mutants
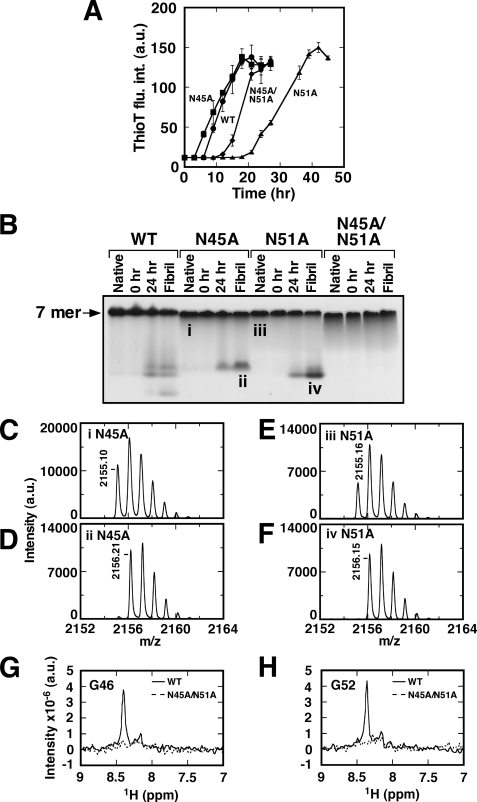
To confirm that the rearrangement reaction in Asn-Gly sequence triggers or accelerates GroES amyloid fibril formation, we generated single N45A and N51A and double N45A/N51A GroES mutants and performed fibril formation experiments (Fig. 5A). All of the GroES mutants were found to form amyloid fibrils eventually, most likely because we selected experimental conditions that greatly encouraged fibril formation. However, the times required for nucleus formation were prolonged in N45A/N51A (12 h) and N51A (22 h) mutants compared with that of wild-type GroES (WT) (6 h), and the time required for the N45A (5 h) mutant was almost the same as the WT. The effect of a single N51A mutation was the greatest, demonstrating that the rearrangement at Asn51-Gly52 plays an important role in accelerating the fibril formation of GroES. Fibril extension rates were only mildly affected by these mutations, suggesting that these Asn-Gly rearrangements mainly affect nucleus formation.
FIGURE 5.
Amyloid fibril formation and the analysis of intermediates formed by various GroES mutants. A, amyloid fibril formation of GroES mutants monitored by Thioflavin-T fluorescence (1 mg/ml GroES, 10 mm sodium phosphate (pH 7.4), 1.6 m Gdn-HCl with linear agitation (90 min−1) at 37 °C). WT, circles; N45A, squares; N51A, triangles; and N45A/N51A, diamonds. B, native-PAGE analysis of GroES native heptamer, incubated GroES (0, 24 h), and GroES mutants resolubilized and refolded from mature amyloid fibrils. The mass spectra of the target peptides of Ser35-Lys55 from GroES N45A and N51A (both (M+H)+ are 2155.18), which were derived from the bands i–iv in native-PAGE gel, are indicated in C–F, respectively. The parent ion mass of the each spectrum is also shown in each panel. G and H, 1H-15N HSQC spectra taken of WT and N45A/N51A incubated for 28 days compared by overlaying 1H slices corresponding to the region Gly46 (G) and Gly52 (H) highlighted by the dotted square in Fig. 3A.
The incubated samples and the mature amyloid fibrils for each mutant were then analyzed by native-PAGE. As shown in Fig. 5B, bands ii and iv, which were observed for WT, became undetectable in the N51A and N45A mutants, respectively, according to their mutation position. No intermediate bands were observed at all for N45A/N51A. In-gel digestion followed by mass spectroscopy measurements for the mutants was also performed. From the peptides derived from native heptameric N45A and N51A mutants (bands i and iii marked in Fig. 5B), peaks of parent ion mass 2155.10 and 2155.16 were observed, respectively (theoretical mass = 2155.18) (Fig. 5, C and E). The peptides from the nonheptameric species of bands ii and iv (marked in Fig. 5B) showed a mass increase of 1 Da, where peaks of parent ion mass 2156.21 and 2156.15 were observed, respectively (Fig. 5, D and F). The results confirmed that each mutation suppressed the β-rearrangement reaction of the corresponding peptide bond, and as a result, prevented the accumulation of intermediate species. This demonstrates that the rearrangement reaction at Asn45 and/or Asn51 significantly affects successful refolding of GroES to native heptamer. Notably, mutation of these two Asn residues does not hamper GroES refolding in a measurable manner.
In NMR measurements of N45A/N51A mutant incubated for 28 days, no resonance peaks of Gly46 (Fig. 5G) and Gly52 (Fig. 5H) were observed. From these results, we concluded that rearrangement reactions at Asn45-Gly46 and/or Asn51-Gly52 occur during nucleus formation, resulting in βAsp45 and/or βAsp51 to be formed. The intermediates, especially βAsp51, affect significantly the formation of fibril nuclei, and interestingly, leave the fibril extension rates almost unchanged (Fig. 5A).
DISCUSSION
Studies of amyloid fibrils have shown that amyloid fibrils are an inherently common structure in proteins and also may be formed under certain conditions in vitro by proteins that are unrelated to diseases (22). We previously found that the co-chaperonin GroES of E. coli (Fig. 1) formed amyloid fibrils from the Gdn-HCl unfolded state (19), and the core sequence of GroES that forms a rigid β-structure in this fibril was identified by protease digestion (20). In addition to elucidating the details of fibril formation and fibril morphology, recent studies indicate the importance of classifying intermediate species formed during the nucleation process, due to their cytotoxicity (14–18). Therefore, the elucidation of characteristics of the nucleus-forming molecular species and the mechanism of formation are quite important and critical issues. Furthermore, as the GroES protein formed typical amyloid fibrils similar to those observed in various neurodegenerative diseases from a completely disordered state, this can be a good model system to understand the amyloid fibril formation mechanism of intrinsically disordered proteins.
Although several NMR measurements of native heptameric GroES have been studied so far (36–40), in our study, we performed 1H-15N HSQC measurements and backbone assignments of GroES in 1.6 m Gdn-HCl, where native GroES heptamer totally unfolds to monomers (19, 24). The 1H-15N resonances of GroES in 1.6 m Gdn-HCl were found to be similar to those of disordered proteins with a limited resonance dispersion of ∼1 ppm in the 1H dimension, but retaining relatively good dispersion covering ∼24 ppm in the 15NH dimension (Fig. 2). From our peak assignments of the unfolded GroES protein, we were able to use GroES as a good model to clarify the mechanism of amyloid fibril formation at an atomic level for the intrinsically disordered proteins.
The detailed peak assignments of GroES in the disordered state allowed us to perform time-lapse measurements of 1H-15N HSQC at the atomic level (Fig. 3, A and B). During the fibrillation process, the peak intensities of the overall residues decreased gradually to 76% after 28 days, indicating that soluble species remained. A notable difference in the plot of the relative peak intensity at 28 day was that the region of Val43-Leu57 showed a significant additional decrease in their intensities. A similar phenomenon was observed for α-synuclein during the initial stage of fibril formation (41), where many cross-peaks were significantly attenuated due to the shortened relaxation times as a consequence of polymerization. In the case of GroES, the decrease in peak intensities within the Val43-Leu57 segment was caused by chemical shift changes in the spectra, which were observed notably for Gly46 and Gly52 and slightly for Gly44, Glu50, Lys55, and Leu57, indicating that structural changes occurred in this region adjacent to the fibril core sequence Asp58-Lys74 (20). Further 15N relaxation experiments indicated that the GroES protein remained as a monomeric state during this time (Fig. 3, C–E).
We found that the NMR resonance changes observed in 1H-15N HSQC (Fig. 3) were due to specific covalent structural changes of Asn45-Gly52 and Asn51-Gly52 (Figs. 4 and 5). In general, asparagine residues with Asn-Gly sequence motifs in peptides and proteins have a tendency to undergo spontaneous deamidation reactions at neutral and alkaline pH. This results in the production of Asp and βAsp (isoAsp) residues through a succinimide intermediate (42). The typical ratio of Asp and βAsp formed after rearrangement is about 1:3, favoring the βAsp form (although the actual ratio is dependent on peptide or protein) (43, 44). These nonenzymatic reactions occur primarily at asparagine and aspartate residues. This deamidation reaction results in a 1-Da increase in the molecular mass. Although the reaction takes place relatively slowly in structured proteins (half-life, 1–500 days) (45), the deamidation rate increases dramatically when the susceptible residues are exposed and flexible (43, 46, 47). After rearrangements had occurred, the flexibilities of Gly46 and Gly52 in the intermediate species were increased substantially compared with the initial state (Fig. 3, C–E, right panels). This was reasonable, because an additional carbon atom is inserted into the polypeptide backbone due to the rearrangement to βAsp. A similar series of reactions is reported to occur also for glutamine and glutamate residues (48). βAsp and βGlu residues have been detected in a variety of proteins, including calmodulin (49), myelin basic protein (50), eye lens crystallin (51, 52), and Aβ peptide (53). Such rearrangements often control not only the biological activity and function of proteins, but also aggregation propensity (54), exemplified by an increased aggregation of α-crystallin (55) and β-sheet structure of Aβ peptide (56).
In the present study, we found that the rearrangement reaction at Asn plays an important role in fibril formation of GroES by accelerating nucleus formation (Fig. 5). The nucleus formation time was significantly prolonged (by 16 h) in the N51A mutant compared with WT, showing that this rearrangement at Asn51-Gly52 contributes greatly to nucleus formation. On the other hand, the nucleus formation time of the N45A mutant was almost the same as WT and that of the N45A/N51A double mutant was shorter than that of the N51A mutant by 10 h. The differences between the various mutants might be attributed to their relative positions with respect to the fibril core region (Asp58-Lys74). Also, we observe that formation of βAsp newly generates a negatively charged side chain along with the rearrangement of the backbone. Because Asn51 is surrounded by negatively charged residues Glu50 and Glu53, the formation of βAsp51 might also give a more significant electrostatic effect that accelerates nucleus formation of the contiguous region.
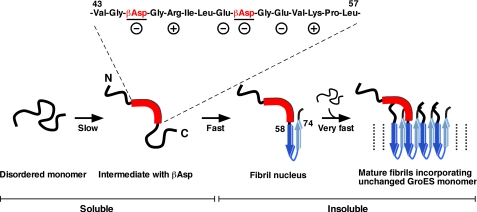
A summary of our findings is shown in Fig. 6 as a possible schematic model of GroES amyloid fibril formation. The residues of Val43-Leu57 have been identified as the region where structural changes occur in the early stages of the fibril formation. This region is distinct from the fibril core region (residues of Asp58-Lys74 denoted a β-strand in ribbon model). Formation of this intermediate is attributed to the Asn45-Gly46 and Asn51-Gly52 rearrangement reactions, whose rate is slow but significant under the conditions we used, and especially the rearrangement at Asn51 resulting in βAsp51 accelerates fibril nucleus formation. The fibril nucleus region is located just after this structurally changed region. The intermediate species are soluble and are detectable in NMR measurements. Once the fibril nucleus is formed, very rapid fibril formation occurs by incorporating random and flexible intact species of GroES monomer. This is why the majority of intact GroES monomers were capable of refolding to native heptamer after being incorporated into mature GroES amyloid fibrils (Fig. 4A). The nucleus formation and the nucleus-dependent extension reactions are too fast to be detected reliably by NMR, and also because of the precipitation of mature fibrils.
FIGURE 6.
Schematic model of the structural changes in early stage of GroES amyloid fibril formation. The region of Val43-Leu57 (denoted by a thick red line) has been identified as a region where structural changes occur early on during fibril formation. This region immediately precedes the fibril core region (Asp58-Lys74: denoted by blue arrows) and is apparently distinct from this region. This intermediate was monomeric, and the structural change was caused by the covalent rearrangement of Asn-Gly sequences. Especially, the formation of βAsp from Asn51 acts as a trigger for the following process. After formation of the intermediate species, a fibril nucleus may be formed fast at the adjacent core region, followed by very fast incorporation of disordered intact monomeric species (nucleus-dependent fibril extension). Therefore the mature amyloid fibrils of GroES contain both altered and unaltered molecular species. For details, see “Results” and “Discussion” sections.
Finally, in the present experiments, we found that the GroES unfolded in Gdn-HCl formed amyloid fibrils triggered by the rearrangement at an Asn-Gly site. As this rearrangement-triggered fibril formation also occurred in 3 m urea containing 1 m NaCl (data not shown), it is necessary for GroES to be completely unfolded for this rearrangement to occur to a significant extent. From this point of view, our results may be broadly relative to intrinsically disordered proteins in general, which are abundant in eukaryotes (∼33%) and play a number of crucial roles in numerous biological processes (3). Recently, it was also reported that some intrinsically disordered proteins are involved in human neurodegenerative diseases (10). It is also noteworthy that many intrinsically disordered proteins related to neurodegenerative diseases, including Aβ peptide (Alzheimer disease) (53), tau-protein (motor neuron disease with neurofibrillary tangles) (57), and prion protein (prion disease) (58, 59), undergo rearrangement reactions to form βAsp within their sequences. Clarifying the relation between this β-rearrangement reaction in intrinsically disordered proteins and various amyloidoses would be quite important when medical treatments for the diseases are considered at the molecular level. In our study, we have assigned the 1H-15N resonances of disordered GroES and found that the contiguous Asn-Gly sequence immediately preceding the fibril core region is a trigger for GroES amyloid fibril formation. These findings may lead to understanding the mechanism of fibril formation related to amyloidoses in general, as well as the structural characteristics and aggregation propensities of the intrinsically disordered proteins, especially those that are retained for long periods in the cell.
Supplementary Material
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Global COE Program, and a grant from the Organization for Regional Industrial Academic Cooperation of Tottori University. Mass spectrometry measurements were performed using the facilities of the Research Center for Bioscience and Technology (Venture Business Laboratory) of Tottori University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- Gdn-HCl
- guanidine hydrochloride
- HSQC
- heteronuclear single quantum coherence
- SSP
- secondary structure propensity
- TOCSY
- total correlation spectroscopy.
REFERENCES
- 1. Wright P. E., Dyson H. J. (1999) J. Mol. Biol. 293, 321–331 [DOI] [PubMed] [Google Scholar]
- 2. Sugase K., Dyson H. J., Wright P. E. (2007) Nature 447, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 3. Ward J. J., Sodhi J. S., McGuffin L. J., Buxton B. F., Jones D. T. (2004) J. Mol. Biol. 337, 635–645 [DOI] [PubMed] [Google Scholar]
- 4. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 5. Yagi H., Kusaka E., Hongo K., Mizobata T., Kawata Y. (2005) J. Biol. Chem. 280, 38609–38616 [DOI] [PubMed] [Google Scholar]
- 6. Uversky V. N., Eliezer D. (2009) Curr. Protein Pept. Sci. 10, 483–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scherzinger E., Lurz R., Turmaine M., Mangiarini L., Hollenbach B., Hasenbank R., Bates G. P., Davies S. W., Lehrach H., Wanker E. E. (1997) Cell 90, 549–558 [DOI] [PubMed] [Google Scholar]
- 8. Chen S., Berthelier V., Hamilton J. B., O'Nuallain B., Wetzel R. (2002) Biochemistry 41, 7391–7399 [DOI] [PubMed] [Google Scholar]
- 9. Ross C. A. (2002) Neuron 35, 819–822 [DOI] [PubMed] [Google Scholar]
- 10. Uversky V. N. (2009) Front. Biosci. 14, 5188–5238 [DOI] [PubMed] [Google Scholar]
- 11. Bhak G., Choe Y. J., Paik S. R. (2009) BMB Rep. 42, 541–551 [DOI] [PubMed] [Google Scholar]
- 12. Jarrett J. T., Lansbury P. T., Jr. (1993) Cell 73, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 13. Wood S. J., Wypych J., Steavenson S., Louis J. C., Citron M., Biere A. L. (1999) J. Biol. Chem. 274, 19509–19512 [DOI] [PubMed] [Google Scholar]
- 14. Laurén J., Gimbel D. A., Nygaard H. B., Gilbert J. W., Strittmatter S. M. (2009) Nature 457, 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakono M., Zako T. (2010) FEBS J. 277, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 16. Kitamura A., Kubota H. (2010) FEBS J. 277, 1369–1379 [DOI] [PubMed] [Google Scholar]
- 17. Nagai Y., Inui T., Popiel H. A., Fujikake N., Hasegawa K., Urade Y., Goto Y., Naiki H., Toda T. (2007) Nat. Struct. Mol. Biol. 14, 332–340 [DOI] [PubMed] [Google Scholar]
- 18. Naiki H., Nagai Y. (2009) J. Biochem. 146, 751–756 [DOI] [PubMed] [Google Scholar]
- 19. Higurashi T., Yagi H., Mizobata T., Kawata Y. (2005) J. Mol. Biol. 351, 1057–1069 [DOI] [PubMed] [Google Scholar]
- 20. Yagi H., Sato A., Yoshida A., Hattori Y., Hara M., Shimamura J., Sakane I., Hongo K., Mizobata T., Kawata Y. (2008) J. Mol. Biol. 377, 1593–1606 [DOI] [PubMed] [Google Scholar]
- 21. Guijarro J. I., Sunde M., Jones J. A., Campbell I. D., Dobson C. M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4224–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobson C. M. (2001) Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uversky V. N., Fink A. L. (2004) Biochim. Biophys. Acta 1698, 131–153 [DOI] [PubMed] [Google Scholar]
- 24. Higurashi T., Hiragi Y., Ichimura K., Seki Y., Soda K., Mizobata T., Kawata Y. (2003) J. Mol. Biol. 333, 605–620 [DOI] [PubMed] [Google Scholar]
- 25. Makio T., Arai M., Kuwajima K. (1999) J. Mol. Biol. 293, 125–137 [DOI] [PubMed] [Google Scholar]
- 26. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 27. Johnson B. A., Blevins R. A. (1994) J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 28. Goddard T. D., Kneller D. G. (2008) SPARKY 3, University of California, San Francisco, CA [Google Scholar]
- 29. Marsh J. A., Singh V. K., Jia Z., Forman-Kay J. D. (2006) Protein Sci. 15, 2795–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shevchenko A., Tomas H., Havlis J., Olsen J. V., Mann M. (2006) Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 31. Yao J., Dyson H. J., Wright P. E. (1997) FEBS Lett. 419, 285–289 [DOI] [PubMed] [Google Scholar]
- 32. Jarymowycz V. A., Stone M. J. (2006) Chem. Rev. 106, 1624–1671 [DOI] [PubMed] [Google Scholar]
- 33. Bertoncini C. W., Rasia R. M., Lamberto G. R., Binolfi A., Zweckstetter M., Griesinger C., Fernandez C. O. (2007) J. Mol. Biol. 372, 708–722 [DOI] [PubMed] [Google Scholar]
- 34. Wu K. P., Kim S., Fela D. A., Baum J. (2008) J. Mol. Biol. 378, 1104–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Higurashi T., Nosaka K., Mizobata T., Nagai J., Kawata Y. (1999) J. Mol. Biol. 291, 703–713 [DOI] [PubMed] [Google Scholar]
- 36. Landry S. J., Zeilstra-Ryalls J., Fayet O., Georgopoulos C., Gierasch L. M. (1993) Nature 364, 255–258 [DOI] [PubMed] [Google Scholar]
- 37. Shewmaker F., Maskos K., Simmerling C., Landry S. J. (2001) J. Biol. Chem. 276, 31257–31264 [DOI] [PubMed] [Google Scholar]
- 38. Fiaux J., Bertelsen E. B., Horwich A. L., Wüthrich K. (2002) Nature 418, 207–211 [DOI] [PubMed] [Google Scholar]
- 39. Fiaux J., Bertelsen E. B., Horwich A. L., Wüthrich K. (2004) J. Biomol. NMR 29, 289–297 [DOI] [PubMed] [Google Scholar]
- 40. Horst R., Wider G., Fiaux J., Bertelsen E. B., Horwich A. L., Wüthrich K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15445–15450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tashiro M., Kojima M., Kihara H., Kasai K., Kamiyoshihara T., Uéda K., Shimotakahara S. (2008) Biochem. Biophys. Res. Commun. 369, 910–914 [DOI] [PubMed] [Google Scholar]
- 42. Clarke S. (1987) Int. J. Pept. Protein Res. 30, 808–821 [DOI] [PubMed] [Google Scholar]
- 43. Geiger T., Clarke S. (1987) J. Biol. Chem. 262, 785–794 [PubMed] [Google Scholar]
- 44. Aswad D. W., Paranandi M. V., Schurter B. T. (2000) J. Pharm. Biomed. Anal. 21, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 45. Robinson N. E., Robinson A. B. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12409–12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bischoff R., Kolbe H. V. (1994) J. Chromatogr. B Biomed. Appl. 662, 261–278 [DOI] [PubMed] [Google Scholar]
- 47. Robinson N. E., Robinson M. L., Schulze S. E., Lai B. T., Gray H. B. (2009) Protein Sci. 18, 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wright H. T. (1991) Crit. Rev. Biochem. Mol. Biol. 26, 1–52 [DOI] [PubMed] [Google Scholar]
- 49. Johnson B. A., Shirokawa J. M., Aswad D. W. (1989) Arch. Biochem. Biophys. 268, 276–286 [DOI] [PubMed] [Google Scholar]
- 50. Chou F. C., Chou C. H., Shapira R., Kibler R. F. (1976) J. Biol. Chem. 251, 2671–2679 [PubMed] [Google Scholar]
- 51. Fujii N., Ishibashi Y., Satoh K., Fujino M., Harada K. (1994) Biochim. Biophys. Acta 1204, 157–163 [DOI] [PubMed] [Google Scholar]
- 52. Sharma K. K., Santhoshkumar P. (2009) Biochim. Biophys. Acta 1790, 1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. (1993) J. Biol. Chem. 268, 3072–3083 [PubMed] [Google Scholar]
- 54. Shimizu T., Watanabe A., Ogawara M., Mori H., Shirasawa T. (2000) Arch. Biochem. Biophys. 381, 225–234 [DOI] [PubMed] [Google Scholar]
- 55. Fujii N., Shimmyo Y., Sakai M., Sadakane Y., Nakamura T., Morimoto Y., Kinouchi T., Goto Y., Lampi K. (2007) Amino Acids 32, 87–94 [DOI] [PubMed] [Google Scholar]
- 56. Fabian H., Szendrei G. I., Mantsch H. H., Greenberg B. D., Otvös L., Jr. (1994) Eur. J. Biochem. 221, 959–964 [DOI] [PubMed] [Google Scholar]
- 57. Watanabe A., Takio K., Ihara Y. (1999) J. Biol. Chem. 274, 7368–7378 [DOI] [PubMed] [Google Scholar]
- 58. Weber D. J., McFadden P. N., Caughey B. (1998) Biochem. Biophys. Res. Commun. 246, 606–608 [DOI] [PubMed] [Google Scholar]
- 59. Sandmeier E., Hunziker P., Kunz B., Sack R., Christen P. (1999) Biochem. Biophys. Res. Commun. 261, 578–583 [DOI] [PubMed] [Google Scholar]
- 60. Koradi R., Billeter M., Wuthrich K. (1996) J. Mol. Graph. 14, 51–55, 29–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.