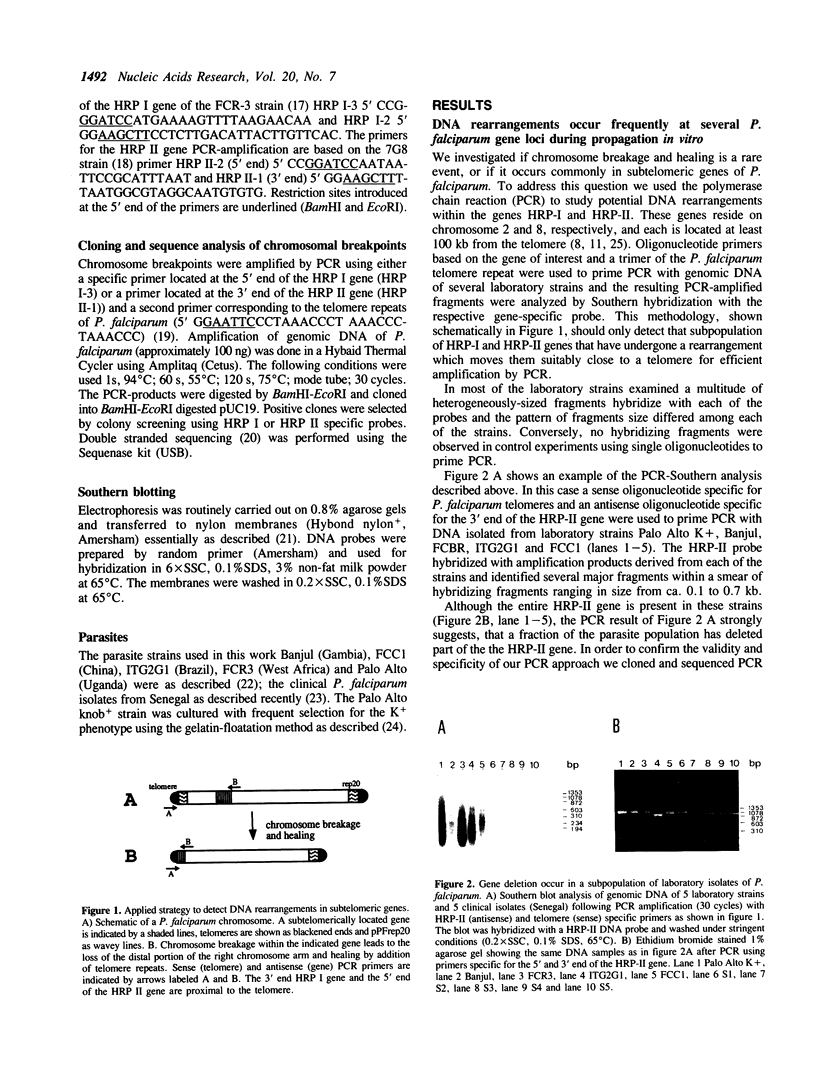
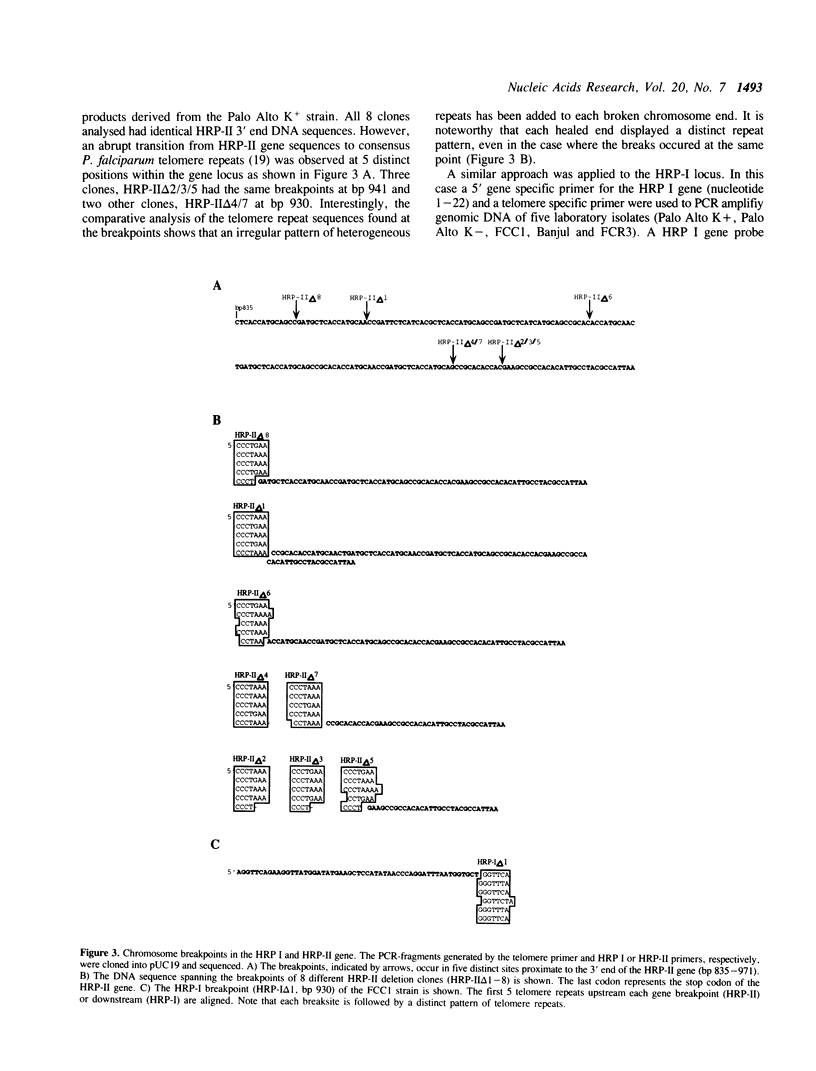
Abstract
We analysed the genetic stability of two subtelomeric genes of the human malaria parasite Plasmodium falciparum. A PCR based assay, using a telomere and a target-gene specific primer was used to detect potential chromosome rearrangements. We show that chromosome breakage and the formation of new telomeres occur frequently in the two genes coding for histidine rich proteins (HRP I and HRP II) in laboratory isolates, but remains undetectable in clinical parasite isolates. This finding suggests an essential role of these genes in vivo and that chromosome breakage is rather an accidental process than a programmed chromosome fragmentation. Cloning and sequencing of 8 chromosome breakpoints of the HRP II gene from one parasite isolate shows that the breakage occurs within a broad region in which new telomere formation appear to take place at random sites. Furthermore, this analysis revealed no obvious sequence similarities of sites of telomere addition. Finally, we show that an irregular pattern of heterogeneous telomere repeats is added at each broken end and that each healed chromosome contains a distinct pattern of repeats. We discuss a model for telomere formation in P. falciparum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggs B. A., Kemp D. J., Brown G. V. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Forsyth K. P., Bianco A. E., Brown G. V., Kemp D. J. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986 Jan 17;44(1):87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kemp D. J. Chromosomes of malaria parasites. Trends Genet. 1989 Oct;5(10):337–342. doi: 10.1016/0168-9525(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Gritzmacher C. A., Reese R. T. Reversal of knob formation on Plasmodium falciparum-infected erythrocytes. Science. 1984 Oct 5;226(4670):65–67. doi: 10.1126/science.6382613. [DOI] [PubMed] [Google Scholar]
- Harrington L. A., Greider C. W. Telomerase primer specificity and chromosome healing. Nature. 1991 Oct 3;353(6343):451–454. doi: 10.1038/353451a0. [DOI] [PubMed] [Google Scholar]
- Hommel M., Hughes M., Bond P., Crampton J. M. Antibodies and DNA probes used to analyze variant populations of the Indochina-1 strain of Plasmodium falciparum. Infect Immun. 1991 Nov;59(11):3975–3981. doi: 10.1128/iai.59.11.3975-3981.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F., Walliker D. Genetic diversity in Plasmodium falciparum. Adv Parasitol. 1990;29:75–149. doi: 10.1016/s0065-308x(08)60105-0. [DOI] [PubMed] [Google Scholar]
- Knapp B., Shaw A., Hundt E., Enders B., Küpper H. A. A histidin alanine rich recombinant antigen protects Aotus monkeys from P. falciparum infection. Behring Inst Mitt. 1988 Apr;(82):349–359. [PubMed] [Google Scholar]
- Morin G. B. Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature. 1991 Oct 3;353(6343):454–456. doi: 10.1038/353454a0. [DOI] [PubMed] [Google Scholar]
- Müller F., Wicky C., Spicher A., Tobler H. New telomere formation after developmentally regulated chromosomal breakage during the process of chromatin diminution in Ascaris lumbricoides. Cell. 1991 Nov 15;67(4):815–822. doi: 10.1016/0092-8674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- Patarapotikul J., Langsley G. Chromosome size polymorphism in Plasmodium falciparum can involve deletions of the subtelomeric pPFrep20 sequence. Nucleic Acids Res. 1988 May 25;16(10):4331–4340. doi: 10.1093/nar/16.10.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C., Nelson R., Magowan C., Wollish W., Jensen J., Leech J. The mature erythrocyte surface antigen of Plasmodium falciparum is not required for knobs or cytoadherence. Mol Biochem Parasitol. 1989 Aug;36(1):61–65. doi: 10.1016/0166-6851(89)90200-4. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Pavlovec A., Shio H., Ravetch J. V. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7139–7143. doi: 10.1073/pnas.84.20.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Prensier G., Slomianny C. The karyotype of Plasmodium falciparum determined by ultrastructural serial sectioning and 3D reconstruction. J Parasitol. 1986 Oct;72(5):731–736. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scherf A., Mattei D., Sarthou J. L. Multiple infections and unusual distribution of block 2 of the MSA1 gene of Plasmodium falciparum detected in west African clinical isolates by polymerase chain reaction analysis. Mol Biochem Parasitol. 1991 Feb;44(2):297–299. doi: 10.1016/0166-6851(91)90016-y. [DOI] [PubMed] [Google Scholar]
- Sinnis P., Wellems T. E. Long-range restriction maps of Plasmodium falciparum chromosomes: crossingover and size variation among geographically distant isolates. Genomics. 1988 Nov;3(4):287–295. doi: 10.1016/0888-7543(88)90117-6. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]