Abstract
Objective:
Motor impairment after stroke has been related to infarct size, infarct location, and integrity of motor tracts. To determine the value of diffusion tensor imaging (DTI) as a predictor of motor outcome and its role as a structural surrogate marker of impairment in chronic stroke, we tested correlations between motor impairment and DTI-derived measures of motor tract integrity.
Methods:
Thirty-five chronic stroke patients with varying degrees of recovery underwent DTI and motor impairment assessments. Fibers originating from the precentral gyrus were traced and separated into pyramidal tract (PT) and alternate motor fibers (aMF). Asymmetry indices of fiber number and regional fractional anisotropy (FA) values comparing lesional with nonlesional hemispheres were correlated with motor impairment scores and compared to an age-matched control group.
Results:
Fiber number and regional FA value asymmetry significantly differed between the groups with lower values in the patients' lesional hemispheres. Both measures significantly predicted motor impairment with stronger predictions when all motor tracts were combined as compared to predictions using only the PT. The pattern of motor tract damage (PT only vs PT and aMF) led to a classification of mild, moderate, or severe impairment with significant between-group differences in motor impairment scores.
Conclusions:
Diffusion tensor imaging-derived measures are valid structural markers of motor impairment. The integrity of all descending motor tracts, not merely the pyramidal tract, appears to account for stroke recovery. A 3-tier, hierarchical classification of impairment categories based on the pattern of motor tract damage is proposed that might be helpful in predicting recovery potential.
GLOSSARY
- aMF
= alternate motor fibers;
- DTI
= diffusion tensor imaging;
- FA
= fractional anisotropy;
- FLAIR
= fluid-attenuated inversion recovery;
- MCA
= middle cerebral artery;
- MRC
= Medical Research Council;
- PLIC
= posterior limb of the internal capsule;
- PT
= pyramidal tract;
- ROI
= region of interest;
- TMS
= transcranial magnetic stimulation;
- UE-FM
= Upper Extremity Fugl-Meyer assessment;
- WMFT
= Wolf Motor Function Test.
Motor impairment after stroke has been related to lesion site and size,1,2 amount of previous lesion burden, and other factors like age or comorbidities.3,4 It has also been shown that the integrity of motor fibers, with the pyramidal tract (PT) as the main descending fiber bundle, but also the cortico-rubro-spinal or cortico-reticulo-spinal systems,5,6 plays an important role for the degree of motor impairment which can be measured with standardized, reliable, and valid clinical assessments.7,8 However, mere lesion location may not sufficiently demonstrate the extent to which motor fibers remain intact and a considerable amount of variance remains unexplained.
Diffusion tensor imaging (DTI) allows for the visualization and quantitative examination of fiber tracts and their integrity in vivo9 so that the topographic relation of lesion location and corticospinal fibers can be evaluated10–14 and fiber degeneration can be revealed.15–18 In the chronic stroke phase, a nonsignificant trend between motor fiber number asymmetry and fine motor performance was previously observed in mildly to moderately affected patients.19 DTI-derived measures such as fractional anisotropy (FA) values in the posterior limb of the internal capsule (PLIC) were shown to correlate with motor impairment and to increase the predictive power of transcranial magnetic stimulation (TMS).20
The aim of our study was to correlate DTI-derived structural measures of differential motor tract integrity with functional motor impairment scores in chronic stroke patients to establish DTI-derived surrogate markers of motor impairment and to relate patterns of motor tract damage to different levels of motor impairment.
METHODS
Subjects.
Our group consisted of 35 chronic stroke patients (8 women; 32 right and 3 left handed; 22 patients with left and 13 with right hemispheric lesions); mean age 56.6 years (SD 12.7); mean time post stroke 30.5 months (SD 30.3; range: 5-170 months) for whom we had imaging data and a detailed neurologic examination from the acute stroke phase. Inclusion criteria consisted of 1) occurrence of first ischemic stroke in the middle cerebral artery (MCA) territory at least 5 months prior to enrollment; 2) no previous or subsequent cerebral ischemia or hemorrhage; 3) Medical Research Council (MRC) strength grade of ≤3/5 in extensor muscles of the affected upper extremity in the acute phase (first 24 hours after symptom onset); 4) standard physical and occupational therapy post stroke. An age-matched control group of 10 healthy subjects (4 women; all right-handed; mean age 58.2 years [SD 12.2]) with no history of neurologic or psychiatric disorders was recruited to compare DTI-derived measures between patients and healthy controls.
Standard protocol approvals, registration, and patient consents.
The study was approved by the local ethical standard committee, and all participants gave written informed consent.
Motor assessments.
Each patient underwent the Upper Extremity Fugl-Meyer assessment (UE-FM) and the Wolf Motor Function Test (WMFT). The UE-FM, a 30-item motor function assessment,21 is a frequently used clinical motor impairment test in stroke rehabilitation.22 The scores range from 0 to 66 with higher scores reflecting more complete functional recovery. The modified WMFT consists of 15 time-based tasks varying in complexity and 2 tests of strength.23,24 It has been used in neurorehabilitation trials as an outcome measure of upper extremity motor function.25 Similar to previous studies, completion times were logarithmized to account for skewed data distribution.25 The resulting score has a maximum value of 1.97 seconds[log] with lower values reflecting better function of the affected arm. UE-FM scores were obtained for all patients whereas 6 patients were missing a full set of WMFT scores.
Image acquisition.
All patients and healthy controls underwent MRI using a 3T GE scanner, which included T1-weighted images (resolution: 0.9375 × 0.9375 × 1.5 mm3), DTI (resolution: 1.87 × 1.87 × 5.0 mm3 with 25 noncollinear diffusion directions with a b-value of 1,000 s/mm2, and one with a value of 0 s/mm2; 30 axial slices covered the entire brain including the brainstem), and a fluid-attenuated inversion recovery (FLAIR) sequence (resolution: 0.5 × 0.5 × 5 mm3). T1-weighted images were spatially normalized to determine lesion size and location. Patients with lacunar stroke were excluded based on lesion volume (diameter of less than 15 mm and volume of less than 1.8 mL),26 typical location, and clinical stroke syndrome. T1-weighted and FLAIR images were compared with acute phase images to exclude patients with subsequent strokes.
Diffusion tensor image analysis.
FA value, a measure of the degree of directional preference of water diffusion, was calculated for each brain voxel. Using MedINRIA, version 1.7 (http://www-sop.inria.fr/asclepios/software/MedINRIA/), diffusion tensors were calculated for all voxels within the brain, and fibers were traced by connecting adjacent voxels with similar principal eigenvectors, applying a standard threshold FA value of 0.2 for continuous fiber reconstruction.27
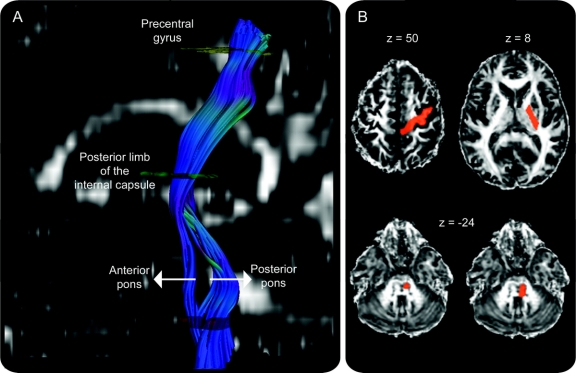
Regions of interest (ROIs) were drawn on the color-coded FA images. The analysis was started with an ROI in the PLIC through which motor fibers descend.28,29 This ROI was drawn on an axial slice corresponding approximately to z = 8 of a spatially normalized brain in Talairach space.30 It was ensured that ROIs had equal volumes between the hemispheres (1.16 ± 0.14 and 1.19 ± 0.15 mL for the ipsilesional and contralesional ROIs). The next, ipsilateral ROI was drawn at a pontine level using a slice just below the level of the superior cerebellar peduncles, corresponding approximately to z = −24. We added a logical “and” function so that only fibers passing through both ROIs were considered for further analysis. We drew 2 different ROIs at that level. A narrower ROI included only the basis pontis (0.30 ± 0.11 mL for ipsilesional and 0.31 ± 0.08 mL for contralesional ROIs), resulting in a fiber bundle that most likely corresponded to the PT according to neuroanatomic31 and DTI-derived atlases.32 A broader pontine ROI (0.63 ± 0.17 mL for ipsilesional and 0.64 ± 0.19 mL for contralesional ROIs) comprised the basis pontis as well as posterior parts of the pons (tegmentum pontis) to include additional corticofugal tracts to the spinal cord. We will refer to these fibers as alternate motor fibers5 (aMF). Any fibers extending to the cerebellum were excluded using axial not-ROIs (exclusion masks) placed in the superior and middle cerebellar peduncles. A third ROI included the precentral gyrus and its underlying white matter (3.16 ± 0.74 for ipsilesional and 3.40 ± 0.85 mL for contralesional ROIs) at a level that corresponded to z = 50 of a spatially normalized brain. A logical “and” function was also added for this ROI so that only fibers that started in the precentral gyrus and passed through the PLIC and the pons were traced, with separate reconstructions for the narrow and broad pontine ROIs (figure 1). Two-tailed paired t tests revealed no significant between-hemispheric or between-group (patients vs controls) differences in ROI volumes.
Figure 1 Visualization of motor fibers originating from the precentral gyrus and traveling through posterior limb of the internal capsule and pons
(A) The descending tracts differentiate into 2 different components in the pons with an anterior component corresponding to the pyramidal tract (PT) and a posterior component consisting of alternate or supplementary motor fibers (aMF). (B) Examples of regions of interest (ROIs), overlaid onto a normalized fractional anisotropy image. The cortical ROI encompassed the precentral gyrus and its underlying white matter (z = 50), which could have also included some corticospinal fibers originating from premotor cortex since there are no clear gross-anatomic markers to discriminate between primary motor cortex and premotor cortex. The ROI encompassing the posterior limb of the internal capsule was drawn at z = 8 and the pontine ROIs were drawn just below the superior cerebellar peduncle at approximately z = −24. (Coordinates are given in Talairach space.)
After reconstructing fiber tracts using the ROIs outlined above, we determined the total number of fibers for each individual tract, hemisphere, and patient/control subject. This parameter has to be understood as a quantitative measure of connectivity between anatomic locations as determined by the ROIs. In addition, we used the ROIs drawn in the PLIC to extract mean regional FA values, calculate asymmetry indices between the lesional and contralesional hemispheres, and compare these values between the patient and control groups as well as with values in the literature.19 Fiber number asymmetry scores (Valueunaff − Valueaff)/(Valueunaff + Valueaff) were calculated separately for the narrow and the broad pontine ROIs. The resulting scores range from −1 to 1 with positive values indicating a reduced fiber count in the affected hemisphere, a value of 0 indicating an equal fiber number between the 2 hemispheres, and positive values indicating fewer fibers in the lesional hemisphere as compared to the contralesional hemisphere. Fiber number and FA value asymmetry indices for the control group were calculated using the following equation: (Valueleft − Valueright)/(Valueleft + Valueright).
RESULTS
Clinical and lesion data.
The group mean scores were 33.6 for UE-FM (SD 18.2) and 0.95 seconds[log] for WMFT (SD 0.56). Lesion sites varied from cortical and immediate subcortical locations (2 patients) suggesting a distal MCA branch occlusion to large striatocapsular (13 patients) and large hemispheric strokes with extensive white and gray matter involvement (20 patients) suggesting an occlusion of an MCA division.
Comparing DTI-derived measures between patients and controls.
Ipsilesional fiber number (for both PT and aMF) and PLIC FA values were lower in the patient group as compared to the control group (p < 0.001) while measurements did not show any differences when the patients' nonlesional hemisphere was compared with an average of both hemispheres of the control group (p = 0.839 for fiber number and p = 0.599 for FA values). Patient and control groups differed with regard to fiber number asymmetry (p < 0.001) and regional FA value asymmetry of the PLIC (p < 0.001).
Within the patient group, the 2 hemispheres differed in absolute fiber numbers and regional FA values. The number of reconstructed fibers was lower in the ipsilesional relative to the contralesional motor tracts (p < 0.001); the ipsilesional FA values of the PLIC were decreased as compared to the nonlesional hemisphere (p = 0.011).
Pattern of ipsilesional motor tract damage.
The lesional hemisphere showed particular patterns of tract damage. The fiber asymmetry scores (between lesional and nonlesional hemispheres) ranged from +0.95 to −0.01 in those patients (22 of 35 patients) in whom fibers extending from the precentral gyrus to the pons could be reconstructed in both hemispheres (including PT and aMF). The fiber number asymmetry scores were 1.0 in the remaining 13 patients with no detectable fibers originating from the ipsilesional precentral gyrus and descending to the pons. These 2 subgroups (i.e., the 2 groups with and without corticospinal fibers in the lesional hemisphere) differed in their motor impairment scores (p < 0.001).
When the narrower brainstem ROI (PT only) was used for tract reconstruction, we found that 21 of the 35 patients had no traceable fibers. This group included the abovementioned 13 patients (who were identified using the broader pontine ROI), as well as 8 additional patients in whom only fibers descending in the posterior pons were traceable (aMF). Again, this subgroup differed from the remaining 14 patients in motor impairment scores (p < 0.001). No patient showed the inverse pattern with traceable anterior fibers but no posterior fibers.
A classification based on the pattern of ipsilesional motor tract damage.
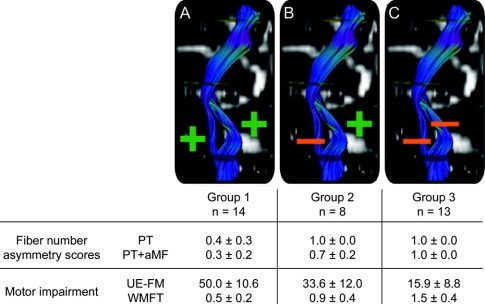
The pattern of ipsilesional motor tract integrity allowed us to divide the patients into 3 groups depending on whether descending fibers were traceable in the anterior (PT) and posterior portion of the pons (aMF).
In the first group, fibers of both the anterior and the posterior portion of descending corticospinal tracts were traceable in the affected hemisphere. In the second group, no anterior fibers were traceable, but fibers passing through the posterior portion of the pons could be reconstructed. In the third group, the tracts maintained no integral connections with the precentral region.
A multivariate analysis of covariance controlling for patient age, time post stroke, and lesion size revealed overall differences among the 3 groups for both UE-FM [F(5,23) = 17.199, p < 0.001] and WMFT [F(5,23) = 10.262, p < 0.001]; note that only 29 out of 35 patients had a full set of WMFT scores. Bonferroni-corrected post hoc tests revealed that all groups showed significant differences between one another for both motor impairment scores (figure 2).
Figure 2 Three impairment groups
An asymmetry score of 1.0 indicates that no fibers could be traced in the ipsilesional hemisphere. Hence, in group 1 (A) fibers originating from the precentral gyrus were traceable both in the anterior pons (pyramidal tract [PT]) and in the posterior pons (alternate motor fibers [aMF]); in group 2 (B) only fibers passing through the posterior pons could be traced (aMF), but no fibers in the anterior pons (PT); in group 3 (C) no fibers passing through either the anterior or posterior part of the ipsilesional pons (PT or aMF) were traceable. Significant between-group differences were found in motor impairment scores for all groups (Bonferroni-corrected post hoc tests). All values are given as mean scores ± SD; Wolf Motor Function Test (WMFT) scores are given in sec[log]. UE-FM = Upper Extremity Fugl-Meyer assessment.
Correlations between DTI-derived measures and motor impairment.
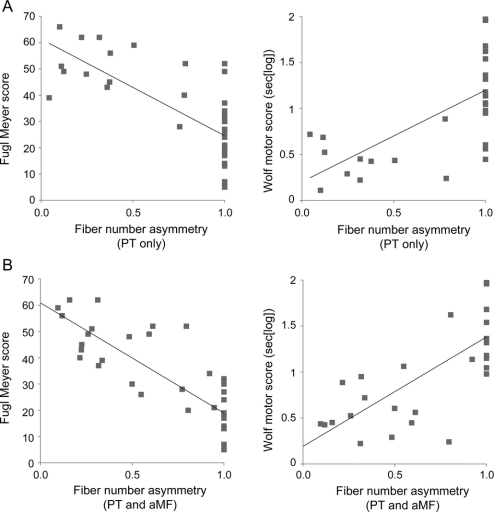
Fiber number asymmetry scores (combining PT and aMF) showed a strong correlation with UE-FM (R = −0.80; p < 0.001) and with WMFT (R = 0.71; p < 0.001; figure 3A) controlling for effects of patient age, time post stroke, and lesion size. The correlations were found to be slightly less prominent (R = −0.69 for UE-FM [p < 0.001] and R = 0.61 for WMFT [p < 0.001]; figure 3B) when the PT fiber asymmetry scores were used. To account for a possible ceiling effect in the regression analyses due to the number of patients with asymmetry scores of 1.0, we ran additional partial correlations after excluding this subgroup (group 3). The correlations remained significant albeit with slightly lower R scores: R = −0.73 for UE-FM and R = 0.55 for WMFT when PT and aMF were combined, and R = −0.64 for UE-FM and R = 0.62 for WMFT when PT fiber asymmetry scores were used.
Figure 3 Regressing fiber number asymmetry with motor impairment scores
(A) Top row: Fiber number asymmetry scores using the narrow pontine region of interest (ROI) (including only pyramidal tract [PT] fibers), and (B) bottom row: using the broad pontine ROI (including PT fibers and alternate motor fibers [aMF]).
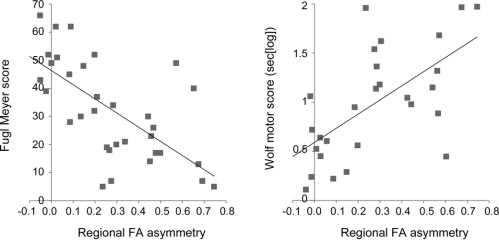
Partial correlations (controlling for patient age, time post stroke, and lesion size) revealed that FA asymmetry scores in the PLIC predicted UE-FM scores (R = −0.71; p < 0.001) and WMFT scores (R = 0.68; p < 0.001) (figure 4).
Figure 4 Regressing regional fractional anisotropy (FA) value asymmetry of the posterior limb of the internal capsule with motor impairment scores
Hemispheric asymmetry of mean FA values in the internal capsule region of interest (also used for tractography of pyramidal tract fibers and alternate motor fibers).
DISCUSSION
Our analysis revealed highly significant correlations between structural measures of fiber tract integrity and functional motor impairment scores in chronic stroke patients. Interestingly, it was the differential integrity of all corticospinal motor tracts rather than the integrity of the PT alone that was associated with motor impairment.
A linear relationship between fine motor skills of the affected limb and fiber number asymmetry has already been found in a group of 10 mildly to moderately affected chronic stroke patients.19 Our quantitative analysis of 35 patients with varying degrees of motor recovery yielded a highly significant relationship between DTI-derived parameters and motor impairment scores. It complements previous studies that associated the pattern of corticospinal tract damage with motor impairment using qualitative DTI approaches10–14,33 or related the intersection of a lesion with the presumed location of corticospinal fibers to motor impairment using structural MRI.1,2 With regard to the functional integrity of corticospinal tracts and its relation to motor outcome, TMS has been shown to be a good predictor in the acute and subacute phase whereas its value in chronic stroke varied between studies.34 The combination of DTI parameters (i.e., regional FA values of the internal capsule) and TMS-derived measures has also been reported to be potentially useful in estimating recovery potential.20 However, our results suggest that DTI-derived measures alone are valid structural surrogates of motor impairment after stroke.
One of the most important findings of our investigation is the possible contribution of alternate corticospinal fibers to recovery. Patients with traceable fibers descending in the posterior pons (aMF) but no intact PT fibers surprisingly revealed only a moderate motor impairment on average, whereas patients with no traceable fibers descending in either the anterior or the posterior pons were severely impaired. The physiologic significance of these alternate fibers has not been fully explored; however, their possible contribution to recovery has already been suggested in patients with complete disruption of the PT who still showed muscular excitability in the affected limb after cortical stimulation6 or had fine finger muscle control.5 The degree of aMF integrity might also explain some of the variability in motor recovery previously described.35
We used the differential integrity of the PT and aMF to develop a 3-tier classification system of motor impairment whose predictive value with regard to a patient's recovery potential can now be tested in the acute recovery phase. Patients with traceable PT fibers and aMF (group 1) achieved the highest UE-FM scores and shortest WMFT completion times. Patients who had traceable aMF but no PT fibers were more severely affected (group 2). However, their UE-FM and WMFT scores still indicated a moderate recovery. Finally, patients with no traceable fibers descending either in the anterior or posterior pons (group 3) were most severely impaired as reflected in lower UE-FM and higher WMFT scores when compared to the other groups (see figure 2 for details). This classification system of impairment categories for chronic stroke patients might be helpful in estimating recovery potential of subacute patients at the outset of their natural recovery or of patients entering experimental neurorehabilitation trials. Furthermore, the classification could be used to refine inclusion criteria for experimental studies.
No patient in our sample showed the inverse pattern of motor tract damage (i.e., traceable corticospinal fibers in the anterior but not in the posterior pons). This is most likely due to the composition of the tracts descending in the posterior pons, which presumably contain a heterogeneous group of polysynaptic fibers. Lesions that severely damage these fibers might be so large that they always involve the PT.
However, limitations of DTI tractography should be considered. While the path of pyramidal fibers is well known31 and the PT can be reliably reconstructed,32 the course of what we termed alternate motor fibers5 (aMF) is not yet based on anatomic studies in humans.36,37 Dedicated high-resolution DTI would be necessary to determine whether these fibers actually belong to the cortico-reticulo-spinal and cortico-rubro-spinal tracts as we suspect.
In this context, it should be emphasized that DTI tractography provides a probabilistic measure of connectivity, but does not reach the anatomic validity of myeloarchitectonic studies and currently cannot differentiate individual axons or synaptic connections.38 The absence of traceable fibers does not necessarily indicate a complete tract disruption, but rather suggests that the amount of tract damage is more severe than in those patients in whom fibers could be traced.
Lower UE-FM scores and longer WMFT completion times also correlated with higher FA asymmetry scores in the PLIC which is attributed mainly to the variability of FA values in ipsilesional PLIC while contralesional values did not differ significantly from those obtained in the control group in our study. However, FA alterations in a part of the contralesional motor tract have been found when a group of 10 patients was split into 2 subgroups; those with a higher level of recovery had higher FA values than normal controls and vice versa for patients with a lower level of recovery, suggesting that remodeling of the contralesional motor system also contributes to recovery.39 Our study differs methodologically, in that we used FA values of the entire PLIC instead of tract FA values so that intact neighboring corticofugal and corticopetal fibers28,29 might have masked potential FA alterations.
With regard to the lesional hemisphere, our findings are in agreement with studies showing a correlation between FA values of the internal capsule or regions in the cerebral peduncle of the lesional hemisphere and motor outcome in subacute or chronic stroke patients.13,16,17 In another study, PLIC FA asymmetry has been shown to offer predictive value for determining motor outcome in chronic stroke patients.20 Our data suggest a linear correlation between PLIC FA asymmetry and motor impairment, explaining approximately 50% of the variance of this correlation. FA value changes remote from an ischemic lesion have been attributed to degeneration and remodeling.13,17,18,40 Other possible contributors to the variance could be intact ascending and descending fiber tracts that traverse through the PLIC in close proximity,28,29 which might mask the effect of FA reduction caused by the ischemic lesion to some degree.
DISCLOSURE
Dr. Lindenberg, Dr. Renga, L. Zhu, and F. Betzler report no disclosures. Dr. Alsop serves as Deputy Editor of Magnetic Resonance in Medicine; may accrue revenue on US Patents 7,545,142 (issued: 6/9/2009), 7,369,888 (issued: 5/6/2008), 6,980,845 (issued: 12/27/2005), and 6,717,405 (issued: 4/6/2004): Methods for perfusion imaging with arterial spin labeling MRI and US Patent 6,252,403 (issued: 6/26/2001): RF coil for high field MRI; receives research support from GE Healthcare, Merck Serono, the NIH [1 R01 CA115745-01A1 (PI), 1 R01 AG027435-01 (Co-I), 1 R21 CA121570A1 (PI), R01-EB004582 (Co-I), R01 MH80729-01A2 (Co-I), R01 MH077073-01A2 (Co-I), R01DC008796-01A1 (Co-I), R01 NS047029-04A2 (Co-I), and 1P50 CA101942-01 (Co-I)]; has received license fee payments from Siemens Medical for US Patents 7,369,888 and 6,980,845: Multi-slice perfusion imaging; and may receive royalty payments through a licensing agreement with GE Healthcare for US Patent 7,545,142: Arterial spin labeling with pulsed radio frequency sequences. Dr. Schlaug receives research support from the NIH [NINDS 1R01NS045049 (PI), NIDCD 1RO1 DC008796 (PI), and NIDCD R01 DC009823-01 (PI)], and from the NSF BCS0518837 (PI).
Supplementary Material
Address correspondence and reprint requests to Dr. Gottfried Schlaug, Department of Neurology, Beth Israel Deaconess Medical Center/Harvard Medical School, 330 Brookline Ave., Boston, MA 02215 gschlaug@bidmc.harvard.edu
Editorial, page 276
Study funding: Supported by a grant from NIH/NINDS (NS045049).
Disclosure: Author disclosures are provided at the end of the article.
Received May 27, 2009. Accepted in final form October 8, 2009.
REFERENCES
- 1.Pineiro R, Pendlebury ST, Smith S, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke 2000;31:672–679. [DOI] [PubMed] [Google Scholar]
- 2.Schiemanck SK, Kwakkel G, Post MW, Kappelle LJ, Prevo AJ. Impact of internal capsule lesions on outcome of motor hand function at one year post-stroke. J Rehabil Med 2008;40:96–101. [DOI] [PubMed] [Google Scholar]
- 3.Duncan PW. Stroke disability. Phys Ther 1994;74:399–407. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SC, Seitz RJ. Imaging functional recovery from stroke. In: Handbook of Clinical Neurology 2008;94:1097–1117. [DOI] [PubMed] [Google Scholar]
- 5.Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol 2004;92:2802–2810. [DOI] [PubMed] [Google Scholar]
- 6.Fries W, Danek A, Witt TN. Motor responses after transcranial electrical stimulation of cerebral hemispheres with a degenerated pyramidal tract. Ann Neurol 1991;29:646–650. [DOI] [PubMed] [Google Scholar]
- 7.Platz T, Pinkowski C, van Wijck F, Kim IH, di Bella P, Johnson G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test, and Box and Block Test: a multicentre study. Clin Rehabil 2005;19:404–411. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair 2005;19:194–205. [DOI] [PubMed] [Google Scholar]
- 9.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konishi J, Yamada K, Kizu O, et al. MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005;64:108–113. [DOI] [PubMed] [Google Scholar]
- 11.Kunimatsu A, Aoki S, Masutani Y, Abe O, Mori H, Ohtomo K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiol 2003;45:532–535. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Han MK, Kim SH, Kwon OK, Kim JH. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. Neuroimage 2005;26:771–776. [DOI] [PubMed] [Google Scholar]
- 13.Nelles M, Gieseke J, Flacke S, Lachenmayer L, Schild HH, Urbach H. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol 2008;29:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada K, Ito H, Nakamura H, et al. Stroke patients' evolving symptoms assessed by tractography. J Magn Reson Imaging 2004;20:923–929. [DOI] [PubMed] [Google Scholar]
- 15.Kang DW, Chu K, Yoon BW, Song IC, Chang KH, Roh JK. Diffusion-weighted imaging in Wallerian degeneration. J Neurol Sci 2000;178:167–169. [DOI] [PubMed] [Google Scholar]
- 16.Lindberg PG, Skejo PH, Rounis E, et al. Wallerian degeneration of the corticofugal tracts in chronic stroke: a pilot study relating diffusion tensor imaging, transcranial magnetic stimulation, and hand function. Neurorehabil Neural Repair 2007;21:551–560. [DOI] [PubMed] [Google Scholar]
- 17.Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 2004;22:1767–1774. [DOI] [PubMed] [Google Scholar]
- 18.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 2000;69:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage 2008;39:1370–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–180. [DOI] [PubMed] [Google Scholar]
- 21.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: 1: a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 22.van Wijck FM, Pandyan AD, Johnson GR, Barnes MP. Assessing motor deficits in neurological rehabilitation: patterns of instrument usage. Neurorehabil Neural Repair 2001;15:23–30. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 2001;32:1635–1639. [DOI] [PubMed] [Google Scholar]
- 24.Morris DM, Uswatte G, Crago JE, Cook EW 3rd, Taub E. The reliability of the Wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil 2001;82:750–755. [DOI] [PubMed] [Google Scholar]
- 25.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006;296:2095–2104. [DOI] [PubMed] [Google Scholar]
- 26.Gerraty RP, Parsons MW, Alan Barber P, Darby DG, Davis SM. The volume of lacunes. Stroke 2001;32:1937–1938. [DOI] [PubMed] [Google Scholar]
- 27.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kretschmann HJ. Localisation of the corticospinal fibres in the internal capsule in man. J Anat 1988;160:219–225. [PMC free article] [PubMed] [Google Scholar]
- 29.Zarei M, Johansen-Berg H, Jenkinson M, Ciccarelli O, Thompson AJ, Matthews PM. Two-dimensional population map of cortical connections in the human internal capsule. J Magn Reson Imaging 2007;25:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 31.Nieuwenhuys R, Voogd J, Huijzen CV. The Human Central Nervous System, 4th ed. Berlin: Springer; 2008. [Google Scholar]
- 32.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology 2004;230:77–87. [DOI] [PubMed] [Google Scholar]
- 33.Newton JM, Ward NS, Parker GJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas: relevance to stroke recovery. Brain 2006;129:1844–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol 2006;117:1641–1659. [DOI] [PubMed] [Google Scholar]
- 35.Cramer SC. Repairing the human brain after stroke: I: mechanisms of spontaneous recovery. Ann Neurol 2008;63:272–287. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey: II: the effects of lesions of the descending brain-stem pathways. Brain 1968;91:15–36. [DOI] [PubMed] [Google Scholar]
- 37.Schmahmann JD, Ko R, MacMore J. The human basis pontis: motor syndromes and topographic organization. Brain 2004;127:1269–1291. [DOI] [PubMed] [Google Scholar]
- 38.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex 2008;44:936–952. [DOI] [PubMed] [Google Scholar]
- 39.Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009;30:3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13:1174–1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.