Summary
An outstanding model to study how neurons differentiate from among a field of equipotent undifferentiated cells is the process of R8 photoreceptor differentiation during Drosophila eye development. We show that in senseless mutant tissue, R8 differentiation fails and the presumptive R8 cell adopts the R2/R5 fate. We identify senseless repression of rough in R8 as an essential mechanism of R8 cell fate determination and demonstrate that misexpression of senseless in non-R8 photoreceptors results in repression of rough and induction of the R8 fate. Surprisingly, there is no loss of ommatidial clusters in senseless mutant tissue and all outer photoreceptor subtypes can be recruited, suggesting that other photoreceptors can substitute for R8 to initiate recruitment and that R8-specific signaling is not required for outer photoreceptor subtype assignment. A genetic model of R8 differentiation is presented.
Introduction
The process of selection and differentiation of a single neuron from amongst a field of equipotent and uncommitted cells is a complicated and important developmental challenge. This task requires differential gene expression between the presumptive neuronal cell and the surrounding uncommitted tissue. One way organisms achieve neural specification is by establishing a zone of neuronal competency and then choosing a single cell from within this zone to differentiate as a neuron. Such a sequence of events occurs during sensory organ precursor (SOP) selection in the developing embryonic nervous system of Drosophila. In imaginal discs, proneural clusters of 15–20 cells define neuroectodermal fields that are competent to differentiate as neurons. These clusters express a family of proneural genes. Eventually, a single SOP expresses proneural genes at a higher level than the surrounding cells. The basic helix-loop-helix proteins encoded by the proneural genes then initiate the process of neuronal selection in the SOP (Cubas et al., 1991; Culi and Modolell, 1998; Jarman et al., 1993; Skeath and Carroll, 1991; Van Doren et al., 1992). Thus, only the SOP expresses the proper genes and has the appropriate cues to differentiate along the neural pathway. In the developing Drosophila eye, neuronal selection occurs by a similar mechanism, and the first neuron to differentiate is the R8 photoreceptor (Jarman et al., 1994; Tomlinson and Ready, 1987a).
The adult retina of Drosophila is a highly structured and specialized lattice of 750–800 repeating units or ommatidia. Each ommatidium contains eight photoreceptors, termed R1–R8. Ommatidia develop sequentially from undifferentiated imaginal tissue as the morphogenetic furrow (MF) progresses anteriorly across the eye disc during the third larval instar (Ready et al., 1976). The passage of the MF results in the selection of a single founder cell per ommatidium, the R8 photoreceptor (Tomlinson and Ready, 1987a). Once R8 differentiates, the recruitment of all other photoreceptors occurs in a precise order, beginning with photoreceptors R2 and R5, followed by R3 and R4, R1 and R6, and finally R7 (Ready et al., 1976). R8 differentiation is tightly regulated, and a number of genes, including Notch, scabrous (sca), and rough (ro), negatively regulate the process of R8 differentiation. Loss of Notch in the MF causes nearly all cells to differentiate as R8 photoreceptors, while the loss of sca or ro affects R8 differentiation at a later stage, resulting in two or three R8 photoreceptors per ommatidium (Baker et al., 1990; Baker and Rubin, 1992; Cagan and Ready, 1989; Heberlein et al., 1991). These data suggest that the R8 photoreceptor differentiates from among a group of uncommitted cells that are all competent to become the R8 photoreceptor and that this group of equipotent cells is progressively reduced to a single cell per ommatidium. Moreover, Notch and sca appear to work as members of the same pathway (Baker et al., 1990; Baker and Zitron, 1995; Lee et al., 2000; Powell et al., 2001), while ro expression is not dependent on Notch signaling (Dokucu et al., 1996).
Negative regulation of R8 differentiation is opposed by positive regulation under the control of the proneural gene atonal (ato) (Jarman et al., 1994). ato is required for R8 differentiation, and ato loss-of-function mutations cause a complete failure of both R8 differentiation and all subsequent photoreceptor recruitment (Jarman et al., 1994, 1995). The protein expression pattern of Ato reflects its function in R8 and is akin to proneural expression patterns observed during SOP selection. Ato is first expressed in a broad stripe within and just anterior to the MF. Gradually, the protein is resolved posteriorly into evenly spaced clusters of cells of decreasing number, and it is ultimately expressed exclusively in the single cell that is destined to become R8 (Jarman et al., 1995). There are multiple identifiable stages to this refinement, and they correlate with the gradual reduction in the number of cells competent to differentiate as R8 (Baker et al., 1996; Dokucu et al., 1996). Immediately prior to R8 differentiation, Ato is detected in a two to three cell cluster (Figure 1A). There is both gain- and loss-of-function evidence that the two to three cell cluster represents the final group of cells that are fully competent to differentiate as R8, and this cluster has thus been termed the R8 equivalence group (Dokucu et al., 1996). ro, a homeodomain encoding gene, is an important negative regulator of this final stage of R8 differentiation, and it is believed that ro is required to ensure that only one R8 cell differentiates from each R8 equivalence group (Figure 1A) (Dokucu et al., 1996; Heberlein et al., 1991). Ro, which is expressed exclusively in the developing eye, is expressed in the developing R2, R3, R4, and R5 photoreceptors, but not in R8 (Kimmel et al., 1990). In addition, within the MF, Ro is expressed in all cells except for the R8 equivalence group and R8, a pattern that is mutually exclusive with that of Ato (Dokucu et al., 1996). These complementary patterns of expression, in conjunction with work demonstrating that Ro negatively regulates ato expression, suggest a mutual antagonism of ato and ro in the developing eye (Dokucu et al., 1996). However, despite these relationships with ato, the mechanism governing ro repression of R8 differentiation is not understood.
Figure 1. Senseless Marks the R8 Equivalence Group and R8 and Is Genetically Downstream of atonal.
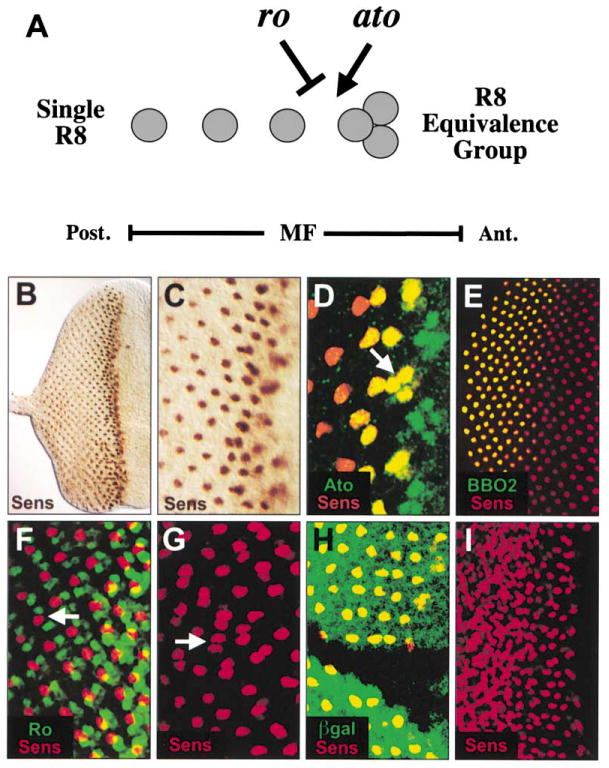
(A) Regulation of R8 differentiation. atonal (ato) is expressed in the three cells of the R8 equivalence group and in the R8 photoreceptor (gray circles). The cells of the R8 equivalence group are equally competent to differentiate as R8, and ato is required for R8 selection (arrow). rough (ro) represses R8 differentiation at the stage of the R8 equivalence group (inhibitory arrow) to ensure that only one of the three cells becomes an R8 photo-receptor. This regulation occurs within the morphogenetic furrow (MF). (B–I) All panels show eye imaginal discs of late third instar larvae, and in this and in all subsequent figures, posterior is to the left. (B) Wild-type disc stained for Sens (brown). Sens is first expressed in the MF and then in a single nucleus per ommatidium posterior to the MF. (C) Higher magnification of (B). (D) Wild-type disc stained for Sens (red) and Ato (green). Ato is broadly expressed in the MF anterior to the onset of Sens expression. Sens and Ato colocalize (yellow) in the R8 equivalence group (arrow). Colocalization continues in R8 until Ato expression ceases. (E) Wild-type disc stained for Sens (red) and the late R8-specific enhancer trap, BBO2 (green). Sens expression precedes BBO2 in the R8 photoreceptor, and BBO2 expression later colocalizes with Sens in R8 (yellow). (F) Wild-type disc stained for Sens (red) and Ro (green). Ro is initially expressed broadly in the MF and does not colocalize with Sens. Immediately posterior to the MF, Ro is expressed in photoreceptors R2 and R5, while Sens is expressed only in R8 (arrow). (G) roX63 mutant disc stained for Sens (red). Sens is expressed in multiple R8 cells in many ommatidia (arrow). (H) Disc containing ato1 mutant clone stained for Sens (red) and negatively marked with β-galactosidase (green). Sens is not expressed within the ato1 mutant clone. (I) A sevenless-GAL4 × UAS-ato disc stained for Sens. Misexpression of ato induces Sens expression.
Recently, the senseless (sens) gene product, a C2H2 type zinc finger protein, has been shown to be both necessary and sufficient for neural development. In the PNS, proneural genes induce sens expression, and sens is in turn required to further activate and maintain high levels of proneural expression (Nolo et al., 2000). Furthermore, sens is expressed in all imaginal tissues, including the third instar eye disc where it is expressed in the R8 photoreceptor (Nolo et al., 2000). This expression in R8, the established relationship between sens and proneural genes in the PNS, and the essential role of sens in PNS development suggest that sens may also function in R8 differentiation.
We have used mutations in sens to decipher mechanisms of R8 differentiation and have found that sens is both necessary and sufficient for R8 differentiation in the Drosophila eye. sens acts downstream of ato in the developing eye, and when sens function is removed, R8 differentiation fails during the resolution of the R8 equivalence group into a single R8 photoreceptor. Moreover, in sens mutant ommatidia, the presumptive R8 cell rapidly and consistently adopts characteristics of the R2/R5 photoreceptor. We identify sens repression of ro in the developing R8 photoreceptor as an essential mechanism of R8 differentiation, suggesting that the mutual antagonism of ato and ro is sens mediated. We also demonstrate that sens misexpression in non-R8 photoreceptors results in both repression of ro and competency to adopt the R8 fate. Strikingly, ommatidia still develop in sens mutant ommatidia despite the absence of R8, and all outer photoreceptor subtypes are successfully recruited. This indicates that other photoreceptors can substitute for R8 to initiate recruitment and rules out all models for outer photoreceptor subtype specification that involve R8-specific signaling, either in the form of spatial cues or ligand/receptor interactions.
Results
Expression of sens in the Developing Drosophila Retina
sens is expressed in the R8 photoreceptor during third instar eye development beginning within the MF (Figures 1B and 1C) (Nolo et al., 2000). As ato is expressed throughout the MF and is the earliest known marker of R8 differentiation, we used Ato expression to precisely determine when Sens expression begins. The expression of Ato and Sens colocalize beginning with the R8 equivalence group, indicating that Sens is expressed prior to the selection of a single R8 photoreceptor (Figure 1D), and Ato and Sens are detected together in the same cell until Ato expression ceases after the third column of photoreceptor development. The overlapping pattern of Sens and a later R8 marker, BBO2, demonstrates that Sens is expressed in R8 throughout larval eye development (Figure 1E). Furthermore, Sens does not colocalize with Ro (Figure 1F), which is never expressed in the cells of the R8 equivalence group or in R8 (Dokucu et al., 1996; Kimmel et al., 1990).
We sought to determine if Sens continues to mark R8 in mutant backgrounds that contain multiple R8 photo-receptors per ommatidium, as is the case for other R8-specific markers (Dokucu et al., 1996; Heberlein et al., 1991). We therefore examined Sens expression in both roX63 (Figure 1G) and scaBP2 (not shown) eye imaginal discs. In both cases, Sens is detected in multiple R8 cells per ommatidium. Thus, Sens remains a faithful marker of R8 in mutant backgrounds.
Since the expression pattern of Sens overlaps that of Ato and sens lies downstream of proneural genes in the PNS, we sought to confirm this relationship in the developing eye. We generated ato1 mutant clones in the eye using the FLP/FRT system (Golic and Lindquist, 1989; Xu and Rubin, 1993) and found that Sens is not detected within the clone (Figure 1H). Similarly, Sens is not detected in eye discs dissected from ato1 mutant larvae (Nolo et al., 2000). We also expressed UAS-ato (Jarman and Ahmed, 1998) under the control of sevenless-GAL4, which is expressed in all photoreceptor cells except R8, R2, and R5, and found that expression of Sens is strongly activated in response to ectopic ato (Figure 1I). Taken together, these data place sens downstream of ato in the developing eye.
Normal R8 Differentiation Requires sens
The genetic relationship between ato and sens, the importance of ato in R8 development, and the early detection of Sens in the developing R8 photoreceptor suggest that sens might play a role in normal R8 differentiation. We tested this by generating large sens mutant clones using the FLP/FRT system in a Minute background (Golic and Lindquist, 1989; Morata and Ripoll, 1975; Xu and Rubin, 1993). Within a single ommatidium of the adult retina, photoreceptors have a characteristic, highly regular arrangement. The rhabdomeres of the six outer photoreceptors (R1–R6) are large in size and form a trapezoid. Centrally placed within the trapezoid are the small rhabdomeres of R7 and R8 (Figures 2A and 2B, inset). This precise organization allows unambiguous identification of all photoreceptor subtypes within a normally constructed ommatidium. Moreover, cells within a single ommatidium are not derived from a fixed cell lineage (Lawrence and Green, 1979; Ready et al., 1976). Thus, ommatidia located at the border of clones containing both wild-type and mutant photoreceptors may still be normally constructed. Analysis of such “mosaic” ommatidia reveals which photoreceptors, if any, require the function of a gene in question for normal ommatidial development. We found that normally constructed ommatidia containing photoreceptors mutant for sens could form only if the R8 photoreceptor had at least one functional copy of sens (Figures 2A and 2B). Of 91 mosaic ommatidia scored, no normally constructed ommatidia containing a sens mutant R8 were recovered, and there was no significant preference for any other photoreceptor to retain sens function (Figure 2C).
Figure 2. The R8 Photoreceptor Is Absent in senseless Mutant Adult Ommatidia.
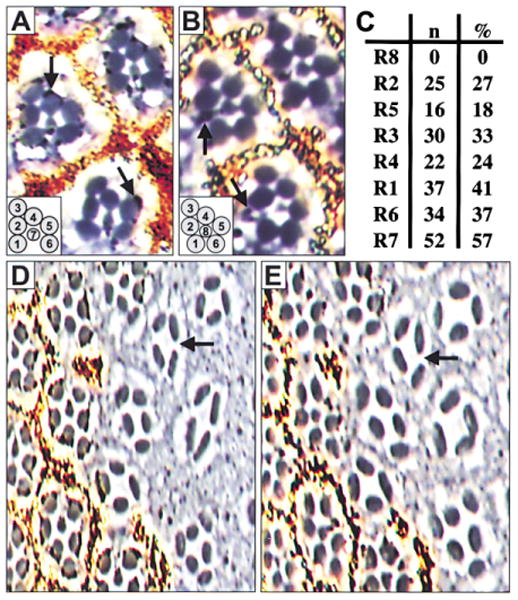
Mitotic clones are negatively marked by the absence of pigment granules. (A and B) High power magnification of three ommatidia at the level of R7 (A) and of R8 (B). Two normally constructed ommatidia with wild-type photoreceptors (top right and left). The pigment for photoreceptors R1–R7 is detected at the level of R7 (arrows, [A]), while pigment for R8 is seen at the level of R8 (arrows, [B]). Mosaic ommatidium containing numerous sens mutant photoreceptors (R1–R4) (bottom). Note pigment associated with R8. ([A and B], inset) Normal configuration of photoreceptor rhabdomeres within an ommatidium at the level of R7 (A) and R8 (B). (C) Data for 91 mosaic ommatidia scored. (n = number of ommatidia mutant for that photoreceptor; % = percentage of ommatidia mutant for that photoreceptor.) Mosaic ommatidia with a sens mutant R8 are not recovered. (D and E) A large sens mutant clone at the level of R7 (D) and R8 (E). Photoreceptors with small rhabdomeres are not detected, suggesting the absence of both R8 and R7. The arrow points to an ommatidium containing four photoreceptors with large rhabdomeres, the most commonly observed configuration.
Large patches of retina containing ommatidia entirely mutant for sens are readily recovered in adults. While ommatidia in such clones are disorganized and of variable size and configuration, the number of ommatidia and the spacing between them is not changed from the surrounding wild-type tissue. However, all ommatidia have one striking similarity: they do not contain morphologically discernable R8 or R7 photoreceptors (Figures 2D and 2E; Table 1). Small rhabdomeres are extremely rare (<0.2%) and could not be identified as part of either an R8 or an R7 photoreceptor (not shown). As R8 is believed to be required for subsequent photoreceptor recruitment during normal eye development, the presence of outer photoreceptors despite the absence of a morphologically distinct R8 within sens mutant ommatidia is a puzzling observation.
Table 1. Failure of R8 Differentiation Results in Ommatidia with Fewer Photoreceptors.
| Genotype | Number of Photoreceptors per Ommatidium | |||||||
|---|---|---|---|---|---|---|---|---|
| 8 | 7 | 6 | 5 | 4 | 3 | Other | Average | |
| sensE2/sensE2 | 1 | 85 | 56 | 84 | 119 | 45 | 7 | 5.1 ± 1.3 |
To avoid scoring mosaic ommatidia, only clusters at least one ommatidium width away from the clone border were counted. Results were pooled from eleven independent clones. Ommatidia of sens mutant clones range in size from three to eight photoreceptors. The most common ommatidial configuration contains four photoreceptors (see Figures 2D and E). The column marked “other” includes rare ommatidia containing rhabdomeres that are positioned sideways and ommatidia that could not be accurately scored.
At least four models could explain the presence of photoreceptors in sens mutant ommatidia despite the apparent absence of R8. First, the R8 photoreceptor could differentiate, begin the process of photoreceptor recruitment, and then die. Second, recruitment of photoreceptors could occur without a differentiated R8. Third, the R8 photoreceptor could differentiate with characteristics of both R8 and another photoreceptor such that it could not be detected as an R8 cell in the adult, yet could still enable photoreceptor recruitment to occur. Fourth, the R8 cell could initiate differentiation but then undergo a fate change into another photoreceptor subtype, while still enabling photoreceptor recruitment to occur.
To test the first model of R8 differentiation followed by death, we looked at expression of an enhancer trap in sca that is normally expressed strongly in the R8 photoreceptor throughout the larval eye disc posterior to the MF. This expression pattern is unlike that of Sca protein, which normally cannot be detected after about the fourth column of ommatidia (Mlodzik et al., 1990), and is likely a consequence of perdurance of either lacZ transcript or β-galactosidase protein well after enhancer activity ceases. Thus, the enhancer trap in sca is a marker for R8 selection that is maintained throughout larval development. Within sens mutant clones, β-galactosidase protein is detected within a single cell per ommatidium all the way to the posterior margin of the eye disc, as in wild-type tissue (Figure 3A). This suggests that a single R8 cell is selected and that this presumptive R8 photoreceptor does not die during larval development during the period when all other photoreceptors are recruited. Thus, the first model is not correct.
Figure 3. R8 Photoreceptor Differentiation Fails in senseless Mutant Ommatidia.
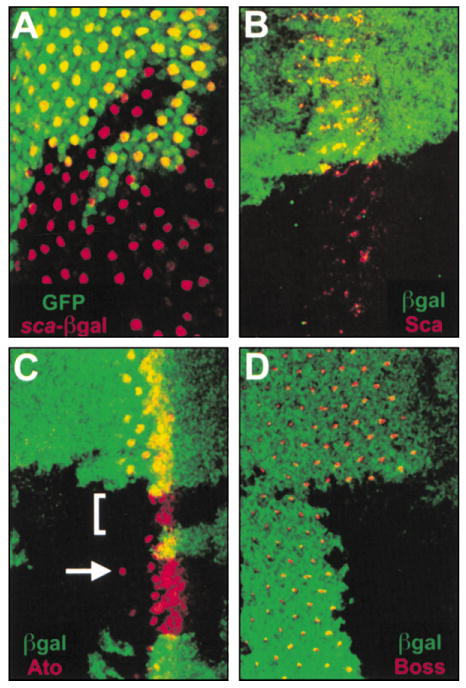
All panels show late third instar eye imaginal discs containing clones of sens mutant tissue marked by the absence of either GFP (A) or β-galactosidase (B–D), both in green. (A) An R8-specific enhancer trap in sca (red) is normally expressed in one cell per ommatidium throughout the eye disc posterior to the MF. As in the wild-type, the enhancer trap is expressed in one cell per ommatidium throughout sens mutant tissue posterior to the MF. (B) Sca protein (red) is normally expressed in the MF and in all R8 cells for the first few columns. Sca is expressed in sens mutant clones at decreased intensity and in fewer cells. (C) Ato (red) is normally expressed in an anterior broad stripe, the R8 equivalence group, and in R8. Within sens mutant clones, Ato is expressed normally through the R8 equivalence group but is absent at the single R8 stage in 75% of ommatidia (bracket). Ato is occasionally detected in R8 (arrow). (D) Boss (red) is normally expressed in all R8 cells posterior to the MF but is never detected within sens mutant clones.
The second model of recruitment without R8 differentiation was addressed by examining other R8-specific markers within sens mutant clones. Normally, Sca protein can be detected in the earliest identifiable R8 photoreceptor, but only for a few columns. Within sens mutant tissue Sca is expressed, but at a lower level of intensity and in fewer cells than in the wild-type (Figure 3B). Expression of another early marker of R8 differentiation, Ato, is also altered. Ato is expressed normally from its early broad expression through the R8 equivalence group (Baker et al., 1996; Dokucu et al., 1996). However, at the stage where Ato normally resolves into a single R8 cell, reduced expression is routinely observed in sens mutant clones. Specifically, Ato expression is absent at the single R8 stage in 75% of ommatidia but rarely can be detected in single cells as late as the third column of development (Figure 3C). The persistence of the enhancer trap in sca suggests that R8 selection occurs, but the observed patterns of Sca and Ato indicate that the process of R8 differentiation is aborted within the MF, most often during resolution of the R8 equivalence group to a single R8 cell, an interval of about one and a half hours. Later markers for R8, Boss (Figure 3D) and BBO2 (not shown), are always absent within sens mutant clones. Thus, while the R8 photoreceptor may initiate differentiation, the process is rapidly aborted and the presumptive R8 always ceases to express R8-specific genes. These findings do not unequivocally rule out the second model of recruitment without a differentiated R8, but they do disprove the third model of dual fate because R8-specific gene expression is rapidly and consistently lost.
Recruitment of All Outer Photoreceptor Subtypes Can Occur Despite Early Abortion of R8
Photoreceptor Differentiation
As the R8 cell may initiate differentiation but does not subsequently die in sens mutant clones, we considered our fourth model that R8 might undergo a fate change. To test this, we studied the expression of photoreceptor subtype-specific markers within sens mutant clones using an R2/R5-specific enhancer trap to mark R2 and R5 (Figure 4A), Spalt (Sal) to mark R3 and R4 (Figure 4C), and BarH1 to mark R1 and R6 (Figure 4D). The most significant finding is that the R2/R5-specific enhancer trap is expressed in three cells per ommatidium instead of the usual two in > 99% of sens mutant ommatidia. In contrast, although photoreceptors of the R3, R4, R1, and R6 subtypes were indeed recruited, they were generally present in reduced numbers (Table 2). These data suggest that, despite rapid loss of R8-specific gene expression in sens mutant clones, all outer photoreceptor subtypes can be successfully recruited. We also found that levels of dual phosphorylated extracellular signal regulated kinase (dpERK), the activated MAP kinase of the Ras signaling cascade, are reduced but not eliminated within sens mutant clones (Figures 4E–4G). As EGFR signaling is responsible for nearly all Ras signaling in the developing eye and is required for all non-R8 photoreceptor recruitment (Freeman, 1996; Kumar et al., 1998; Tio and Moses, 1997; Yang and Baker, 2001), the reduction in dpERK levels is consistent with, and may be the cause of, diminished photoreceptor recruitment in sens mutant tissue.
Figure 4. The Presumptive R8 Photoreceptor Becomes an R2/R5 Photoreceptor and Is Sufficient to Recruit All Outer Photoreceptor Subtypes in senseless Mutant Ommatidia.
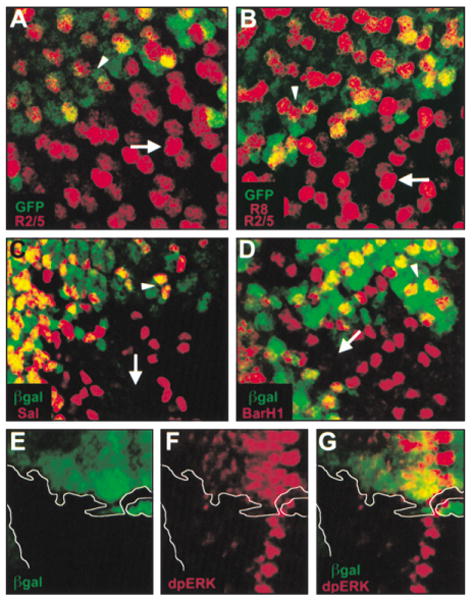
sens mutant clones are negatively marked with either GFP (A and B) or β-galactosidase (C–G), both in green. (A) R2/R5 photoreceptors are marked with an enhancer trap specific to R2/R5 (red). The enhancer trap is expressed in two cells per ommatidium in wild-type tissue (arrowhead) and in three cells per ommatidium within the clone (arrow; Table 2). (B) R2/R5 photoreceptors are marked as in (A), and R8 is marked with the R8-specific enhancer trap in sca (also in red). The combination of the two enhancer traps is normally expressed in three cells per ommatidium (arrowhead). In the clone, three cells per ommatidium continue to express the combination of enhancer traps, but the centrally placed cell stains with greater intensity (arrow). (C) R3/R4 photoreceptors are marked with Sal (red), which is expressed initially in two photoreceptors per ommatidium in wild-type tissue (arrowhead) and in a variable number of cells within the clone. An ommatidium completely lacking Sal is indicated (arrow). (D) R1/R6 photoreceptors are marked with BarH1 (red), and BarH1 is expressed in two cells per ommatidium in wild-type tissue (arrowhead) and in two or fewer cells within the clone. An ommatidium completely lacking BarH1 is indicated (arrow). (E–G) dpERK (red) identifies regions of activated Ras signaling and marks areas of differentiating photoreceptors. Within the clone, dpERK levels are reduced but not absent posterior to the MF.
Table 2. R8 Differentiation Is Not Required to Induce Outer Photoreceptor Subtypes.
| Number of Photoreceptors per Ommatidium | ||||||
|---|---|---|---|---|---|---|
| Subtype | sensE2/sensE2 | wildtype | ||||
| 0 | 1 | 2 | 3 | Average | Average | |
| R2/R5 | 0 | 0 | 2 | 264 | 3.0 ± 0.10 | 2 |
| R3/R4 | 39 | 24 | 30 | 9 | 1.1 ± 1.0 | 2 |
| R1/R6 | 26 | 51 | 50 | 0 | 1.2 ± 0.75 | 2 |
To avoid scoring mosaic ommatidia, only clusters at least one ommatidium width away from the clone border were counted. Results were pooled from at least five independent clones for each subtype. R2 and R5 were identified with an R2/R5-specific enhancer trap, R3 and R4 were identified by Spalt (Sal) expression, and R1 and R6 were identified by BarH1 expression. In the wild type, each of these is detected in only two photoreceptors per ommatidium. In sens mutant ommatidia, three R2/R5 cells are nearly always observed, and R3, R4, R1, and R6 photoreceptors successfully differentiate but in reduced numbers (see Figures 4A, 4C, and 4D).
The fourth model predicts that the extra R2/R5 photoreceptor observed in sens mutant ommatidia might be the presumptive R8 cell. To test this hypothesis, we took advantage of the R8-specific enhancer trap in sca, which persists in the presumptive R8 photoreceptor even within sens mutant tissue (Figure 3A). We determined the expression of both the R2/R5- and the R8-specific enhancer traps in the same sens mutant clone and found that in the neighboring wild-type tissue the two enhancer traps together mark three cells per ommatidium at comparable levels of intensity (Figure 4B). Although three cells per ommatidium continue to express the two en-hancer traps within sens mutant clones, the centrally positioned cell is consistently marked with greater intensity (Figure 4B). Therefore, the R2/R5 marker is misexpressed in the presumptive R8 photoreceptor.
R8 Differentiation Is Mediated by sens Repression of ro
In addition to the R2/R5-specific enhancer trap described above, we examined another R2/R5 marker, Rough (Ro), to confirm and extend our hypothesis that the presumptive R8 photoreceptor was becoming an R2 or R5 photoreceptor. Ro is normally expressed in the R2/R5 photoreceptors and later expands to include the R3/R4 photoreceptors. Within sens mutant clones, Ro is detected abnormally in three cells per ommatidium at the time when it should only be detected in the R2/ R5 photoreceptor pair (Figure 5A). Moreover, when the presumptive R8 cell is marked with the R8-specific enhancer trap in sca (Figure 5B), the enhancer trap is consistently expressed in one of the three Ro-expressing cells (Figure 5C). These data suggest that sens normally represses Ro in the differentiating R8 photoreceptor.
Figure 5. R8 Differentiation Requires senseless-Mediated Repression of Rough.
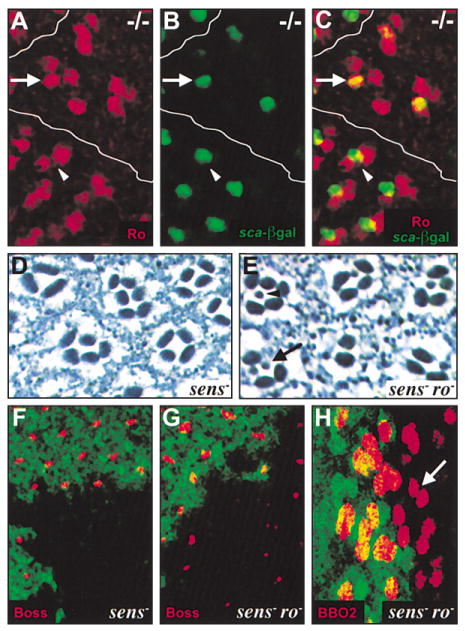
(A–C) sens mutant clones are positioned close to the MF and are marked with a white line. (A) Ro (red) is expressed outside the clone in two cells per ommatidium (arrowhead) but in three cells per ommatidium within the clone (arrow). (B) The R8-specific enhancer trap in sca (green) is expressed in one cell per ommatidium within the clone (arrow) and marks the presumptive R8 photoreceptor (see Figure 3A). (C) Merge of (A) and (B). The R8-specific enhancer trap in sca (green) and Ro (red) colocalize (yellow) in one cell per ommatidium within the clone (arrow) but not in the wild-type tissue (arrowhead), indicating that Ro is expressed inappropriately in the presumptive R8 photoreceptor. (D and F) sens mutant clone. (E, G, and H) sens mutant clone induced in a roX63 mutant background. (D) No photoreceptors with small rhabdomeres are present. (E) Photoreceptors with small rhabdomeres are detected. Some clusters have only one photoreceptor with a small rhabdomere in a given section (arrowhead), but others have multiple photoreceptors with small rhabdomeres (arrow). (F–H) Clones are negatively marked with GFP (green). (F) Boss (red) is not expressed within the clone. (G) Boss expression (red) is restored within the clone. (H) Expression of BBO2, an R8-specific enhancer trap (red), is restored within the clone. In some cases, BBO2 is detected in more than one cell per ommatidium (arrow).
As ro is not normally expressed in R8 (Figure 1F) (Dokucu et al., 1996; Kimmel et al., 1990), and misexpression of Ro induces changes in cell fate (Kimmel et al., 1990), we hypothesized that Ro misexpression in the presumptive R8 cell is responsible for the loss of R8 observed in sens mutant clones. To test whether ro is epistatic to sens, we generated sens mutant clones in a ro mutant background. We found that many ommatidia mutant for both sens and ro contain photoreceptors with small rhabdomeres, suggesting the presence of either R8 or R7 photoreceptors (Figure 5E). Furthermore, some double mutant ommatidia contain more than one photo-receptor with a small rhabdomere, similar to the ro mutant phenotype (Figure 5E). To determine if the photoreceptors with small rhabdomeres were R8 cells, we looked at the expression of two late markers of R8 differentiation, Boss, and an R8-specific enhancer trap, BBO2, in sens mutant clones generated in a ro mutant background. We found that while Boss is never expressed in tissue mutant only for sens (Figure 3D and 5F), Boss expression is restored in many ommatidia in the double mutant tissue (Figure 5G). BBO2 expression is also restored in the double mutant tissue, sometimes in two photoreceptors within the same cluster, suggesting the presence of more than one R8 photoreceptor (Figure 5H). Thus, inappropriate Ro expression in the presumptive R8 cell is likely responsible for the early abortion of R8 differentiation and adoption of the R2/ R5 fate in sens mutant ommatidia.
Since sens-mediated repression of ro plays a critical role in R8 differentiation, we tested if sens misexpression was sufficient to repress ro and induce additional photoreceptors to adopt the R8 fate. sens was misexpressed in clones posterior to the MF using a variation on the MARCM system (Table 3; Experimental Procedures) (Lee and Luo, 1999). Within sens misexpression clones, Ro is repressed in those cells showing the highest levels of Sens (Figures 6A–6D). sens misexpression is also sufficient to induce Boss expression in multiple cells per ommatidium and at higher levels than it is normally expressed (Figures 6E–6H). Finally, in the adult retina, misexpression of sens causes ommatidial disruption near the center of the clone and induces the formation of ectopic small rhabdomeres within ommatidia near the clonal border (Figures 6I and 6J). Thus, sens is capable of repressing Ro when misexpressed, and is sufficient to induce both R8-specific gene expression and rhabdomere morphology.
Table 3. Fly Strains.
| Genotype | Source |
|---|---|
| Canton S | Bloomington Stock Center |
| w; sensE2 FRT80B/TM6B | This work |
| w; sensE1 FRT80B/TM6B | This work |
| w; roX63 | (Kimmel et al., 1990) |
| w; ato1 | (Jarman et al., 1995) |
| w; FRT82 ato1 | (Chen and Chien, 1999) |
| BBO2 enhancer trap (R8) | (Hart et al., 1990) |
| rI234 enhancer trap (sca) | G. Rubin |
| rM104 enhancer trap (R2/R5) | U. Gaul |
| UAS-ato (1) | (Jarman and Ahmed, 1998) |
| y w; UAS-sens (c5) | (Nolo et al., 2000) |
| w; sensE2 FRT80B rM104/TM6B | This work |
| w; rI234; sensE2 FRT80B/TM6B | This work |
| y w hsFLP122; P{w+mC = arm-lacZ} FRT40A | (Vincent et al., 1994) |
| y w hsFLP122; FRT82 P{w+mC=arm-lacZ} | (Vincent et al., 1994) |
| w; P{y+}66E P{w+}70C FRT80B/TM3 | J. Treisman |
| w; P{w+mC=ubi-GFP}61EF FRT80B/TM3 | (Davis et al., 1995) |
| w; M(3)i(55) P{w+}70C, FRT80B/TM6B | (Newsome et al., 2000) |
| y w hsFLP122; P{w+mC=ubi-GFP}61EF M(3)i(55) P{w+}70C FRT80B/TM6B | This work |
| y w hsFLP122; M(3)i(55) P{w+mC=arm-lacZ}70C FRT80B/TM6B | This work |
| w; sensE2 FRT80B roX63/TM6B | This work |
| y w hsFLP122; P{w+mC=ubi-GFP}61EF, M(3)i(55) P{w+}70C FRT80B roX63/TM6B | This work |
| hsFLP122 P{w+mW.hs=GawB}elavC155 P{w+mC=UAS-mCD8::GFP}; P{w+mC=tubP-GAL80} FRT40A | (Lee and Luo, 1999) |
Figure 6. Rough Is Repressed and Ectopic R8 Photoreceptors Are Induced by Misex-pression of senseless.
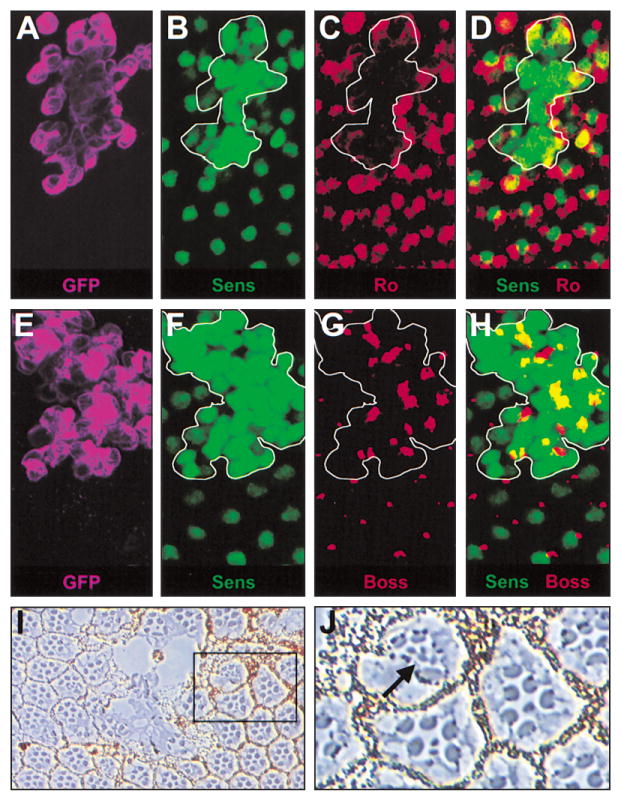
(A–J) A variation of the MARCM system was used to generate clones of tissue misexpressing sens posterior to the MF in an otherwise wild-type background (Experimental Procedures). (A and E) Clone boundaries are marked by membrane-associated GFP (purple). (B, D, F, and H) Sens (green) expression is induced within the clones at a high level compared to wild-type levels. (A–D) Ro (red) is expressed at normal levels and in its expected pattern outside of the clone but is reduced where Sens expression is highest within the clone (white border). (E–H) Boss is expressed in a single cell per ommatidium outside of the clone but is detected in additional cells per ommatidium and at greatly increased levels where sens is misexpressed (white border). (I) Misexpression of sens disrupts ommatidia in the center of the clone. (J) Near the border of the clone, ommatidia containing multiple photoreceptors with small rhabdomeres are detected.
Discussion
In Drosophila, R8 photoreceptor differentiation is a highly regulated process that requires complex interactions among many positively and negatively acting factors. However, despite the importance of R8, both in the Drosophila eye and as a model for the study of nervous system development, the molecular mechanism controlling R8 differentiation has not been well established. We demonstrate that R8 differentiation requires sens-mediated repression of ro, an eye-specific repressor of R8 differentiation, and thus identify the mechanism of repression of a cell fate repressor as an essential component of the process of R8 differentiation. Moreover, we show that when R8 differentiation fails within sens mutant ommatidia, the presumptive R8 cell inappropriately expresses Ro and differentiates as an R2/R5 photoreceptor. Despite adoption of the R2/ R5 fate by the presumptive R8 cell, all subsequent outer photoreceptor subtypes can be recruited, demonstrating that non-R8 photoreceptors can substitute for R8 in recruitment, and ruling out all models for outer photoreceptor subtype specification that involve R8-specific signaling, either in the form of spatial cues or ligand/ receptor interactions.
sens-Mediated Repression of ro Is Essential for R8 Differentiation
We have demonstrated that sens lies downstream of ato and that sens expression, which begins in the R8 equivalence group, is maintained in R8 at least through the completion of larval development (Figures 1B–1E). Consistent with this expression pattern, there is a cell-autonomous requirement in R8 for sens, but no requirement for sens in any other photoreceptor (Figure 2). Interestingly, in sens mutant ommatidia, a presumptive R8 photoreceptor is selected, but it appears that R8 differentiation does not occur in the majority of ommatidia, and in the minority of ommatidia where R8 differentiation may initiate, the process is quickly aborted within the MF (Figures 3A–3D). As R8-specific gene expression is never observed or is rapidly lost in sens mutant tissue, sens function is thus required to ensure proper R8 differentiation and to maintain the R8 fate immediately following the stage of the R8 equivalence group, but is not required for R8 selection.
In sens mutant ommatidia, the presumptive R8 cell rapidly expresses R2/R5-specific genes and adopts the fate of an R2/R5 photoreceptor in essentially all cases (Figures 4A, 4B, and 5A–5C). One R2/R5-specific marker that is abnormally expressed in the presumptive R8 within sens mutant ommatidia is Ro. Previous work has established that ro acts as a repressor of R8 differentiation and that Ro is not normally expressed in R8 (Dokucu et al., 1996; Jarman et al., 1994; Kimmel et al., 1990; Tomlinson et al., 1988). The consistent misexpression of Ro within the presumptive R8 photoreceptor in sens mutant tissue suggests that sens represses Ro in R8 (Figures 5A–5C). Such a relationship between sens and ro is further supported by the observation that sens misexpression causes the repression of Ro in outer photoreceptors (Figures 6A–6D). Moreover, it is clear that repression of Ro in R8 is of functional significance because loss of ro function is sufficient to rescue the R8 loss observed in sens mutant clones (Figures 5D–5H). These data imply that ro is epistatic to sens and that sens-mediated repression of Ro is essential for R8 differentiation. Thus, we identify repression of a cell-fate repressor as a major mechanism of R8 differentiation. Our findings are also consistent with the observations that sens acts as a repressor in the Drosophila CNS (Nolo et al., 2000) and that the sens homologs Gfi-1 (murine) and pag3 (C. elegans) function as repressors as well (Grimes et al., 1996; Jia et al., 1997).
In addition to its role as a repressor of R8 differentiation, ro has previously been demonstrated to be sufficient to induce changes in subtype specification, and it is thought that ro acts downstream of photoreceptor recruitment to specify photoreceptor subtype identity as an R2/R5 cell (Kimmel et al., 1990). Moreover, it is clear that in ro mutant ommatidia the presumptive R2/ R5 photoreceptors adopt the fate of an R1, R3, R4, or R6 photoreceptor (Heberlein et al., 1991). Similarly, both loss- and gain-of-function experiments reveal that sens function is not required for establishment or maintenance of neural fate in the developing eye, but specifically for directing a cell to follow the R8 differentiation pathway (this work). Thus, sens and ro seem to have analogous roles in directing the specification of specific photoreceptor cell fates. The transcriptional and genetic relationships we have identified between sens and ro imply that the process of R8 differentiation involves a hierarchical interaction where sens normally represses ro to prevent both ro repression of R8 and ro induction of R2/R5. When sens function is removed, ro is abnormally expressed in the presumptive R8 cell and the R2/R5 fate is adopted (Figure 7A).
Figure 7. Regulation of Photoreceptor Differentiation in the Drosophila Eye.
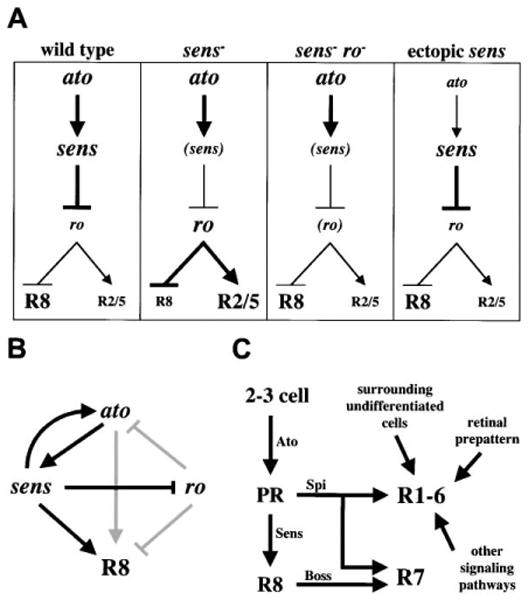
(A) Summary of genetic relationships controlling R8 differentiation. Wild-type R8: ato induces sens, which represses ro, resulting in R8 differentiation and failure of R2/R5 induction. sens mutant R8: ro is not repressed, resulting in R2/R5 induction and failure of R8 differentiation. sens, ro double mutant R8: despite the lack of sens, absence of ro results in R8 differentiation and failure of R2/R5 induction. Ectopic sens: misexpression of sens posterior to the morphogenetic furrow represses ro and induces ectopic R8 differentiation. (B) Model for the genetic regulation of R8 differentiation. Arrows in black indicate novel relationships identified in this paper while arrows in gray indicate those previously identified (see text for details).
(C) Model for non-R8 photoreceptor recruitment. ato function leads to selection of the presumptive R8 photoreceptor (PR). This PR secretes Spitz (Spi) to initiate the process of photoreceptor recruitment but does not need to differentiate as R8 to perform this task. Other factors act in coordination with Spi to induce specific photoreceptor fates. sens function is specifically required for the presumptive R8 cell to differentiate as R8. After R8 differentiates, Boss is expressed and interacts with Sevenless to induce R7 differentiation in conjunction with Spi activation of the EGFR.
We thus propose a new model for the genetic regulation of R8 differentiation that includes the relationships among ato, sens, and ro (Figure 7B). In this model, ato induces sens within the R8 equivalence group and R8, and sens is in turn required for maintenance of ato expression. As R8 may transiently differentiate in sens mutant clones, ato is likely sufficient to confer specificity to R8 differentiation, whereas sens is required to “lock-in” and maintain this program of R8 differentiation, primarily via the repression of ro. Thus, mutual antagonism of ato and ro is likely mediated by sens. sens presumably has a ro-independent role in R8 differentiation as well, as loss of ro function does not completely rescue the sens mutant phenotype. The relationship between ato and ro presented in this model has been established elsewhere (Dokucu et al., 1996).
Two of our findings suggest that while R8 differentiation and SOP selection are similar in principle, there are fundamental differences between the two. First, there is no direct evidence that repression of a repressor is a mechanism used during SOP selection to specify neuronal fates, while this mechanism is of great importance during R8 differentiation. Second, while the relationships between sens and proneural genes are maintained both in the developing eye and the emerging SOP, the sens loss-of-function phenotype is quite different in the eye and the embryonic nervous system. In the embryonic nervous system, loss of sens function results in cell death and complete neural loss. However, loss of sens function in the developing eye leads to altered cell fate decisions, but cells remain viable as neurons. These differences between the eye and the embryonic nervous system are not entirely unexpected, as successful R8 selection and differentiation hinges on the unique phenomenon of differential patterns of gene expression with the passage of the MF. For example, whereas the SOP is surrounded by largely equivalent cells on all sides as it is selected, the emerging R8 cell is surrounded by a graded environment–a field of R8 competent cells immediately anteriorly, equivalently staged regions of R8 differentiation dorsally and ventrally, and more mature ommatidia posteriorly. Moreover, cells in each of these environments exert specific effects upon the process of R8 differentiation (for examples, see Dokucu et al., 1996; Dominguez, 1999). The continued analysis of this unusual developmental strategy may thus unveil principles of nervous system development that are not accessible by the study of SOP development or other systems.
R8 Cell-Type-Specific Events Are Separable from Its Recruiting Function
Our analysis of sens mutant ommatidia reveals that the process of R8 differentiation fails very early in development. Despite this, recruitment and differentiation of outer photoreceptors occurs. These findings are unique and paradoxical because R8 is thought to initiate the recruitment of all other photoreceptors, although ato-independent photoreceptor differentiation has been observed (Dominguez et al., 1998; Freeman, 1994; Jarman et al., 1994; Sun et al., 2000; Tio et al., 1994; Tomlinson and Ready, 1987a). Moreover, loss-of-function mutations in all other genes known to be cell-autonomously required in R8 for normal eye development lead to the complete failure of photoreceptor recruitment (Freeman, 1994; Jarman et al., 1994; Tio et al., 1994; Tio and Moses, 1997; Wasserman et al., 2000). How can photoreceptor recruitment, a process known to require R8, occur without an R8 cell?
At present, R8 is believed to have two distinct functions in the process of photoreceptor recruitment. First, R8 is thought to recruit photoreceptors by providing the initial source of Spitz (Spi), a positive ligand for the EGFR during eye development (Freeman, 1994; Lesokhin et al., 1999; Tio et al., 1994). Spi activates the EGFR which induces Ras signaling and differentiation of all photoreceptors (except R8), cone cells, and pigment cells (Freeman, 1996; Kumar et al., 1998; Spencer et al., 1998; Tio and Moses, 1997). Second, R8 recruits the R7 photoreceptor via direct Boss/Sevenless interactions (reviewed in Cagan, 1993; Raabe, 2000; Zipursky and Rubin, 1994). The second function of R8 is clearly abrogated in sens mutant clones. This is expected because R7 is induced by physical contact with the Boss ligand, which is normally expressed solely on R8, and Boss is never expressed within sens mutant clones. However, the first function of R8, while somewhat compromised, is not eliminated.
Current models predict that after Spi is first secreted from R8, it binds to the EGFR and induces Ras signaling in two neighboring cells, the emerging R2 and R5 photo-receptors. Subsequently, Spi is also secreted from R2 and R5 (and later R3 and R4) and the increased Spi concentration leads to recruitment of all later photoreceptors. In one model, it is specifically the timing of induction of the EGFR pathway that determines photoreceptor subtype (Freeman, 1996). However, an equally plausible model for the recruitment of R2 and R5 (and perhaps later photoreceptors) is one that is similar to R8-mediated induction of R7, where both activation of the EGFR pathway by Spi and ligand/receptor interactions (Boss/Sevenless) are required together for induction of the R7 fate (Tio and Moses, 1997). We have found that all outer photoreceptor subtypes can be recruited in sens mutant ommatidia. As sens mutant ommatidia lack a differentiated R8 cell, our observations rule out all models for subtype specification that involve any R8-specific signaling, either in the form of spatial cues or ligand/receptor interactions. Other models that rely on timing, signaling from other photoreceptors (Wolff and Ready, 1993), retinal prepatterning (Dickson et al., 1992; Freeman, 1996), combinatorial signaling (Flores et al., 2000; Xu et al., 2000), the actions of surrounding undifferentiated cells (Huang and Fischer-Vize, 1996), or instructive signaling from the presumptive R8 prior to overt R8 differentiation remain possible, likely in combination with one another.
In sens mutant ommatidia, the selection of the presumptive R8 cell is not affected, but the presumptive R8 differentiates as an R2/R5 cell at approximately the same time as R8 would normally differentiate. Thus, it is likely that while the identity of the cell initially producing Spi is different in the absence of sens function, the timing of initiation of Spi secretion remains more or the less the same. As all photoreceptors are recruited, it therefore appears that an R2 or R5 photoreceptor can largely fulfill the previously presumed function of R8 in outer photoreceptor recruitment. Thus, we have specifically demonstrated that R8 is dispensable for photoreceptor recruitment and it is likely that Spi produced from an alternate source (in this case R2/R5) at roughly the same time is entirely sufficient to initiate the process of recruitment. However, as fewer photoreceptors are recruited in sens mutant tissue, it is clear that activation of recruitment from this alternative source is suboptimal. Indeed, decreased levels of dpERK expression in sens mutant ommatidia reflect a reduction in Ras signaling, which is perhaps due to decreased secretion of Spi (Figures 4E–4G). Nevertheless, it is now certain that activation of the recruiting pathway mediated by Spi occurs independently of R8 differentiation (Figure 7C).
Experimental Procedures
Fly Strains and Clonal Analysis
Fly strains used in this work are indicated in Table 3. Clonal analysis was conducted with the sensE2 null allele (Nolo et al., 2000) and findings were confirmed with sensE1, a second null allele (R.N. and H.B., unpublished data). In all cases tested, the phenotypes of the two alleles are identical. To induce sens mutant clones in the eye imaginal disc, we used FLP-mediated mitotic recombination in a Minute background to enhance the size of the clones (Golic and Lindquist, 1989; Morata and Ripoll, 1975; Xu and Rubin, 1993). First instar larvae from a cross between w; sensE2 FRT80B/TM6B and either y w hsFLP122; P{y+}66E P{w+}70C FRT80B/TM3 (mosaic analysis); y w hsFLP122; P{w+mC=ubi-GFP}61EF M(3)i(55) P{w+} 70C FRT80B/TM6B (mosaic analysis, large adult clones, third instar analysis with enhancer trap lines); or y w hsFLP122; M(3)i(55) P{w+mC= arm-lacZ}70C FRT80B/TM6B (third instar analysis without enhancer trap lines) were heat shocked at 38°C for one hour. Mutant clones are marked by the absence of pigment granules in adults and by the loss of either a ubi-GFP reporter or an arm-lacZ reporter in imaginal discs (Davis et al., 1995; Vincent et al., 1994). Analysis of sens mutant clones in a ro mutant background was carried out as above but with progeny of a cross between the following stocks: w; sensE2 FRT80B roX63/TM6B and y w hsFLP122; P{w+mC=ubi-GFP}61EF, M(3)i(55) P{w+}70C FRT80B roX63/TM6B.
Misexpression of sens employed a variation on the MARCM system in which clones were generated that lacked the tubulin-GAL80 repression construct but did not contain any mutations (Lee and Luo, 1999). y w; UAS-sens (c5) females were crossed to y w hsFLP122; P{w+mC=arm-lacZ} FRT40A males to generate males of the following genotype: y w; P{w+mC=arm-lacZ} FRT40A/+; UAS-sens/+. These males were then crossed to hsFLP122 P{w+mW.hs=GawB}elavC155 P{w+mC=UAS-mCD8::GFP}; P{w+mC=tubP-GAL80} FRT40A females and first instar larvae were subjected to a one hour heat shock at 38°C. Within the clone, GAL80 repression of GAL4 is lost, and both UAS constructs are expressed. The elavC155 GAL4 driver is not neural-specific in the developing eye imaginal disc and is expressed in a ubiquitous pattern posterior to the MF.
Antibody Staining, Adult Retina Preparation, and Microscopy
The following primary antibodies were used: guinea pig anti-Sens (1:800); rabbit anti-Ato (1:5000), mouse anti-Ro (1:100), mouse anti-Sca (1:200), mouse anti-Boss (1:2000), rabbit anti-Sal (1:100), rabbit anti-BarH1 (1:100), mouse anti-dpERK (1:250) (Sigma), rabbit anti-β-galactosidase (1:500) (Cappel), mouse anti-β-galactosidase (1:1000) (Promega), and rabbit and mouse anti-GFP (1:1000) (Molecular Probes). Conjugated goat anti-mouse, rabbit, and guinea pig fluorescent secondary antibodies were ALEXA 488 (Molecular Probes), Cy3 (Jackson Immunochemicals), or Cy5 (Jackson Immunochemicals).
Eye imaginal discs from wandering third instar larvae were dissected in cold PBS (0.1 M phosphate [pH 7.2], 150 mM NaCl). Discs were then fixed in PEMF (0.1 M PIPES [pH 7.0], 1 mM MgSO4, 2 mM EGTA + 4% formaldehyde, diluted from 16% Methanol Free Ultrapure Formaldehyde [Polysciences, Inc.]) for Sens, Ro, Sal, BarH1, β-galactosidase and GFP or in PLP (2% paraformaldehyde, 10 mM NaIO4, 75 mM lysine, 3.5 mM NaPO4 [pH 7.2]) for Boss, dpERK, β-galactosidase, and GFP. All fixes were for 20 min on ice. Discs fixed in PEMF were then permeabilized with PAXD (PBS containing 1% BSA, 0.3% Triton X-100, and 0.3% sodium deoxycholate) two times for 20 min on ice and then once for 20 min on ice with PAXDG (PAXD containing 5% normal goat serum) and incubated overnight at 4°C in primary antibody in PAXDG. Discs were washed three times for 20 min on ice in PAXDG and incubated in secondary antibody in PAXDG for 1–2 hr at 4°C. Discs were then washed at room temperature in PAXDG, in PAXD, and in PBS (once for 10 min each). After postfixing in PEMF for 15 min at room temperature, discs were washed twice in PBS and equilibrated in Vectashield for a minimum of 3 hr before mounting. Discs fixed in PLP were washed in 0.1 M sodium phosphate (pH 7.2) containing 0.1% Saponin and 5% normal goat serum. This buffer was used to dilute both primary and secondary antibodies and for all washes with times as above. Conditions for anti-Ato and anti-Sca were as described (Lee et al., 1996). Images were captured with a Zeiss LSM 510 confocal microscope. Adult eyes were fixed, embedded, and sectioned as previously described (Tomlinson and Ready, 1987b). All images were processed with Adobe Photoshop software.
Acknowledgments
We thank Nick Baker, Ulrike Heberlein, Andrew Jarman, Cheng-Ting Chien, Ulrike Gaul, Larry Zipursky, Gerry Rubin, Barry Dickson, Jessica Treisman, Ron Davis, Kevin Moses, Kwang Choi, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for providing fly stocks, antibodies, and protocols. We thank especially Bassem Hassan and Georg Halder for comments on the manuscript and members of the Mardon lab for support and advice. We also thank Sam Wu for gracious use of the confocal microscope. B.J.F. is supported by a grant from the McNair Foundation. This work was supported by funds awarded to G.M. from the National Eye Institute (R01 EY11232-01), the Retina Research Foundation, and the Moran Foundation, and an Ophthalmology Core Grant from the National Eye Institute (EY02520).
References
- Baker NE, Rubin GM. Ellipse mutations in the Drosophila homologue of the EGF receptor affect pattern formation, cell division, and cell death in eye imaginal discs. Dev Biol. 1992;150:381–396. doi: 10.1016/0012-1606(92)90250-k. [DOI] [PubMed] [Google Scholar]
- Baker NE, Zitron AE. Drosophila eye development: Notch and Delta amplify a neurogenic pattern conferred on the morphogenetic furrow by scabrous. Mech Dev. 1995;49:173–189. doi: 10.1016/0925-4773(94)00314-d. [DOI] [PubMed] [Google Scholar]
- Baker NE, Mlodzik M, Rubin GM. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990;250:1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–1301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- Cagan R. Cell fate specification in the developing Drosophila retina. Dev Suppl. 1993:19–28. [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Chen CK, Chien CT. Negative regulation of atonal in proneural cluster formation of Drosophila R8 photoreceptors. Proc Natl Acad Sci USA. 1999;96:5055–5060. doi: 10.1073/pnas.96.9.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P, de Celis JF, Campuzano S, Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991;5:996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Culi J, Modolell J. Proneural gene self-stimulation in neural precursors—an essential mechanism for sense organ development that is regulated by Notch signaling. Genes Dev. 1998;12:2036–2047. doi: 10.1101/gad.12.13.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I, Girdham CH, O'Farrell PH. A nuclear GFP that marks nuclei in living Drosophila embryos; maternal supply overcomes a delay in the appearance of zygotic fluorescence. Dev Biol. 1995;170:726–729. doi: 10.1006/dbio.1995.1251. [DOI] [PubMed] [Google Scholar]
- Dickson B, Sprenger F, Hafen E. Prepattern in the developing Drosophila eye revealed by an activated torso—sevenless chimeric receptor. Genes Dev. 1992;6:2327–2339. doi: 10.1101/gad.6.12a.2327. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Zipursky SL, Cagan RL. atonal, rough and the resolution of proneural clusters in the developing Drosophila retina. Development. 1996;122:4139–4147. doi: 10.1242/dev.122.12.4139. [DOI] [PubMed] [Google Scholar]
- Dominguez M. Dual role for Hedgehog in the regulation of the proneural gene atonal during ommatidia development. Development. 1999;126:2345–2353. doi: 10.1242/dev.126.11.2345. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Wasserman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Curr Biol. 1998;8:1039–1048. doi: 10.1016/s0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- Flores GV, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U. Combinatorial signaling in the specification of unique cell fates. Cell. 2000;103:75–85. doi: 10.1016/s0092-8674(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Freeman M. The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech Dev. 1994;48:25–33. doi: 10.1016/0925-4773(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Grimes HL, Gilks CB, Chan TO, Porter S, Tsichlis PN. The Gfi-1 protooncoprotein represses Bax expression and inhibits T-cell death. Proc Natl Acad Sci USA. 1996;93:14569–14573. doi: 10.1073/pnas.93.25.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AC, Kramer H, Van Vactor DL, Jr, Paidhungat M, Zipursky SL. Induction of cell fate in the Drosophila retina: the bride of sevenless protein is predicted to contain a large extracellular domain and seven transmembrane segments. Genes Dev. 1990;4:1835–1847. doi: 10.1101/gad.4.11.1835. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Mlodzik M, Rubin GM. Cell-fate determination in the developing Drosophila eye: role of the rough gene. Development. 1991;112:703–712. doi: 10.1242/dev.112.3.703. [DOI] [PubMed] [Google Scholar]
- Huang Y, Fischer-Vize JA. Undifferentiated cells in the developing Drosophila eye influence facet assembly and require the Fat facets ubiquitin-specific protease. Development. 1996;122:3207–3216. doi: 10.1242/dev.122.10.3207. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Ahmed I. The specificity of proneural genes in determining Drosophila sense organ identity. Mech Dev. 1998;76:117–125. doi: 10.1016/s0925-4773(98)00116-6. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369:398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- Jia Y, Xie G, McDermott JB, Aamodt E. The C. elegans gene pag-3 is homologous to the zinc finger proto-oncogene gfi-1. Development. 1997;124:2063–2073. doi: 10.1242/dev.124.10.2063. [DOI] [PubMed] [Google Scholar]
- Kimmel BE, Heberlein U, Rubin GM. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Green SM. Cell lineage in the developing retina of Drosophila. Dev Biol. 1979;71:142–152. doi: 10.1016/0012-1606(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Lee EC, Hu XX, Yu SY, Baker NE. The scabrous gene encodes a secreted glycoprotein dimer and regulates proneural development in Drosophila eyes. Mol Cell Biol. 1996;16:1179–1188. doi: 10.1128/mcb.16.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu SY, Baker NE. The scabrous protein can act as an extracellular antagonist of notch signaling in the Drosophila wing. Curr Biol. 2000;10:931–934. doi: 10.1016/s0960-9822(00)00622-9. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lesokhin AM, Yu SY, Katz J, Baker NE. Several levels of EGF receptor signaling during photoreceptor specification in wild-type, Ellipse, and null mutant Drosophila. Dev Biol. 1999;205:129–144. doi: 10.1006/dbio.1998.9121. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Powell PA, Wesley C, Spencer S, Cagan RL. Scabrous complexes with Notch to mediate boundary formation. Nature. 2001;409:626–630. doi: 10.1038/35054566. [DOI] [PubMed] [Google Scholar]
- Raabe T. The sevenless signaling pathway: variations of a common theme. Biochim Biophys Acta. 2000;1496:151–163. doi: 10.1016/s0167-4889(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Carroll SB. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991;5:984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
- Spencer SA, Powell PA, Miller DT, Cagan RL. Regulation of EGF receptor signaling establishes pattern across the developing Drosophila retina. Development. 1998;125:4777–4790. doi: 10.1242/dev.125.23.4777. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jan LY, Jan YN. Ectopic Scute induces Drosophila ommatidia development without R8 founder photoreceptors. Proc Natl Acad Sci USA. 2000;97:6815–6819. doi: 10.1073/pnas.110154497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio M, Moses K. The Drosophila TGF-alpha homolog spitz acts in photoreceptor recruitment in the developing retina. Development. 1997;124:343–351. doi: 10.1242/dev.124.2.343. [DOI] [PubMed] [Google Scholar]
- Tio M, Ma C, Moses K. spitz, a Drosophila homolog of transforming growth factor-alpha, is required in the founding photoreceptor cells of the compound eye facets. Mech Dev. 1994;48:13–23. doi: 10.1016/0925-4773(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Neuronal differentiation in the Drosophila ommatidium. Dev Biol. 1987a;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987b;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Kimmel BE, Rubin GM. rough, a Drosophila homeobox gene required in photoreceptors R2 and R5 for inductive interactions in the developing eye. Cell. 1988;55:771–784. doi: 10.1016/0092-8674(88)90133-x. [DOI] [PubMed] [Google Scholar]
- Van Doren M, Powell PA, Pasternak D, Singson A, Posakony JW. Spatial regulation of proneural gene activity: auto- and cross-activation of achaete is antagonized by extramacrochaetae. Genes Dev. 1992;6:2592–2605. doi: 10.1101/gad.6.12b.2592. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Girdham CH, O'Farrell PH. A cell-autonomous, ubiquitous marker for the analysis of Drosophila genetic mosaics. Dev Biol. 1994;164:328–331. doi: 10.1006/dbio.1994.1203. [DOI] [PubMed] [Google Scholar]
- Wasserman JD, Urban S, Freeman M. A family of rhomboid-like genes: Drosophila rhomboid-1 and roughoid/rhomboid-3 cooperate to activate EGF receptor signaling. Genes Dev. 2000;14:1651–1663. [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1326. [Google Scholar]
- Xu C, Kauffmann RC, Zhang J, Kladny S, Carthew RW. Overlapping activators and repressors delimit transcriptional response to receptor tyrosine kinase signals in the Drosophila eye. Cell. 2000;103:87–97. doi: 10.1016/s0092-8674(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yang L, Baker NE. Role of the EGFR/Ras/Raf pathway in specification of photoreceptor cells in the Drosophila retina. Development. 2001;128:1183–1191. doi: 10.1242/dev.128.7.1183. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–397. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]


