Abstract
Summary: A syndemic is defined as the convergence of two or more diseases that act synergistically to magnify the burden of disease. The intersection and syndemic interaction between the human immunodeficiency virus (HIV) and tuberculosis (TB) epidemics have had deadly consequences around the world. Without adequate control of the TB-HIV syndemic, the long-term TB elimination target set for 2050 will not be reached. There is an urgent need for additional resources and novel approaches for the diagnosis, treatment, and prevention of both HIV and TB. Moreover, multidisciplinary approaches that consider HIV and TB together, rather than as separate problems and diseases, will be necessary to prevent further worsening of the HIV-TB syndemic. This review examines current knowledge of the state and impact of the HIV-TB syndemic and reviews the epidemiological, clinical, cellular, and molecular interactions between HIV and TB.
INTRODUCTION
A syndemic is defined as the convergence of two or more diseases that act synergistically to magnify the burden of disease. The syndemic interaction between the human immunodeficiency virus (HIV) and tuberculosis (TB) epidemics has had deadly consequences around the world. This review examines current knowledge of the state and impact of the HIV-TB syndemic and reviews epidemiological, clinical, cellular, and molecular interactions between HIV and TB.
Scale of the Problem: Millions Affected and Millions of Lives Lost
HIV-associated TB contributes substantially to the burden of TB-associated morbidity and mortality. Of the estimated 33.4 million people living with HIV in 2008, nearly 30% were estimated to have latent or active TB infection (111, 249, 250, 262). Conversely, of the 9.4 million cases of incident TB worldwide, an estimated 1.4 million (15%) were coinfected with HIV in 2008 (250). HIV infection is the strongest known risk factor for TB. High HIV prevalence rates are significantly correlated with high TB incidence rates (Fig. 1) (263). The confluence of the two epidemics has hit hardest in sub-Saharan Africa, which constituted 79% of all cases of incident TB in persons with HIV infection in 2007 (62, 263). South Africa alone accounted for 24% of all incident HIV-associated TB cases worldwide in 2008, even though its estimated population is less than 1% of the global population (262). Of the 15 countries with the highest estimated TB incidence rates in 2007, over half were sub-Saharan African countries with HIV prevalence rates of over 10% in the general population (263).
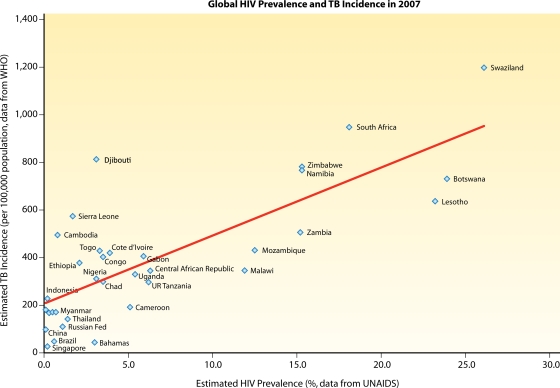
Fig. 1.
Higher HIV prevalence rates are associated with higher TB incidence rates. We used data from 132 countries from the UNAIDS/WHO 2008 report on the global AIDS epidemic for HIV prevalence (250) and from the WHO 2009 report on global tuberculosis control for TB incidence (263) and generated a scatter plot showing a positive linear correlation. The Pearson correlation coefficient (r) was 0.799, with a (two-tailed) P value of <0.01 using SPSS statistical software.
HIV-associated TB accounts for a disproportionate share of TB-associated mortality. In 2008, HIV-associated TB accounted for 29% of deaths among incident TB cases, even though it contributed to 15% of all incident TB cases (Fig. 2) (111, 263). The estimated case-fatality rate of incident TB was more than 2-fold higher for people infected with HIV (37%) than for those without HIV (16%) (Fig. 2) (111, 263). The higher case-fatality rate of TB in HIV-infected individuals is likely due to a combination of factors associated with HIV coinfection: (i) the rapid progression of disease due to the failure of immune responses to restrict the growth of Mycobacterium tuberculosis, (ii) delayed diagnosis and treatment of TB infection due to atypical presentation and lower rates of sputum smear positivity (33, 53, 122), (iii) delayed diagnosis of HIV infection due to stigma or insufficient uptake of HIV testing in TB clinics (122, 262), (iv) delayed start or lack of access to combination antiretroviral therapy (ART) (122), and (v) higher rates of multidrug-resistant (MDR) TB (MDR-TB) leading to a delayed initiation of effective therapy (104). Deaths due to TB accounted for one-quarter of the estimated 2 million HIV-related deaths worldwide in 2008, and TB is the leading cause of death for people living with HIV in low- and middle-income countries (155, 250, 262, 263). In 2007, the burden of deaths from HIV-associated TB was highest in South Africa, Nigeria, India, Zimbabwe, Ethiopia, the United Republic of Tanzania, Mozambique, Uganda, and Kenya and accounted for the majority of TB-associated mortality in most of these countries (Table 1) (262, 263).
Fig. 2.
HIV-associated TB contributes disproportionately to TB-related deaths. (Data are from WHO Global Tuberculosis Control: a Short Update to the 2009 Report [262].) Although HIV-associated TB accounted for 15% of all incident TB, it contributed to 29% of deaths among incident TB cases in 2008. The estimated case-fatality rate of incident TB was more than 2-fold higher for people infected with HIV (37%) than for those without HIV (16%).
Table 1.
Estimated burden of HIV and tuberculosis in 2007 in select countriesa
| Country | No. of incident TB cases | TB incidence rate (per 100,000 population per yr) | HIV prevalence in adult population (%) | HIV prevalence in incident TB cases (%) | No. of TB-related deaths in HIV-infected individuals | % HIV-infected individuals of all TB-related deaths |
|---|---|---|---|---|---|---|
| South Africa | 460,600 | 948 | 18.1 | 73 | 93,700 | 84 |
| Nigeria | 460,149 | 311 | 3.1 | 27 | 58,970 | 43 |
| India | 1,962,000 | 168 | 0.3 | 5.3 | 29,500 | 9 |
| Zimbabwe | 104,400 | 782 | 15.3 | 69 | 28,410 | 80 |
| Ethiopia | 314,267 | 378 | 2.1 | 19 | 23,280 | 31 |
| United Republic of Tanzania | 120,291 | 297 | 6.2 | 47 | 19,830 | 63 |
| Mozambique | 92,296 | 431 | 12.5 | 47 | 17,480 | 64 |
| Uganda | 101,785 | 330 | 5.4 | 39 | 16,110 | 56 |
| Kenya | 132,357 | 353 | NA | 48 | 14,590 | 60 |
Urban Population Growth May Escalate the HIV and TB Syndemic
In 2008, the composition of the world's population tipped such that the majority lived in urban areas instead of rural areas for the first time in history. Urban population growth in Africa and Asia is expected to drive the majority of future global population growth, with concomitant increases in slum areas and levels of urban poverty (251). In developing countries, the majority of urban populations live in slums: 72% of the urban population in sub-Saharan Africa and 56% in South Asia (251). Key features of slum life, such as crowded housing, working conditions with poor ventilation, poor nutrition, and lack of access to quality health care, continue to drive TB transmission (20, 209, 251). The association between poverty, urbanization, housing density, and TB incidence is well documented (27, 50, 164, 207). The level of poverty, as measured by the gross domestic product per capita, is directly related to the incidence of TB (133). Many socioeconomic determinants of TB are also drivers of risk behaviors for HIV transmission, such as injection drug use and commercial sex work. A prospective study from New York City found significantly higher rates of TB, AIDS, and death for substance users on welfare than for the general population of New York City (risk ratios of 15, 10, and 5, respectively) (100). In addition, HIV-infected persons living in resource-constrained settings face socioeconomic and behavioral barriers to HIV testing and access to antiretroviral treatment (16). Without adequate urban planning and investment in equitable urban health care systems, including integrated TB and HIV programs, the rise in slum areas and urban poverty will continue to propel the transmission of HIV-associated TB and its associated morbidity and mortality.
Population Mobility Can Shape the Dynamics of Transmission of HIV and TB
Globalization and increasing population mobility have shaped the HIV-TB syndemic. Annual global human migration is estimated to include approximately 84 million migrant workers, 51 million internally displaced persons (e.g., displaced by natural disasters and conflict), 17 million refugees and asylum seekers, 2.4 million immigrants, 2.1 million international students, and 924 million tourists or business travelers (167). Immigration can increase TB in populations with previously low TB prevalences. For example, in Madrid, the proportion of immigrants in a cohort of TB-HIV-coinfected patients increased from 8% in 1999 to 39% in 2006 (253). Likewise, in a French cohort of HIV-infected patients enrolled in care, over half of those with HIV-TB coinfection were immigrants, the majority from sub-Saharan Africa (5). Traditionally, reactivation TB was thought to be the cause of active TB in immigrants who originated from areas with high TB prevalences and who are now living in countries with low TB prevalences (26). However, cluster analyses in Spain suggested that recent transmission can be a significant cause of TB infections in immigrants (11, 174). While immigration can influence the dynamics of the HIV-TB syndemic, its effect is less marked than that of the smaller-scale internal migration of selected high-risk populations, such as migrant workers in South Africa. South African migrant workers who lived in overcrowded hostels and had sex with commercial sex workers were at a high risk of acquiring and transmitting HIV and TB in the cities where they worked, and they subsequently transmitted the infections to their wives and families during regular visits back to their hometowns (2). On the other hand, the impact of individuals displaced by conflicts and natural disasters, such as the 2010 Haiti earthquake, on the transmission dynamics of the HIV-TB syndemic remains unknown.
TB AND HIV: INTERACTIONS AT THE POPULATION LEVEL
Individuals with a new diagnosis of TB are nearly 19 times more likely to be coinfected with HIV than those without TB (0.8% HIV prevalence in adults aged 15 to 49 years and 15% HIV prevalence in incident TB cases) (249, 250). Conversely, people living with HIV are 20 to 30 times more likely to develop TB than those without HIV. The TB incidence rate ratio (IRR), the relative risk of TB developing in HIV-infected persons compared to that in HIV-uninfected persons, varies according to HIV prevalence. Countries with a generalized HIV epidemic have a TB IRR of 20.6. Countries with concentrated HIV epidemics (HIV prevalence, 0.1% to 1%) have a TB IRR of 26.7, and countries with a low prevalence of HIV infection (HIV prevalence less than 0.1%) have a TB IRR of 36.7 (111, 263). This apparently paradoxical inverse relationship between TB IRR and HIV prevalence likely depends on the interplay between local TB incidence and prevalence rates in the general population, the rate of detection of new TB cases, and other associations between HIV and TB transmission that increase the likelihood of coinfection (20, 62). For example, countries with a generalized HIV epidemic may have high rates of malnutrition and poverty, barriers to basic health care, and high rates of TB exposure (20), leading to a high cumulative lifetime risk for incident TB regardless of HIV status. Therefore, these countries may have less divergence in the incidence rates of TB in HIV-infected individuals compared with the general population. On the other hand, in countries with concentrated or low-prevalence HIV epidemics, the drivers of TB transmission may be more closely linked with specific risk factors for HIV infection (for example, living in grouped housing such as jails, shelters, and psychiatric wards and injection drug use), and thus, the incidence rate of TB infection in HIV-infected individuals is much higher than that in the general population.
HIV Is a Driver of the TB Epidemic
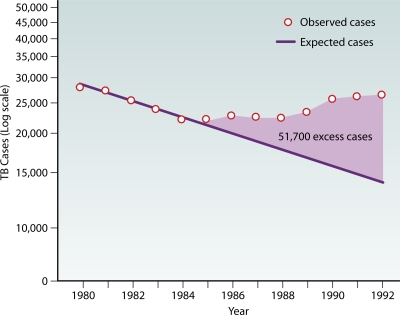
Evidence that HIV serves as a driver of TB at the population level has been noted by multiple epidemiological studies (15, 20). In the United States, numbers of observed TB cases had been declining from 1980 to 1985 but increased by 20% from 1985 to 1992, with an estimated 51,700 excess TB cases attributed to the growing HIV epidemic (Fig. 3) (44). The largest increases in rates of TB occurred in areas and populations heavily affected by the HIV epidemic at the time: New York (84%), California (54%), urban areas (29%), and the 25- to 44-year age group (55%). In San Francisco, the HIV epidemic contributed an additional 14% of TB cases from 1991 to 2002. Most of these cases were due to a reactivation of latent TB, although 41% were attributed to recent transmission (70). In England and Wales, nearly one-third of the increase in numbers of cases of TB from 1999 to 2003 occurred in HIV-infected patients (8). In South Africa, the burgeoning HIV epidemic was associated temporally with a worsening of the TB epidemic in a periurban community. As the HIV prevalence rate increased from 6.3% in 1996 to 22% in 2004, annual TB notification rates increased 2.5-fold, culminating in a staggering 1,468 TB cases per 100,000 persons in 2004. For each 1% increase in HIV prevalence, TB notification rates increased by 55 cases per 100,000 persons in 1998 to 1999 and increased by 81 cases per 100,000 persons in 2004 (154). Biologically, this association makes sense: increasing numbers of individuals immunocompromised by HIV infection lead to a larger reservoir of individuals susceptible to reactivation TB and result in more TB cases.
Fig. 3.
Estimated excess TB cases attributed to the worsening HIV epidemic in the United States from 1985 to 1992. (Reprinted from reference 44.)
Does HIV Alter the Transmission Dynamics of TB?
At the community level, the relationship between HIV and the transmission of TB as measured by the annual risk of TB infection (ARTI) is unclear. Traditionally, TB surveillance programs use the ARTI as an indicator to monitor TB transmission in the community (218). The ARTI is calculated as the prevalence of positive tuberculin skin tests (TSTs) in schoolchildren divided by their average age. One early study in Kenya found that the ARTI increased from 1986 to 1996 in a setting of high HIV prevalence, which suggested an increased rate of transmission of TB (202). However, another study in Tanzania found a decrease in the ARTI in the context of a rising HIV prevalence from 1983 to 2003 (81). A third study from South Africa found that the ARTI was unchanged from 1999 to 2005, while the HIV prevalence increased from 14% to 23% during that period (187).
In light of other studies showing HIV to be a driver of TB incidence, these conflicting findings may be due to the failure of the ARTI to accurately reflect ongoing TB transmission in the adult population. The use of the ARTI to monitor TB transmission in the community assumes that the TST positivity rate in children is proportional to the incidence rate of primary TB infection in children and that the TB incidence in children is reflective of TB transmission in the general population. The first assumption may be problematic in areas with widespread Mycobacterium bovis BCG vaccination or where M. bovis is endemic (218). The second assumption assumes generalizability despite a nonrepresentative sample selection, which may be problematic due to variable levels of social mixing between TB-HIV-coinfected adults and the young children used to determine the ARTI. In support of this, a South African study investigated the change in TB notification rates by age group from 1996 to 2004, a period in which adult HIV prevalence increased from 6% in 1996 to 22% in 2004. The burden of excess TB cases was greatest in adults; adolescents also experienced a significant rise in notified TB cases. However, children did not have a significant increase in TB notification rates despite the rise in HIV prevalence and excess TB cases in adults (154). Due to the limited generalizability, the ARTI may not be an accurate measure of TB transmission in the general population.
A more direct method to investigate the transmission of TB in HIV-infected patients is to evaluate close contacts of index cases. Although initial studies of close contacts of TB patients with HIV infection had conflicting results, more recent studies suggested that TB patients coinfected with HIV may be less infectious than TB patients without HIV infection. A prospective cohort study in the Dominican Republic of over 800 household contacts of 58 HIV-infected and 116 matched HIV-uninfected index cases with newly diagnosed smear-positive or culture-positive pulmonary TB found that HIV-infected index cases were half as likely as HIV-uninfected index cases to transmit TB to their close contacts, even after controlling for the degree of smear positivity (89). Similarly, in Brazil, a prospective cohort study of 360 contacts of 86 patients with smear-positive pulmonary TB found a significantly decreased risk of TST conversion in contacts of HIV-infected index cases, with an odds ratio (OR) of 0.24 (95% confidence interval [CI], 0.09 to 0.65) (36). A meta-analysis of eight studies comparing the prevalences of TST positivity among household contacts of TB index cases found a lower rate of TST positivity among contacts of HIV-infected than among contacts of HIV-uninfected index TB cases (64). A cross-sectional study evaluating the prevalence of positive TSTs in pediatric contacts of adults with TB and HIV infection in Botswana found a lower proportion of TST positivity in children exposed to adult index cases with CD4 counts of <200 cells/μl than in those exposed to index cases with CD4 counts of ≥200 cells/μl (145). In summary, HIV-infected patients with pulmonary TB are less likely than HIV-uninfected patients to transmit TB to their household contacts, and patients with advanced AIDS may be less infectious than patients with earlier stages of HIV infection. Possible explanations for the decreased transmission of TB by HIV-infected patients include less frequent cavitary TB, lower sputum bacillary burden, weakened cough with more severe disease, and greater social isolation.
With or without HIV, TB Infectiousness Is Highly Variable
The transmission of TB is a product of the infectiousness of the index case and the duration of infectiousness (80), both of which can be affected by HIV. Data at the population level obscure the extreme variability of infectiousness of TB in individuals. Seminal studies by Riley et al. in the 1950s revealed a marked variability in the infectiousness of TB patients. In these classical studies, guinea pigs were contained in a penthouse exposed to exhaust air from a TB ward, and their TST conversion was monitored. Only 3 of 77 patients were responsible for over 73% of TB infections in guinea pigs, and half of all TB infections in guinea pigs were caused by one patient with laryngeal TB (91). Similarly, Escombe et al. recreated the in vivo sampling model using guinea pigs housed above a mechanically ventilated HIV-TB ward in Lima, Peru (86, 87). Of the nearly 100 TB-HIV-coinfected inpatients, only 10 were responsible for all characterized cases of TB infections in the guinea pigs. In addition, of the 125 TB-infected guinea pigs for which TB drug susceptibility and spoligotype results were available, 98% were matched to the drug susceptibility and spoligotype patterns of TB from one single HIV-infected patient. TB infectiousness varies widely between different TB-HIV-coinfected patients and is correlated with proven determinants of TB transmission, such as sputum smear positivity, lung cavitation, laryngeal TB, cough frequency, and sputum volume and consistency (86, 87, 92, 165, 206). HIV can affect TB transmission dynamics by altering the traditional determinants of TB transmission. For example, in HIV-infected individuals, TB is more likely to manifest with a lower frequency of cavitation, as smear-negative pulmonary TB, or as extrapulmonary TB (discussed further below) (15, 33, 214).
HIV and the Mean Duration of Infectiousness of TB
A longer duration of infectiousness leads to higher rates of transmission of TB (80). Two studies, one of South African gold miners and one of workers in Harare, Zimbabwe, demonstrated a significantly shorter mean duration of infectiousness of TB in HIV-infected patients than in patients without HIV (58, 59). In a cross-sectional and longitudinal cohort study of over 1,600 South African gold miners recruited from their annual routine fitness-to-work examination from 2000 to 2001, Corbett et al. found a significantly shorter mean duration of infectiousness, defined as smear positivity, in HIV-infected individuals with TB (2 months) than in individuals with TB without HIV infection (14 months) (59). This difference was attributed to the higher rate of progression of TB disease and symptoms and the more rapid presentation and diagnosis of TB in gold miners with HIV infection in the absence of antiretroviral treatment. Similarly, a study of workers recruited from occupational health clinics in Harare found the mean duration of infectiousness of smear-positive pulmonary TB to be 1.5 months for HIV-infected patients, compared to 12 months for those without HIV (58). A subsequent community-based study of suburban Harare, where the HIV prevalence is 21%, estimated a mean duration of infectiousness of TB of 4.5 months for HIV-infected individuals, compared with nearly a year for HIV-uninfected individuals (57).
In contrast, a cross-sectional active case-finding survey of HIV and TB in a periurban slum community in South Africa found the estimated duration of undiagnosed and infectious TB to be slightly higher for HIV-infected persons (∼12 months) than for individuals without HIV (∼9 months) (270). Although the existing TB control program detected 67% of smear-positive TB cases in persons without HIV, it performed poorly for the detection of TB in HIV-infected persons. Of the HIV-infected persons with smear-positive pulmonary TB identified by the survey during a 4-month period in 2005, only 33% had been identified through the existing TB control program's passive case-reporting system. Thus, the longer duration of infectious TB in HIV-infected persons was driven mainly by a higher proportion of undiagnosed TB in HIV-infected persons, likely as a result of increased barriers to care for HIV-infected persons in this periurban slum community.
Taken together, these studies suggest that the mean duration of infectiousness of TB can be markedly affected by access to care and rapidity of diagnosis (80). Where HIV-infected individuals have access to care and are enrolled in a care program and/or where active TB case finding exists, the mean duration of infectiousness of TB is shorter than that of TB in persons without HIV due to a combination of a higher rate of progression, earlier presentation to the health care system, and more rapid diagnosis. The combination of the absence of available care, socioeconomic barriers to care, and passive TB case finding is associated with a longer duration of undiagnosed and infectious TB in both HIV-infected and -uninfected persons, which is more detrimental to HIV-infected persons, who die from TB more rapidly.
In summary, HIV is a driver for TB epidemics by increasing the incidence of TB and TB-related deaths in a population of immunodeficient individuals susceptible to both primary and reactivation TB. However, it remains unclear how much HIV-associated TB contributes to the transmission of TB in the community. HIV may decrease the infectiousness of TB due to a lower likelihood of cavitary disease and higher frequency of smear-negative pulmonary TB and extrapulmonary TB, which lower the mycobacterial load in the sputum. HIV may also decrease the mean duration of infectiousness of TB because of earlier presentation and diagnosis and shorter time to death in persons infected with HIV. On the other hand, barriers to health care access are associated with a longer mean duration of infectiousness of TB in HIV-infected persons. The transmission of TB from HIV-uninfected TB cases, who are more likely to have smear-positive and cavitary pulmonary TB and a longer duration of infectiousness, to susceptible HIV-infected individuals clearly serves as a major driving force for the HIV-TB syndemic (61).
Hospitals, Clinics, and Prisons Have Fueled the Syndemic
Poorly ventilated enclosed facilities, such as hospitals, clinics, and prisons, where people congregate or stay and share the same air can promote the transmission of TB. That hospitals and prisons with poor or inconsistent infection control practices can serve as locations for the spread of TB to persons infected with HIV was documented in the early 1990s in New York and Florida (38, 41, 43, 97, 98). Undiagnosed TB can be highly prevalent in reception areas of clinics where susceptible HIV-infected patients intermingle. In Port-au-Prince, Haiti, a cross-sectional study of 28,261 patients at an HIV voluntary counseling and testing (VCT) center found that 3,708 (13%) patients reported having a cough for at least 5 days, and of these patients, 925 (25%) were diagnosed with suspected or confirmed TB by sputum acid-fast bacillus (AFB) smear, culture, or chest radiography (138). Another study from Santo Domingo, Dominican Republic, found that almost 10% of individuals presenting for HIV testing at a VCT center had undiagnosed active TB (90). In resource-poor settings, the potent mix of high TB prevalence and high HIV prevalence, inconsistent infection control practices, delayed diagnoses, and crowding in poorly ventilated clinics, hospitals, and prisons make such facilities important sites of disease transmission. The role of exogenous infection and nosocomial transmission has been noted in the spread of multidrug-resistant (MDR) TB and extensively drug-resistant (XDR) TB (XDR-TB) in HIV-infected individuals in Tugela Ferry, a rural area of South Africa (12, 103). Together, these studies highlight the need for effective infection control and ventilation as components of comprehensive strategies to prevent the spread of TB and drug-resistant TB to HIV-infected patients in waiting rooms, clinics, and hospital wards (18, 268).
In the United States, the latest CDC guidelines recommend the use of airborne precautions with airborne infection isolation (AII) negative-pressure rooms with at least 12 air changes per hour (ACH) and fitted N95 masks as part of the package of interventions to prevent the transmission of TB in health care settings and correction and detention facilities (39, 42). However, respiratory masks may not be used in a timely fashion due to delayed diagnosis, and negative-pressure ventilation rooms may not be adequately maintained, even if available (88, 268). A study from Lima, Peru, measured air changes per hour and estimated the TB transmission risk for a variety of hospital rooms, including “old-fashioned” rooms with large open windows and high ceilings (built pre-1950), “modern” hospital rooms (built in 1970 to 1990), and more recently constructed mechanical ventilation negative-pressure isolation rooms (built post-2000). Surprisingly, naturally ventilated “old-fashioned” rooms with open windows and doors fared best, with 40 ACH on average, followed by naturally ventilated “modern” rooms, with 17 ACH on average. The worst performers were mechanically ventilated negative-pressure rooms, with an average ACH well below the minimum 12 ACH recommended. In these mechanical ventilation isolation rooms, air extraction and supply fans were found to be unprotected by filters and had poorly maintained motors and corroded fan blades. The calculated risk of TB transmission was three times higher in mechanical ventilation rooms than in “old-fashioned” natural ventilation rooms with large open windows, open doors, and high ceilings. This study highlights the limitations of importing technology from developed countries without first adapting to the realities of a resource-poor setting and provides evidence that natural ventilation may be a cost-effective way to reduce the risk of nosocomial transmission of TB (88).
TB AND HIV: INTERACTIONS AT THE LEVEL OF THE INDIVIDUAL
Effect of Tuberculosis on HIV
Preliminary data from observational and retrospective studies have suggested that TB accelerates the progression of disease in HIV (13, 168, 259). A retrospective study of over 200 patients found a higher mortality rate and a higher incident rate of new AIDS-defining opportunistic infections for HIV-TB-coinfected patients than for HIV-infected patients without active TB who were matched for CD4 cell counts (259). A prospective observational study for South Africa found higher rates of non-TB AIDS-defining illnesses and higher mortality rates for HIV-infected patients with TB than for HIV-infected patients without TB. The difference in survival was significant with higher CD4 counts (>200 cells/μl) and most pronounced with CD4 counts of >400 cells/μl (13). Similarly, a prospective cohort study in Uganda that evaluated the impact of TB on the survival of HIV-infected patients found a 3-fold-higher risk of death for patients whose CD4 counts were greater than 200 cells/μl but did not find a significant effect of TB on mortality for patients with advanced immunodeficiency (260). Whether TB accelerates the progression of disease in HIV remains unproven, as observational data do not establish causality (60). However, there are additional observations that support the hypothesis that TB accelerates the virologic course of HIV (69).
The development of TB is associated with increased HIV replication, as measured by the viral load at the site of TB infection in the lungs as well as by the plasma viral load (246, 247). As described in more detail below, M. tuberculosis activates HIV transcription and enhances viral entry in vitro (55). The heterogeneity of HIV harvested from pulmonary segments infected with TB is higher than that from segments without TB, which is suggestive of higher levels of replication at sites of TB infection (55). However, this finding may be due to the effect of inflammation on HIV replication rather than a specific effect of TB on HIV. Additionally, observational data have shown a higher level of systemic HIV heterogeneity in HIV-TB-coinfected patients than in CD4-matched HIV-infected patients without active pulmonary TB (55). It is thought that the increased proinflammatory response with active TB plays a role in increasing HIV transcription, as higher levels of tumor necrosis factor alpha (TNF-α) are associated with higher viral loads in patients coinfected with HIV and TB (245). On the other hand, the treatment of active TB did not show a significant difference of CD4 counts or HIV viral load in a prospective cohort of 111 TB-HIV-coinfected patients in South Africa (197).
Effect of HIV on Tuberculosis: Increased Susceptibility and Accelerated Progression
The relative risk of TB doubles in the first year after HIV infection, when CD4 counts are still preserved, and continues to increase during the years after seroconversion as CD4 counts decrease (234). HIV increases the risk of progression to active TB in both primary TB infection and the reactivation of latent TB. In populations of immunocompetent people, 3 to 5% will develop active TB in the first 2 years after TB infection (99, 188, 219, 228). HIV coinfection impairs the ability of the immune response to contain TB (discussed further below) and increases the likelihood of developing active TB during the initial period of TB infection (99). During a TB outbreak in an HIV housing facility in San Francisco, half of the HIV-infected persons who were exposed became infected with TB, as evidenced by the development of a newly positive TST or active TB. Of the HIV-infected residents who were infected with M. tuberculosis, 73% (11 of 15) developed active TB within the first 6 months of TB infection (67).
In persons with latent TB, HIV infection accelerates and augments progression to reactivation TB. For HIV-uninfected individuals with latent TB infection (LTBI), the lifetime risk of developing active TB due to reactivation is 8 to 10%. In contrast, this risk is approximately 10% per year for HIV-infected persons (128, 142, 227, 228). A prospective study of injection drug users (IDUs) enrolled in a methadone maintenance program in New York City found that active TB developed in 7 of 49 HIV-infected subjects with a prior positive purified protein derivative (PPD) test and in none of 62 HIV-uninfected subjects with a prior positive PPD test (227). Similarly, a San Francisco study of injection drug users enrolled in a methadone maintenance program found that the rate of developing reactivation TB was over 10 times higher for HIV-infected TST-positive patients (5.0 per 100 person-years) than for TST-positive patients without HIV (0.4 per 100 person-years) (66).
HIV-infected patients appear to be at a higher risk for reinfection with TB, likely because of an impaired capacity to mount long-lasting protective immune responses (115). DNA fingerprinting studies of South African gold miners cured of a first episode of culture-positive TB, who developed recurrent TB, subsequently found that 69% (11 of 14) of culture-positive recurrences in HIV-infected patients were due to reinfection (45). DNA fingerprinting of another cohort of South African mine workers with recurrent TB found that HIV infection was a risk factor for recurrent TB due to reinfection but not relapse (235).
Effect of HIV on Tuberculosis: Atypical Presentation and Extrapulmonary TB
The clinical presentation of TB in HIV-infected persons has been reviewed extensively elsewhere (15, 33, 142, 239). HIV-infected patients with TB commonly present with subacute systemic and respiratory symptoms, including fever (88%), weight loss (79%), cough (79%), and diarrhea, which last 6 weeks on average (33). Lower CD4 counts are associated with more severe systemic symptoms (33). At all stages of HIV infection, pulmonary TB is the most common form of TB (33). In general, HIV-infected patients with high CD4 counts have clinical manifestations of TB similar to those of TB patients without HIV infection. Chest X-ray (CXR) findings for HIV-infected individuals with CD4 counts of >350 cells/μl with pulmonary TB are typically upper lobe infiltrates, cavitations, and/or pleural disease, similar to those with pulmonary TB reactivation in HIV-uninfected patients (33, 102, 210, 214). Additionally, after the initiation of antiretroviral therapy (ART), patients can also present with TB-associated immune reconstitution inflammatory syndrome (discussed further below).
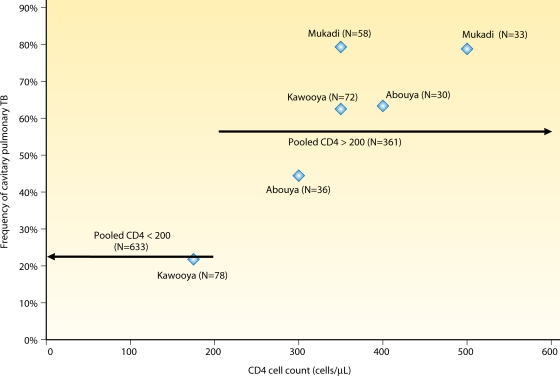
In HIV-infected patients with pulmonary TB, the likelihood of cavitation is correlated with the CD4 count (6, 19, 34, 68, 71, 105, 143, 144, 159, 199, 208, 210). We collated data from 12 studies that examined the frequency of lung cavitation associated with CD4 counts in patients coinfected with HIV and pulmonary TB and found 4-fold-higher odds of having cavitation in patients whose CD4 counts were above 200 cells/μl than for those with CD4 counts below 200 cells/μl (odds ratio, 4.44; 95% CI of the OR, 3.36, 5.88) (Fig. 4). Atypical CXR findings are associated with an altered immunity to reactivation and primary TB infection in HIV-infected patients (110). As the CD4 count falls below 200 cells/μl, HIV-infected patients with pulmonary TB are more likely to have atypical CXR findings, including pleural effusion, lower or middle lobe infiltrates, mediastinal adenopathy, interstitial nodules, or a normal CXR (33, 117, 144, 210, 214). One retrospective study of 133 HIV-infected patients with culture-confirmed pulmonary TB found that 21% of patients with CD4 counts below 200 cells/μl had normal CXR findings, compared with 5% of patients with CD4 counts above 200 cells/μl (117). Cavitary lesions due to TB are rare in patients with advanced HIV, and the presence of cavitary lesions in patients with CD4 counts of less than 200 cells/μl should prompt a search for other etiologies (33, 102, 161, 210).
Fig. 4.
As the CD4 cell count declines, the frequency of cavitation in pulmonary TB decreases. Data were pooled from 12 studies (6, 19, 34, 66, 71, 105, 143, 144, 159, 199, 208, 210) that examined the frequency of lung cavitation associated with CD4 counts in patients coinfected with HIV and pulmonary TB. Patients whose CD4 count was above 200 cells/μl had a 4-fold-higher odds of having cavitary pulmonary TB than those with CD4 counts below 200 cells/μl (odds ratio, 4.44; 95% CI, 3.36, 5.88).
Although pulmonary TB is the most common presentation regardless of the stage of HIV infection, persons with advanced immune suppression are more likely to have extrapulmonary TB than are HIV-infected persons with relatively intact immunity or persons without HIV (15, 33, 83, 136). Unlike persons without HIV, in whom extrapulmonary TB is usually found in the absence of pulmonary TB, HIV-infected persons with extrapulmonary TB are more likely to have concomitant pulmonary TB (15, 212, 229, 255). Symptoms of extrapulmonary TB (with or without concomitant pulmonary TB) in a cohort of 199 HIV-infected patients included fever (95%), respiratory symptoms (66%), lymphadenopathy (62%), gastrointestinal symptoms (37%) with diarrhea and abdominal pain, and neurological symptoms (29%), including confusion and headache (229). Common sites of infection in extrapulmonary TB with HIV coinfection include the following (in decreasing order of frequency): disseminated TB involving bone marrow, blood, or liver; genitourinary TB; peripheral lymphadenitis; pleural TB; mediastinal TB with mediastinal lymphadenopathy and/or pericarditis; central nervous system (CNS) TB with meningitis or parenchymal infection with tuberculous abscesses and tuberculomas; mediastinal and pericardial TB (229); intra-abdominal lymphadenitis or peritonitis; musculoskeletal abscesses or osteomyelitis; or infection at other sites, including the adrenal glands and the gastrointestinal tract (15, 33, 229, 255).
Effect of HIV on Tuberculosis: Higher Mortality
Tuberculosis in a patient with HIV is curable. However, HIV increases the mortality rates associated with TB. A prospective 12-year study in San Francisco showed that the TB case-fatality rate was significantly higher for patients coinfected with HIV than for HIV-uninfected patients (22% and 10%, respectively) in the era prior to ART and that the higher TB case-fatality rate for HIV-infected patients persisted even after the availability of ART in 1997 (70). A study from Côte d'Ivoire showed that mortality rates for coinfected patients prior to the availability of antiretroviral treatment were related to the degree of immune deficiency (7). At follow-up 6 months after the initiation of TB treatment, patients whose CD4 counts were less than 200 cells/μl had a mortality rate of 10%, and those with CD4 counts of between 200 and 499 cells/μl had a mortality rate of 4%, which were 28 and 12 times higher, respectively, than the mortality rate for TB patients without HIV. In other resource-limited settings, the reported case-fatality rates prior to the availability antiretrovirals were extremely high: in the Central African Republic, the case-fatality rate was as high as 58% for HIV-infected patients, compared to 20% for HIV-uninfected patients at 24 months after the initiation of TB treatment (198).
Treatment with Antiretroviral Therapy Decreases Incidence of TB
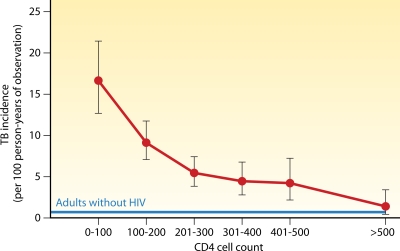
Treatment with antiretroviral therapy (ART) is associated with a decreased incidence of TB. Lawn et al. found that TB incidence rates trended down during the first 5 years of a South African antiretroviral program, from 3.35/100 person-years in the first year of ART to 1.01/200 person-years in the fifth year (156). The use of ART and immunological recovery, a rise in CD4 counts, significantly decreased the incidence of TB, with the greatest effect on patients with restored CD4 cell counts above 500 cells/μl (14, 113, 151, 156, 223). Additionally, treatment with ART and the restoration of cell-mediated immunity are associated with less extrapulmonary involvement in TB infection (112). However, despite ART treatment, the overall TB incidence rate for HIV-infected patients still remained approximately 10-fold higher than that for adults without HIV (156). Even for patients on ART who attained CD4 cell counts above 500 cells/μl, TB incidence rates still remained 2-fold higher than those for adults without HIV (Fig. 5).
Fig. 5.
TB incidence rates decrease with recovery of CD4 cell counts during antiretroviral therapy. TB incidence is defined as incident TB cases per 100 person-years, with 95% confidence intervals shown. The blue line represents baseline TB incidence (0.7 cases/100 person-years) in adults without HIV in a comparable neighboring community. (Adapted from reference 156 with permission of the publisher.)
Screening and Diagnosis of TB in HIV-Infected Patients
Current U.S. guidelines recommend that all persons should be tested for latent TB infection (LTBI) with either a tuberculin skin test (TST) or gamma interferon (IFN-γ) release assays (IGRAs) at the time of HIV diagnosis (4, 142). For those who test positive, a CXR should be obtained. For those with CXR abnormalities and for those who have a normal CXR for whom suspicion of disease is high (patients with symptoms and those originating from an area where the disease is endemic), U.S. guidelines recommend that three sputum samples for AFB smear and culture should be obtained in the morning on different days as part of the initial evaluation for suspected pulmonary TB (142). On the other hand, the WHO recommends symptomatic screening for active TB in HIV-infected patients (261, 267). However, there is no universal agreement on what screening involves, with many national programs screening for cough alone (217). Since a significant proportion of HIV-infected patients with active TB have less specific symptoms or have no symptoms, screening for cough alone will miss the majority of patients with culture-confirmed TB (217). Cain et al. designed an algorithm for TB screening for people infected with HIV that uses a combination of three predictors: cough of any duration, fever of any duration, or night sweats lasting 3 weeks or more during the previous 4 weeks (35). In this study population from Cambodia, Thailand, and Vietnam, combination symptom screening was found to have 93% sensitivity and 36% specificity, with a 97% negative predictive value and a 21% positive predictive value for culture-positive pulmonary TB. Similarly, Corbett et al. assessed the efficacy of provider-initiated symptom screening for TB in HIV-infected patients in Zimbabwe and found that assessing for the presence of any cough, drenching night sweats, or weight loss yielded a sensitivity of 75% and a specificity of 82%, with a 99.2% negative predictive value and a 10.2% positive predictive value for TB (both culture-positive and culture-negative TB) (63). The use of a combination of symptoms to screen for TB appears to be an effective and practical method to rule out active TB in HIV-infected patients.
However, symptom screening for active TB has a low specificity, with a low positive predictive value, and therefore, the diagnosis of active TB presently relies on microscopy and culturing of appropriate specimens. In the study by Cain et al. mentioned above, only 9% of the persons who screened positive for TB symptoms had a positive AFB smear with concentrated sputum, and the rest relied on culture for the diagnosis of TB. Because HIV-infected patients with culture-confirmed pulmonary TB have a lower frequency of cavitary lesions, they have a lower rate of sputum smear positivity (33, 83). However, when adjusted for the presence of cavities, the yield of a sputum smear from HIV-infected persons can be similar to that of a sputum smear from HIV-uninfected persons (33, 175, 233). Smith et al. found the sensitivity of fluorescent microscopy of a single concentrated sputum smear to be 60% for HIV-infected persons with culture-confirmed pulmonary TB. With the presence of cavitary lesions, the sensitivity of a single sputum smear increased to 85% (233). In the United States, approximately 25% of HIV-infected persons with culture-confirmed pulmonary TB have negative sputum smears (142).
However, other studies have found the sputum smear to be much less sensitive, and the results have varied widely. Due to operator dependence on sample collection and processing and interpretation of sputum smears, the sensitivity of the sputum smear ranges from 20% to 60% in resource-constrained settings (37, 217, 236). An evaluation of sputum smears and cultures from HIV-infected persons with culture-confirmed TB in Thailand and Vietnam found the sensitivity of three sputum smears to be only 38% (195, 211). More recently, a multicenter prospective study of 1,748 HIV-infected patients from Cambodia, Thailand, and Vietnam found the sensitivity of either of the first two concentrated sputum smears positive for acid-fast bacilli to be 38% for culture-positive TB (35). In this study population, for which the prevalence of TB was 15%, the positive predictive value of either of the first 2 positive sputum smears was 84%, and the negative predictive value was 90%.
In the study by Cain et al. described above, among patients who screened positive for TB but had two negative sputum smears, only mycobacterial culture was optimally effective in diagnosing TB (35). Although culture is the “gold standard” for the diagnosis of active TB, culture results take 2 to 6 weeks, and culture is not currently an available diagnostic option in most resource-constrained settings (264). In high-burden, resource-constrained countries, the main diagnostic approaches remain suboptimal, with smears of direct, unconcentrated sputum examined by light microscopy and not followed by culture. Thus, the majority of culture-confirmed pulmonary TB cases are likely missed in resource-constrained settings because of the reliance on direct sputum light microscopy as the diagnostic approach.
Simple, rapid diagnostics that can replace direct sputum smear microscopy are urgently needed for resource-constrained health care systems (232). Inexpensive light-emitting diode (LED) microscopes have been developed recently, which are more sensitive, are similar in specificity and require less operator time than conventional light microscopy examination of Ziehl-Neelsen-stained direct smears (256). Microscopic observation drug susceptibility (MODS) assays have been developed for the diagnosis of TB and the detection of drug-resistant TB (191, 192). MODS assays of sputum samples of patients with HIV infection have 98% sensitivity and 100% specificity for the detection of TB and provided results in 14 days for most samples (196, 216). The Xpert MTB/RIF assay is a newly developed automated real-time PCR assay for TB and resistance to rifampin (RIF) with fully integrated sample processing, which can be performed by staff with minimal training (25, 256). With the use of one direct sputum sample, the Xpert MTB/RIF assay demonstrated 98.2% sensitivity for smear-positive TB; 72.5% sensitivity for smear-negative, culture-positive TB; and a specificity of 99.2% (25). In South African study populations with HIV prevalence rates of 71 to 76%, Xpert MTB/RIF testing of three sputum samples (one direct and two concentrated) has a sensitivity of greater than 95% for all culture-positive TB cases, with 99 to 100% sensitivity for smear-positive, culture-positive TB; 87 to 90% sensitivity for smear-negative, culture-positive TB; and 97 to 98% specificity (25). Sensitivity for rifampin resistance was 94 to 100% in South African study populations (25). This highly sensitive and easy-to-use diagnostic system has a rapid turnaround time of less than 2 h and, in combination with MODS assays to detect drug-resistant TB, has the potential to revolutionize the field of TB diagnostics (25, 192, 256).
For extrapulmonary TB in HIV-infected patients, current U.S. CDC guidelines recommend AFB smear and culture of tissue or fluid aspiration or biopsy specimens and mycobacterial blood cultures to evaluate for disseminated disease. Recommendations for the diagnosis of suspected TB in a person infected with HIV in the United States are available from the Department of Health and Human Services (DHHS)/CDC guidelines for the management of opportunistic infections (142). Clinicians in resource-constrained settings should refer to the latest WHO guidelines for improving the diagnosis of smear-negative pulmonary and extrapulmonary TB for HIV-infected persons in resource-limited settings, which can be found at the Evidence-Based Tuberculosis Diagnosis website (http://www.tbevidence.org/guidelines.htm). The 2007 WHO algorithms emphasize the timely diagnosis and treatment of all cases of TB, including smear-negative pulmonary and extrapulmonary TB. For smear-negative pulmonary TB, the revised case definitions emphasize clinical judgment in conjunction with the use of at least two sputum specimens for AFB staining, HIV testing, CXR, and sputum culture if possible (264). For extrapulmonary TB, the revised case definitions and algorithms emphasize HIV testing, sputum smears, CXR, clinical judgment, aspiration of the infected site for AFB staining, and immediate initiation of TB treatment for disseminated TB, TB meningitis, or TB-associated pleural effusion or pericardial effusion (264).
IFN-γ Release Assays for HIV-Infected Patients
IFN-γ release assays (IGRAs) are a recently introduced modality for the detection of cellular immune responses to M. tuberculosis antigens; three assays are currently commercially available: QuantiFERON-TB Gold (QFT-G), QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB. These assays detect the release of the cytokine gamma interferon (IFN-γ) from T lymphocytes after stimulation with the M. tuberculosis-specific antigens early secretory antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) and also TB antigen TB 7.7 for the QFT-GIT test (24, 74). The QuantiFERON assays use anticoagulated whole blood and measure the release of gamma interferon into the supernatant by enzyme-linked immunosorbent assays (ELISAs), while the T-SPOT.TB assay uses isolated blood mononuclear cells and determines the number of gamma interferon-secreting cells in the sample by using an enzyme-linked immunospot (ELISPOT) technique (24, 74). Each test is performed with a negative control and a positive control, and results are reported as being indeterminate if there is a high signal for the negative control or a low signal for the positive control. The performance of IGRAs is impaired for HIV-infected patients with advanced immunodeficiency. Higher rates of indeterminate results are found for patients with CD4 counts below 100 cells/μl, due to low levels of IFN-γ in response to stimulation with the positive control (24).
IGRAs are approved for the diagnosis of LTBI in HIV-infected patients but lack specificity for the diagnosis of active TB (4, 142, 177). For the T-SPOT.TB assay, the sensitivity was between 79 and 95% and the specificity was between 64% and 100% for active TB in HIV-infected patients (74). The sensitivity of the QFT-GIT test for culture-confirmed pulmonary TB in HIV-infected patients ranged from 65% to 91% in previously reported studies, and the likelihood of indeterminate results increased with lower CD4 counts (1, 9, 74, 158, 177, 242, 254). Aabye et al. found that 22% of HIV-infected patients with culture-confirmed pulmonary TB had indeterminate results with the QFT-GIT test, and patients with CD4 counts below 300 cells/μl were nearly 3 times as likely to have indeterminate results as patients with CD4 counts above 300 cells/μl (1). Even after excluding indeterminate results, the QFT-GIT assay missed 12% of HIV-infected Tanzanian patients with culture-confirmed pulmonary TB. Similarly, in a South African cohort of HIV-infected patients, the sensitivity and specificity of the QFT-GIT test were 30% and 63%, respectively, compared with sputum culture results. Of the HIV-infected patients with culture-confirmed pulmonary TB, 50% had false-negative QFT-GIT results and 20% had indeterminate results. Of the HIV-infected patients who had negative sputum culture results, 36.3% had positive QFT-GIT results, probably due to latent TB infection (254). Thus, in a setting of high TB prevalence (123), the utility of IGRAs to rule out active TB is limited by the inadequate negative predictive value of the test.
At this time, although IGRAs are approved for the diagnosis of LTBI, they are not recommended for the diagnosis of active TB in HIV-infected persons (142). In countries with high TB prevalences, the sensitivity and negative predictive value of the IGRA are insufficient to rule out active TB infection in HIV-infected patients. In countries with low TB prevalences, the specificity and positive predictive value of the IGRA are insufficient to rule in active TB. Additionally, IGRAs cannot distinguish between latent and active TB, and the rates of indeterminate results for HIV-infected patients are relatively high, especially for patients with low CD4 counts.
Treatment of TB for HIV-Infected Patients: Rifampin Combination Therapy Improves Survival
The principles of TB drug treatment in HIV-infected patients are the same as those for HIV-uninfected patients: (i) daily rifampin (RIF) as a part of a 4-drug combination regimen during the initial 2-month phase of treatment is critical and life-saving, and (ii) rifampin given at least 3 times weekly as a part of a 2-drug regimen during the continuation phase lowers failure and relapse rates and prevents the development of rifampin resistance (123, 239).
The use of bactericidal rifampin in both the initial and continuation phases of TB treatment is critically important, as its use in TB chemotherapy regimens reduces mortality for HIV-infected patients with pulmonary TB. In a randomized trial comparing rifampin- to thiacetazone-based initial and continuation phase regimens for pulmonary TB in HIV-infected patients, treatment with the rifampin-based regimen improved survival (203). Daily dosing of rifampin in the initial phase of treatment is critical to prevent the development of relapse or treatment failure due to acquired rifampin resistance in HIV-infected patients with TB (146, 160). Rifampin is also essential as a part of the combination treatment regimen for the continuation phase. Two studies which compared the use of RIF to the use of ethambutol (ETB) in the continuation phase after initial treatment with isoniazid (INH)-RIF-pyrazinamide (PZA)-ETB found that those on ETB-INH were 2 to 3 times more likely to experience treatment failure or TB relapse than those on RIF-INH for continuation therapy (135, 204). Several meta-analyses have shown that treatment with a longer duration (6 months or longer) of a rifamycin drug (rifampin or rifabutin [RfB]) resulted in lower rates of failure and recurrence and improved survival during treatment, compared to only 2 months of treatment with a rifamycin drug (146, 150, 186). More recent data suggest that a duration of rifampin treatment even longer than the recommended 6 months may decrease failure and recurrence rates even further (146, 239). In summary, the use of daily rifampin as part of a combination regimen in the initial phase and the use of rifampin during the continuation phase of treatment improve survival and reduce rates of treatment failure, TB relapse, and the development of acquired drug resistance in HIV-infected patients with pulmonary TB.
For drug-susceptible TB in HIV-infected patients, U.S. CDC/Infectious Diseases Society of America (IDSA) 2009 guidelines and WHO 2009 guidelines recommended a 6-month regimen with an initial 2 months therapy consisting of INH, RIF or RfB, PZA, and ETB, followed by 4 months of continuation therapy with INH and RIF or RfB. For cavitary disease with a delayed response to treatment or extrapulmonary TB, the guidelines recommended prolonged continuation therapy with INH and RIF or RfB extended to 7 months, for a total of 9 months of treatment (142). For extrapulmonary disease involving the central nervous system (tuberculoma or meningitis) or bones and/or joints, the guidelines recommended a 12-month course of total treatment. Additionally, HIV-infected patients on INH should receive pyridoxine supplementation to minimize the risk of peripheral neuropathy. Clinicians should refer to the U.S. CDC guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents for current and detailed management recommendations. An online and downloadable version of the guidelines is available (http://www.aidsinfo.nih.gov/Guidelines/Default.aspx?MenuItem=Guidelines). Additionally, the 2009 WHO TB treatment guidelines are available at the WHO website (http://www.who.int/tb/publications/tb_treatmentguidelines/en/index.html).
Rifampin Accelerates Metabolism of Protease Inhibitors, Raltegravir, and Maraviroc
As potent inducers of the cytochrome P450 system, rifampin and rifabutin have significant interactions with many medications, including antiretrovirals. Because protease inhibitors (PIs) are substrates of CYP3A4, and rifampin induces CYP3A4, the combination of rifampin with any protease inhibitor, with or without ritonavir (used to inhibit CYP3A4), greatly accelerates the metabolism of protease inhibitors, resulting in negligible concentrations of protease inhibitors in the serum. Additionally, rifampin induces the activity of the efflux pump P-glycoprotein (P-gp), which further accelerates the elimination of protease inhibitors (123). In the presence of rifampin, the area under the concentration-time curve (AUC) of most protease inhibitors is decreased by over 90%. Even with ritonavir boosting, atazanavir, lopinavir, and indinavir serum concentrations are decreased by 90% (40). For this reason, the combination of rifampin and any protease inhibitors with or without ritonavir boosting is contraindicated.
For integrase inhibitors and entry inhibitors, studies now indicate that rifampin significantly accelerates the metabolism of raltegravir and maraviroc and results in subtherapeutic levels of the antiretroviral drugs when administered at standard doses (85, 258). The use of rifampin with raltegravir or maraviroc should thus be avoided if possible. If coadministered with rifampin, the dose of raltegravir or maraviroc must be increased (85, 258). Rifabutin has less of an effect on raltegravir metabolism and may be more appropriate for coadministration (30).
Rifampin Can Be Used with Efavirenz
The preferred combined regimen for the concomitant treatment of HIV and TB is efavirenz (EFV)-based antiretroviral therapy (ART) with rifampin-based TB treatment (40). Rifampin does reduce the AUC of EFV by 22% by inducing CYP3A4 (40). However, a large prospective cohort study in South Africa and a randomized clinical trial in Thailand failed to show any adverse effect on virologic suppression when a standard dose of efavirenz (600 mg daily) was used with rifampin (29, 170, 171). Although U.S. guidelines do not recommend changing the dose of EFV when used with rifampin, some experts recommend increasing the dose of EFV for patients weighing over 60 kg (40).
Nevirapine (NVP) is the most widely used nonnucleoside reverse transcriptase inhibitor (NNRTI) in resource-constrained settings due to its lower price and availability in fixed-dose combinations (29, 123). Unfortunately, rifampin reduces the AUC of NVP by one-half (40). The use of NVP with rifampin was associated with higher rates of virologic failure starting at 6 months after the initiation of treatment in a South African prospective cohort study (29). A randomized controlled trial in Thailand comparing the use of EFV-based antiretroviral treatment with NVP-based antiretroviral treatment in conjunction with rifampin-based TB treatment found that NVP had a 5-fold-higher risk of having drug levels below the recommended minimum concentration at week 6 (171). Although there was no detectable difference in virologic outcomes with the use of EFV compared with the use of NVP in patients taking concomitant rifampin, that study did show that patients on EFV or NVP with drug levels below the recommended minimum concentration were four times more likely to develop HIV treatment failure. Some experts have suggested avoiding the lead-in phase of NVP (commonly used to decrease side effects) and starting NVP at the twice-daily full dose with concomitant rifampin use in order to minimize the pharmacokinetic impact of rifampin on NVP and to improve virologic outcomes (52, 123). The use of NVP with rifampin-based TB treatment remains an alternative, although inferior, choice to the use of EFV and should be prescribed for coinfected patients who cannot take efavirenz and if rifabutin is not available (40, 52, 123).
Rifabutin Can Be Used with Protease Inhibitors
For patients unable to use efavirenz (first trimester of pregnancy, efavirenz resistance, or other contraindications for efavirenz), U.S. CDC guidelines recommend the use of protease inhibitor (PI)-based ART with rifabutin-based TB treatment (40). Rifabutin is a much less potent inducer of CYP3A4 than rifampin. On the other hand, the protease inhibitors are inhibitors of CYP3A4, in addition to being substrates of CYP3A4. The net effect is that the concomitant use of rifabutin and protease inhibitors increases levels of rifabutin, doubling the AUC of rifabutin on average, and thereby increases significantly the risk of rifabutin toxicities such as uveitis, neutropenia, arthralgia, and hepatitis.
With the concomitant use of most ritonavir-boosted protease inhibitors, U.S. CDC guidelines recommend reducing the dose of rifabutin (40). The use of PI-based antiretroviral treatment with rifabutin-based TB treatment requires vigilant adherence to both ART and TB treatment regimens. Incomplete adherence to PI-based ART regimens, which may require twice-daily dosing, could result in subtherapeutic levels of rifabutin, even in the setting of directly observed TB treatment. Subtherapeutic levels of rifabutin have been associated with the development of acquired rifamycin resistance (257). This is illustrated by case reports of the development of TB relapse due to acquired rifampin resistance after treatment with every-other-day rifabutin and ritonavir-boosted PI-based ART (28, 134). There remains a critical need to better understand the pharmacokinetic interactions between rifabutin and protease inhibitors in HIV-infected patients. Rifabutin drug concentration monitoring may be warranted during treatment when it is feasible (28).
Despite the complexity of drug interactions, rifamycins are crucial for the initial and continuation phases of treatment of TB in HIV-infected patients; these drug interactions can be managed and should not be avoided by delaying treatment. Table 2 summarizes the pharmacokinetic and pharmacodynamic drug-drug interactions between antiretrovirals and rifampin or rifabutin. Detailed reviews of the interactions between rifampin and antiretrovirals have been reported elsewhere (40, 178), and information on the management of drug interactions for the treatment of HIV and TB coinfection can be found at the CDC website (http://www.cdc.gov/tb/publications/guidelines/TB_HIV_Drugs/default.htm) (40). Clinicians requiring more detailed information on specific drug-drug interactions should consult the latest U.S. DHHS guidelines for the use of antiretrovirals, which have detailed tables on the pharmacokinetic interactions and dosage adjustments needed for the coadministration of ARTs and antimycobacterials (75). Additionally, online charts and interactive tools are available at the Johns Hopkins HIV Guide website (http://www.hopkins-hivguide.org/drug/) (17); the University of California at San Francisco (UCSF) HIV InSite website (http://hivinsite.ucsf.edu/InSite?page=ar-00-02), which has an excellent database of antiretroviral drug interactions; the New York City Bureau of Tuberculosis Control Clinical Policies and Protocols website (http://www.nyc.gov/html/doh/html/tb/tb.shtml); and the University of Liverpool HIV-druginteractions.org website (http://www.hiv-druginteractions.org/).
Table 2.
Interactions between antiretrovirals and rifampin or rifabutina
| HIV antiretroviral | Interaction withb: |
|
|---|---|---|
| Rifampin | Rifabutin | |
| Nonnucleoside reverse transcriptase inhibitors | ||
| Efavirenz | EFV ↓ | RfB ↓↓ |
| Nevirapine | NVP ↓↓↓ | RfB ↑ |
| Etravirine | ETR ↓↓↓↓? | ETR ↓↓, RfB ↓ |
| Protease inhibitors | ||
| Atazanavir | ATVr ↓↓↓↓ | ATV ↑↑↑↑↑, RfB↑↑↑↑↑ |
| Darunavir | DRV ↓↓↓↓? | RfB ↑? |
| Fosamprenavir | FPV ↓↓↓↓? | RfB ↑↑? |
| Indinavir | IDV and IDVr ↓↓↓↓ | IDV ↓↓, RfB ↑↑↑↑↑ |
| Lopinavir | LPV and LPVr ↓↓↓↓ | LPV ↑, RfB ↑↑↑↑↑ |
| Nelfinavir | NFV ↓↓↓↓ | NFV ↓↓, RfB ↑↑↑↑↑ |
| Saquinavir | SQVr ↓↓↓ | SQV↓↓ |
| Tipranavir | TPV ↓↓↓↓? | RfB ↑↑↑↑↑ |
| Ritonavir | RTV ↓↓ | RfB ↑↑↑↑↑ (by 400%) |
| Integrase inhibitor | ||
| Raltegravir | RAL ↓↓↓ | No known interaction |
| Entry inhibitors | ||
| Maravaroc | MVC ↓↓↓ | MVC ↓↓? |
| Enfuvirtide | No known interaction | No known interaction |
| Nucleotide/nucleoside reverse transcriptase inhibitors | ||
| Abacavir | No known interaction | No known interaction |
| Didanosine | No known interaction | No known interaction |
| Emtricitabine | No known interaction | No known interaction |
| Lamivudine | No known interaction | No known interaction |
| Stavudine | No known interaction | No known interaction |
| Tenofovir | No known interaction | No known interaction |
| Zidovudine | AZT ↓↓ | No known interaction |
Based on data from reference 17a and the Lexi-Comp Drug-Drug Interaction Analysis online program.
↓/↑, Cmin or AUC decreased/increased by up to 25%; ↓↓/↑↑, Cmin or AUC decreased/increased by 25 to 50%; ↓↓↓/↑↑↑, Cmin or AUC decreased/increased by 50 to 75%; ↓↓↓↓/↑↑↑↑, Cmin or AUC decreased/increased by 75 to 100%; ↑↑↑↑↑, Cmin or AUC increased; ATV, atazanavir; ATVr, ritonavir-boosted atazanavir; DRV, darunavir; FPV, fosamprenavir; IDV, indinavir; IDVr, ritonavir-boosted indinavir; LPV, lopinavir; LPVr, ritonavir-boosted lopinavir; NFV, nelfinavir; SQV, saquinavir; SQVr, ritonavir-boosted saquinavir; TPV, tipranavir; RTV, ritonavir; RAL, raltegravir; MVC, maravaroc; AZT, zidovudine.
TB-Associated Immune Reconstitution Inflammatory Syndrome
Immune reconstitution inflammatory syndrome (IRIS) is a clinical deterioration after the initiation of ART due to inflammatory responses against pathogens or tumors. In TB-associated IRIS (TB-IRIS), the clinical presentation varies widely and includes high fevers, worsening respiratory status, worsening or new lymphadenopathy, breakthrough meningitis, new or worsening CNS lesions, radiological worsening of pulmonary infiltrates or pleural effusions, hepatosplenomegaly, or ascites (54, 96, 123, 142, 182).
There are two clinical forms of TB-associated IRIS: paradoxical TB-associated IRIS and unmasking TB-associated IRIS. Paradoxical TB-associated IRIS is an exaggerated inflammatory response during TB treatment in a patient known to have TB (142), while unmasking TB-associated IRIS is previously undiagnosed TB which is unmasked after the initiation of ART. Incidence rates of paradoxical TB-IRIS range from 8% to 43%, and the majority of TB-IRIS occurs within the first 4 to 8 weeks after the initiation of ART (54, 73, 152, 182). In North American TB Trials Consortium (TBTC) Study 23, of 137 patients who received ART during TB treatment, 25 (18%) developed TB-IRIS (26). In the Starting Antiretroviral Therapy at Three Points in Tuberculosis (SAPIT) trial, IRIS was diagnosed in 53 (12.4%) of 429 patients who had initiated ART during TB treatment, of which 5 required corticosteroid therapy, and there were no deaths attributable to IRIS (7). Risk factors for TB-IRIS include starting ART within the first 2 months of TB treatment, extrapulmonary or disseminated TB, low CD4 counts (<100 cells) at the start of ART, a viral load of >105 log10 copies/ml at the start of ART, a rise in the percentage of CD4 cells during ART, and decreasing viral load during ART (54, 95, 142).
Because there is no definitive laboratory test for TB-IRIS, TB-IRIS remains a clinical diagnosis after thorough investigation to exclude new opportunistic infections, TB treatment failure, drug toxicity, and other possible causes of clinical deterioration in a patient on TB treatment who has initiated antiretrovirals (54, 142, 230). In 2008, the International Network for the Study of HIV-Associated IRIS (INSHI) proposed revised case definitions for TB-associated IRIS for use in resource-limited settings (181). The case definition for paradoxical TB-IRIS uses three components: antecedent requirements, clinical criteria, and the exclusion of alternative explanations for clinical deterioration. The two antecedent requirements that must be met are that (i) the diagnosis of TB must be made prior to starting ART and (ii) there must be an initial response to TB treatment before ART initiation. Clinical criteria are defined as the onset of TB-IRIS manifestations within 3 months of ART initiation, reinitiation, or regimen change because of treatment failure. Additionally, the patient must have at least one major clinical criterion or two minor clinical criteria. Major clinical criteria include (i) new or enlarging lymph nodes, cold abscesses, or other focal tissue involvement; (ii) new or worsening radiological features of TB; (iii) new or worsening CNS TB; or (iv) new or worsening serositis. Minor clinical criteria include (i) new or worsening constitutional symptoms such as fever, night sweats, or weight loss; (ii) new or worsening respiratory symptoms; or (iii) new or worsening abdominal pain with peritonitis, hepatomegaly, splenomegaly, or abdominal adenopathy. Finally, alternative explanations for clinical deterioration must be excluded if possible, including a failure of TB treatment due to drug resistance, poor adherence to TB treatment, another opportunistic infection or neoplasm, or drug toxicity or reactions.
For unmasking TB-IRIS, the INSHI proposed the following provisional definition: a patient not receiving TB treatment when ART is initiated who then presents with active TB within 3 months of ART initiation and who has either a heightened intensity of clinical manifestations, particularly if there is evidence of a marked inflammatory component to the presentation, or a clinical course that is complicated by a paradoxical reaction once established on TB treatment (181). The 2008 INSHI case definition for TB-IRIS has been validated subsequently in a South African cohort of 498 TB-HIV-coinfected patients against expert opinion and in a Thai cohort of 126 patients against the study definition confirmed by an external reviewer (120, 172).
Although much remains unknown regarding the pathogenesis of TB-IRIS, a preliminary mechanism has been proposed. After the initiation of ART in HIV-infected patients on treatment for TB, the manifestations and severity of TB-IRIS likely depend on interactions between the residual burden of M. tuberculosis components, which serve as stimuli for the innate immune response and/or specific T cell responses, the partially reconstituted cellular immune response, and incomplete regulation of the reconstituted immune system (95, 182). Risk factors for TB-IRIS, such as low CD4 cell counts, extrapulmonary TB, and disseminated TB, are thought to reflect a high burden of TB at the time of diagnosis, leading to a high residual burden of M. tuberculosis components after the initiation of TB treatment, which, in turn, stimulates and triggers an intense reaction from the partially reconstituted and incompletely regulated innate and/or adaptive immune responses (95). Full immune recovery in HIV-infected patients entails (i) reconstitution of immune cells depleted by HIV infection, (ii) regeneration of lymphoid organs damaged by HIV-induced inflammation, (iii) restoration of pathogen-specific cellular immunity, and (iv) appropriate regulation of the reconstituted immune system (95). The exaggerated granulomatous inflammatory response in TB-IRIS is hypothesized to be due to the partial reversal of HIV-induced immune defects and an imbalanced regulation of effector and regulatory cellular immune responses against TB-specific antigens (95). TB-IRIS inflammation is characteristic of a Th1 immune response, although a Th17 response has not been excluded (82, 95, 184). Evidence for immune dysregulation in TB-IRIS remains elusive: preliminary studies have not been able to detect a difference in the numbers of circulating regulatory T cells in patients with TB-IRIS compared with those without TB-IRIS (95, 182). However, since these studies depended on the use of surface markers and not functional assays to quantify regulatory T cells, it remains possible that a functional defect of regulatory T cells contributes to IRIS.
The clinical course of paradoxical TB-IRIS is usually self-limited, and symptoms can be managed with nonsteroidal anti-inflammatory agents with the continuation of TB treatment and ART (142, 182). However, supportive treatment may be needed if symptoms are severe. The use of corticosteroids to manage severe TB-IRIS has been recommended based on anecdotal experience and case reports (54, 142, 182). More recently, a double-blind placebo-controlled randomized trial evaluating the use of prednisone for TB-IRIS in 109 study participants in South Africa (median CD4 count, 53 cells/μl) found a reduction in days of hospitalization and numbers of procedures for study participants who received prednisone (173, 185). There was no difference in mortality between the two groups, although the study may have been underpowered to detect a mortality difference, and patients with life-threatening TB-IRIS including neurological involvement, airway compression, and respiratory failure were excluded. It is critically important to rule out other causes of clinical deterioration prior to the diagnosis of TB-IRIS, as the use of corticosteroids may worsen an undiagnosed underlying infection or drug-resistant TB. In one cohort of 100 patients with suspected TB-IRIS, 7 had an alternative opportunistic disease, and 13 had rifampin-resistant TB (183).
Timing of Initiation of Antiretrovirals during Treatment for Tuberculosis
The decision regarding when to start ART for people with active TB has to weigh the risk of TB-IRIS or adverse effects of drugs against the risk of HIV disease progression. Recent studies have demonstrated that starting ART during treatment for TB reduces mortality in ART-naïve patients despite the increased risk of TB-IRIS (3, 22, 75, 142, 266). Observational data from South Africa revealed that 71% of deaths in TB-HIV-coinfected patients on TB treatment occurred in patients waiting to start ART who had CD4 counts of less than 100 cells/μl or WHO stage 4 disease (157). A prospective cohort study of HIV-infected TB patients in Thailand found that those who received ART during TB treatment had one-sixth the risk of death compared to those who did not receive ART (222). A retrospective cohort analysis of 1,003 Thai patients coinfected with HIV and TB found that the initiation of ART within 6 months of TB diagnosis reduced mortality (169). Similarly, a retrospective analysis from Spain found that TB-HIV-coinfected patients who started ART within 8 weeks of initiation of TB treatment had a significant reduction in mortality (9.3% mortality with starting ART within 8 weeks and 19.7% mortality with starting ART after 8 weeks), even though there was no difference in median initial CD4 counts between the two groups (253). A comparison of three hypothetical cohorts of TB-HIV-coinfected patients in whom ART was initiated during the first 8 weeks or during weeks 8 through 24 or not initiated during TB treatment predicted that early ART initiation had the highest survival benefit (33 estimated deaths per 1,000 patients) compared to the group with deferred ART treatment (48 estimated deaths per 1,000 patients) and compared to those who did not initiate ART (147 estimated deaths per 1,000 patients) (225).
The SAPIT trial, conducted in Durban, South Africa, showed that for TB-HIV-coinfected patients with CD4 counts of up to 500 cells/μl, the initiation of ART during TB treatment reduced mortality compared to waiting for the completion of TB treatment before the initiation of ART. The SAPIT trial was an open-label randomized controlled trial designed to address the optimal timing for the initiation of antiretroviral therapy with 642 TB-HIV-coinfected patients randomized to one of three arms (3). The first arm was assigned to start ART within 4 weeks after the start of TB treatment (integrated therapy). In the second arm, ART was started within 4 weeks after the completion of the 2-month intensive phase of TB treatment (integrated therapy). In the third arm, ART was started after the completion of 6 months of TB treatment (sequential therapy). Baseline characteristics, including CD4 counts and viral loads, were similar between the two integrated-therapy groups and the sequential-therapy group. During a planned interim analysis, the researchers found that starting ART during TB treatment (integrated therapy) reduced mortality by 56% compared with sequential therapy. When stratified by CD4 counts, this mortality reduction was significant for CD4 counts of less than 200 cells/μl and for CD4 counts of between 200 and 500 cells/μl. The data and safety-monitoring committee therefore recommended that all patients in the sequential-therapy group be started on ART as soon as possible prior to the completion of the SAPIT study.
The Cambodian Early versus Late Introduction of Antiretroviral Drugs (CAMELIA) clinical trial showed significantly increased survival with the initiation of ART at 2 weeks compared to 8 weeks after starting TB treatment in severely immunocompromised patients with HIV infection. The study participants were 661 ART-naïve Cambodian adult patients coinfected with TB and HIV whose CD4 counts were less than 200 cells/μl who were randomized to groups starting ART either 2 weeks or 8 weeks after the start of TB treatment. By the end of the 50-week follow-up period, there was a 33% difference in mortality, with 59 deaths in 332 patients in the early arm and 87 deaths in 329 patients in the late arm. A retrospective review of 69 patients with HIV and TB coinfection found that an earlier initiation of ART after 2 weeks of TB treatment in patients with CD4 counts below 100 cells/μl had a significant mortality reduction compared with patients with CD4 counts below 200 cells/μl who started ART after 8 weeks of TB treatment (4.5% versus 27.7%, respectively; P = 0.03) (243). Some experts on the U.S. guideline panel recommend an initiation of ART after 2 weeks of TB treatment for those with CD4 counts below 100 cells/μl (75).
Drug-Resistant Tuberculosis and HIV: a Deadly Syndemic
Multidrug-resistant TB (MDR-TB) is defined as resistance to at least 2 drugs, including isoniazid (INH) and rifampin (RIF), while extensively drug-resistant TB (XDR-TB) is a TB isolate resistant to INH and RIF plus any fluoroquinolone and at least one of the three injectable second-line drugs (capreomycin, kanamycin, or amikacin) (127, 265). Of the estimated 450,000 cases of MDR-TB in 2008, 360,000 cases (80%) were incident TB cases (accounting for 3.6% of all incident TB cases), and 94,000 (20%) were acquired MDR-TB in patients who had been previously treated for TB (265). XDR-TB is estimated to account for 5.4% of all MDR-TB cases (172, 265). Deaths from MDR-TB were estimated to be 150,000 patients, with 53,000 (35%) of the deaths in HIV-coinfected patients (265). HIV and MDR-TB appear to have a syndemic relationship, although data are limited (265). The 2010 WHO report on global surveillance for drug-resistant TB has data available for only eight countries, mostly from Eastern Europe and North America. The burden of MDR-TB and XDR-TB in HIV-infected patients in much of Africa remains unknown.
The deadly synergy between HIV and drug-resistant TB is most clearly illustrated by the XDR-TB outbreak in Tugela Ferry, KwaZulu Natal, South Africa. Gandhi et al. investigated the prevalence rates of MDR-TB and XDR-TB in a region with HIV prevalence rates of 20% in maternity wards and 37% among women receiving antenatal care (103, 104). Of the 1,539 patients tested, 542 (35%) had culture-positive TB, with MDR-TB in 221 (41%) of those with culture-positive TB. Of the MDR-TB cases, 53 (24%) had XDR-TB, of which all of the 44 patients who were tested for HIV were infected with HIV, with a median CD4 count of 63 cells/μl. The mortality rate associated with XDR-TB was 98% (52 of 53 patients), with a median survival time of 16 days from the time of specimen collection to death. Seventy percent of patients with XDR-TB died within 30 days from the time of sputum collection before the diagnosis of XDR-TB was even made or confirmed. This deadly outbreak was likely due to nosocomial transmission: most of the patients had not been treated for TB previously, two-thirds of the patients had been hospitalized in the previous 2 years, 2 health care workers died from XDR-TB during this outbreak, and 85% of the genotyped XDR-TB samples were of the same KwaZulu-Natal (KZN) genetic family of strains.
A subsequent retrospective study of all patients with MDR-TB or XDR-TB from Tugela Ferry from 2005 to 2007 found that of the 272 MDR-TB and 382 XDR-TB cases, 90% and 98% were coinfected with HIV with median CD4 counts of 41 cells/μl and 36 cells/μl and median HIV viral loads of 160,000 and 135,500 log10 copies/ml, respectively (103, 104). The majority of patients were not receiving ART at the time of TB diagnosis. By 30 days after sputum collection, 40% of patients with MDR-TB and 51% of patients with XDR-TB had died. Since the diagnosis of MDR-TB or XDR-TB can take weeks to months after sputum collection to establish, approximately one-half of these coinfected patients had died before the diagnosis was confirmed, and they never received second-line TB treatment. Only 37% of those with MDR-TB and 25% of those with XDR-TB were referred for second-line TB treatment. Additionally, delays in establishing the diagnosis of MDR-TB or XDR-TB meant delays in or lack of infection control: many of these patients were hospitalized in communal wards with other patients. Overall mortality rates were extremely high: up to 76% of HIV-infected patients with MDR-TB and 85% of HIV-infected patients with XDR-TB died within 1 year of sputum collection.
TB AND HIV: INTERACTIONS AT THE CELLULAR AND MOLECULAR LEVELS
Mechanisms of Immunity to Mycobacterium tuberculosis
The cellular and molecular mechanisms of innate and adaptive immunity to M. tuberculosis are incompletely understood. Nevertheless, work with humans and experimental animals has identified some of the major cellular elements, interactions, and essential molecular mediators that participate in the immune control of M. tuberculosis infection and that are direct or indirect targets of HIV.
M. tuberculosis is a facultative intracellular pathogen. Evidence that M. tuberculosis resides in macrophages has been known at least since the studies of Sabin and colleagues in the 1920s (65, 220), although the spectrum of cells in which the bacteria reside in vivo has been recently found to include myeloid dendritic cells (129, 244, 269). Macrophages and dendritic cells serve as cells that present antigen to T lymphocytes and secrete cytokines that direct the differentiation and modulation of T lymphocytes. As an intracellular pathogen, M. tuberculosis is inaccessible to antibodies for most of its life cycle, and therefore, T lymphocytes, particularly CD4 and CD8 T cells, have essential roles in adaptive immunity to M. tuberculosis. Experiments in mice have demonstrated that the absence or depletion of CD4 T cells leads to a poor control of M. tuberculosis growth in the lungs and to accelerated death (194). M. tuberculosis-infected CD8 T cell-deficient or -depleted mice survive longer than CD4-deficient mice but succumb earlier than M. tuberculosis-infected immunocompetent mice (194). Likewise, the depletion of CD4 (76) or CD8 (48) cells from M. tuberculosis-infected macaques compromises the control of bacterial replication. Therefore, it is not surprising that HIV-infected humans are more susceptible to TB and that mortality and frequency of disseminated and extrapulmonary TB are inversely related to CD4 T cell counts (153). CD4 T cells recognize foreign peptides bound to human leukocyte antigen (HLA) class II molecules on the surface of antigen-presenting cells. In macrophages infected with M. tuberculosis, most (200) but not all (252) of the bacteria reside in membrane-bound phagosomes, which, through a vesicular pathway, deliver peptide antigens bound to HLA class II molecules to the cell surface. Thus, the predominant intracellular location of the bacteria is consistent with a central role for CD4 cells in the immune control of TB. Consequently, it is highly likely that a CD4 T cell deficiency in HIV-infected people is the predominant and direct cause of the dramatic increase in susceptibility to TB.
In addition to CD4 T cells, several specific cytokines, especially interleukin 12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor (TNF), are essential for human immunity to M. tuberculosis. Studies of HIV-uninfected humans with recurrent and severe infections with mycobacteria of low virulence, such as Mycobacterium avium complex, Mycobacterium fortuitum, and the attenuated vaccine strain M. bovis BCG, have revealed mutations in the gene encoding a subunit of the IFN-γ receptor (139), and further studies have revealed IFN-γ receptor mutations in certain HIV-negative patients with severe and/or recurrent TB (139, 140). These findings clearly demonstrate a critical role for the cytokine IFN-γ in the immune control of TB in humans and are consistent with the results of experiments with mice that revealed essential roles for IFN-γ (56, 94) and IFN-γ receptors (72) in the control of M. tuberculosis infection.
HIV Effects on TB Immunity: CD4 T Cell Depletion
The best-characterized and most important effect of HIV on immunity to TB and other infections is the depletion of CD4 T cells from secondary lymphoid tissues, peripheral blood, and mucosal sites such as the intestinal lamina propria (118, 180). HIV depletes CD4 T cells by several mechanisms, but the induction of apoptosis of directly infected and bystander (i.e., not productively infected) cells exerts the greatest quantitative effect (reviewed in reference 124). A recent study using human lymphoid aggregated cultures (HLACs) revealed a marked and selective depletion of CD4 T cells after inoculation with a CXCR4-tropic HIV-1 strain. Despite the extensive depletion of CD4 cells from the cultures, no more than 5% of the CD4 cells exhibited evidence of productive infection. The vast majority of cell death occurred in CD4 cells that were abortively infected (i.e., bystander cells). Additional studies revealed that incomplete reverse transcription intermediates triggered caspase 3-dependent apoptosis accompanied by the caspase 1-dependent release of IL-1β. In other words, after HIV had entered bystander CD4 T cells and hijacked the cell's reverse transcription machinery, the host bystander CD4 T cells sensed the partial HIV DNA transcripts and induced apoptosis in order to block further reverse transcription and replication of HIV. These in vitro studies provide a molecular mechanism for the profound depletion of abortively infected CD4 T cells as well as a mechanism for an accompanying inflammatory response that may contribute further to the manifestations of HIV infection (78). Additional mechanisms of CD4 T cell destruction, such as targeting by antibodies and by autoreactive T cells, have been described but are thought to make smaller contributions to the overall loss of CD4 T cells. In addition to increased CD4 T cell death induced by HIV, a decreased thymic output of naïve CD4 T cells in HIV-infected subjects results in an inability to replace CD4 T cells at the rate that they are destroyed (125, 126).
During the early stages of HIV infection, the CD4 T cell population in the intestinal lamina propria is depleted preferentially and markedly; a large fraction of the cells lost in this initial phase of infection are CD4 Th17 effector memory cells (31). Th17 cells recognize bacterial and fungal (and, less commonly, viral) antigens, secrete IL-17 and IL-22, and contribute to immunity to yeasts, extracellular bacteria (114, 166, 190), and, possibly, M. tuberculosis (226). After the initial phase of massive depletion of intestinal Th17 cells, the cells that are most markedly depleted from peripheral blood and secondary lymphoid tissues are effector memory cells, without known specificity for those with Th1, Th2, or Th17 phenotypes (21). The loss of CD4 effector memory cells can account for the decrease in the immune control of latent infections and allows the reactivation of TB and opportunistic infections such as cytomegalovirus (CMV) and Pneumocystis jirovecii. With late-stage infection, perhaps due to the cumulative effect of decreased thymic output and due to the emergence of HIV that utilizes CXCR4 as a coreceptor, naïve CD4 T cells also decrease in number and frequency, which can contribute to ineffective responses to new infections and vaccinations.
HIV Effects on TB Immunity: CD4 T Cell Dysfunction
In addition to quantitatively depleting the CD4 cell population, HIV infection also impairs the qualitative function of the remaining CD4 T cells. An early study characterized functional responses (assayed as IL-2 production) of peripheral blood T cells from HIV-infected patients with CD4 T cell counts of ≥400/μl and described a time-dependent progression of defective responses, with a loss of responses to foreign recall antigens (influenza virus and tetanus toxoid) that preceded the loss of allogeneic responses or responses to phytohemagglutinin (PHA), a polyclonal T cell activator (51). This pattern is consistent with an early loss of memory CD4 cell function, followed by the loss of responses by naïve CD4 T cells.
More recently, CD4 cells from HIV-infected patients who had undetectable HIV in plasma after treatment with ART for a minimum of 1 year were found to exhibit defective IFN-γ secretion in response to stimulation with M. tuberculosis purified protein derivative (PPD) (241). Defective IFN-γ responses could not be attributed to a lack of PPD-responsive CD4 cells, as CD4 cells from ART-treated patients were able to proliferate in response to PPD. The defective IFN-γ responses observed were more severe in chronically infected, ART-treated patients than in a group with primary HIV infection, suggesting that chronic infection caused a persistent defect that was not restored with effective HIV treatment. Deficient IFN-γ responses were restored by the addition of IL-12 to the assays, indicating that the PPD-reactive cells had the capability of producing IFN-γ but did not do so under the conditions of the assays used. Further investigations of in vitro responses to M. tuberculosis antigens by cells from ART-treated chronically infected patients will be necessary to understand the clinical relevance of these findings.
In vitro studies have also revealed that HIV infection decreases the surface expression of the CD4 molecule through an effect of the HIV negative factor (Nef) protein on the endocytosis of CD4 molecules at the cell surface (10). Perhaps even more significant are the observations that the HIV Nef protein downregulates HLA classes I and II at the cell surface (47), which, in turn, has been found to impair antigen-specific T cell activation in vitro (240). The HIV Nef protein has also been reported to downregulate the surface expression of the costimulatory molecules CD80 and CD86 on antigen-presenting cells, which results in an impaired activation of naïve CD4 T cells (46). By disrupting antigen presentation and by undermining the essential molecular interactions critical for CD4 cell recognition and stimulation, HIV has evolved the ability to evade the host immune system and, incidentally, to impair host CD4 T cell recognition of non-HIV antigen-bearing target cells, such as those infected with M. tuberculosis, as well. While the results of these in vitro studies are clear, the quantitative contribution of these mechanisms to the pathogenesis of HIV infection or HIV-related opportunistic infections such as TB in vivo is less well defined.
HIV Effects on TB Immunity: Evidence for and against Selective Depletion of M. tuberculosis-Specific T Cells
Since TB occurs with an increased incidence early after HIV infection, prior to a measurable depletion of CD4 T cells from peripheral blood (234), there is substantial interest in determining whether HIV infection disproportionately depletes M. tuberculosis-specific CD4 T cells. However, the available studies have yielded conflicting results. One study found that the frequency of IFN-γ-producing CD4 T cells responsive to the highly immunogenic ESAT-6 or CFP-10 peptide or to an ESAT-6/CFP-10 fusion protein exhibited a negative correlation with the total CD4 T cell count in HIV-infected, antiretroviral-naïve subjects, implying that CD4 T cells responsive to these antigens are relatively spared during the progressive depletion of total CD4 T cells by HIV (121). In contrast, another study analyzed the frequency of IFN-γ-secreting cells after stimulation with recombinant ESAT-6 or CFP-10 proteins and reported that a lower proportion of HIV-infected individuals had specific responses above a defined cutoff of responding cells (109). In that cross-sectional study, those authors found no correlation between the number of ESAT-6- or CFP-10-responsive cells and the total CD4 T cell count in HIV-infected or -uninfected subjects. Since the subjects in the cross-sectional study were not selected in advance for evidence of latent TB infection, it is unclear how the results might have been confounded by including subjects that were not infected with M. tuberculosis. In an additional effort, those investigators performed longitudinal studies of 5 subjects with latent TB infection at high risk for HIV infection and analyzed CD4 memory T cell (CD4+ CD27− CD45RO+) responses to ESAT-6, CFP-10, or PPD. This revealed that when expressed as a fraction of CD4 memory cells, the frequency of IFN-γ-producing cells declined with time after HIV seroconversion in 4 of the 5 subjects, none of whom developed active TB during the period of follow-up. Ironically, the fifth subject, for whom the frequency of M. tuberculosis antigen-responsive IFN-γ-producing cells did not decline, developed active TB, which was accompanied by an increased frequency of M. tuberculosis antigen-responsive IFN-γ-producing CD4 memory cells. An additional recent study provided compelling evidence for the selective depletion of M. tuberculosis-specific CD4 cells in HIV-infected individuals by comparing the frequencies of CD4 cells specific for M. tuberculosis or for CMV antigens (108). That study also revealed that M. tuberculosis-specific cells more frequently expressed IL-2, while CMV-specific cells expressed macrophage inflammatory protein 1β (MIP-1β), and IL-2 promoted HIV infection, while MIP-1β blocked infection, providing a potential mechanism for the selective infection and depletion of M. tuberculosis-specific CD4 cells compared with CMV-specific cells. Taken together, these results indicate that (i) some individuals maintain populations of M. tuberculosis-specific CD4 T cells even while their overall population of CD4 T cells is declining, (ii) certain individuals disproportionately lose M. tuberculosis-specific CD4 T cells capable of secreting IFN-γ early in the course of HIV infection, (iii) there is substantial variation between HIV-infected individuals in the rate of loss of M. tuberculosis-specific CD4 T cells, and (iv) IFN-γ production by antigen-specific peripheral blood T cells is an imperfect correlate of protective immunity to M. tuberculosis, at least in the context of HIV coinfection. A resolution of the discordant results reported thus far will benefit substantially from further studies with well-characterized subjects, standardized assay conditions, an examination of a broader repertoire of M. tuberculosis antigens, and the use of M. tuberculosis peptide-loaded HLA tetramers to probe the frequency of antigen-specific T cells so that their frequency can be compared to their functional responses.
While the above-mentioned studies of peripheral blood T cell responses yielded conflicting results regarding the early loss of peripheral blood CD4 T cell responses to M. tuberculosis antigens, a recent study of lung cells from HIV-infected persons in an area with a high prevalence of TB revealed evidence for an especially marked effect of HIV infection on M. tuberculosis-specific CD4 T cells in the lungs (141). As expected, the HIV-infected subjects studied had fewer peripheral blood CD4 T cells than did the HIV-negative controls (median, 226 versus 786 cells/μl, respectively). The frequency of CD4 T cells (as the percentage of total T cells) in bronchoalveolar lavage (BAL) fluid was lower in the lungs than in the blood of both HIV-infected and -uninfected subjects, without a disproportionate reduction in HIV-infected subjects. However, M. tuberculosis antigen-specific CD4 T cells were significantly less frequent in BAL fluid from HIV-infected subjects than in BAL fluid from subjects without HIV. The use of intracellular cytokine staining (ICS) to quantitate the frequency of polyfunctional (IFN-γ-, TNF-, and/or IL-2-producing) mycobacterial antigen-specific CD4 T cells also revealed a significant reduction in the amount of polyfunctional CD4 T cells in BAL fluid of HIV-infected versus HIV-negative subjects. These important studies provide evidence that local adaptive immune responses to mycobacterial antigens are disproportionately reduced compared with peripheral blood cell responses in HIV-infected individuals and provide evidence that compromised local lung adaptive immune responses may contribute to susceptibility to a reactivation of latent TB, especially early after HIV infection.
HIV Effects on TB Immunity: Are M. tuberculosis-Specific CD8 T Cells Affected?
While a crucial role for CD4 T cells in human immunity to M. tuberculosis is widely accepted, strong evidence that CD8 T cells contribute to the control of TB in humans is emerging. Human CD8 T cells specific for M. tuberculosis antigens are capable of recognizing and lysing M. tuberculosis-infected cells in vitro (49, 137, 238) and contribute to the killing of mycobacteria, in part through the action of a cytolytic T cell granule protein, granulysin (237). CD8 T cells are essential for protective immunity to M. tuberculosis in mice (194) and nonhuman primates (48), suggesting that they contribute to the control of TB in humans. Moreover, recent work has revealed evidence that the treatment of rheumatoid arthritis patients with the anti-TNF monoclonal antibody infliximab specifically depletes a subset of CD8 T cells that express granulysin and contributes to mycobacterial killing in in vitro assays (32, 189). Taken together, these observations provide strong evidence that CD8 T cells are important for immunity to M. tuberculosis in humans.
HIV may indirectly cause a loss of M. tuberculosis antigen-specific CD8 T cells through the depletion of M. tuberculosis antigen-specific CD4 T cells. In mice, CD4 T cells contribute to the optimal development and maintenance of functional CD8 T cells (176), and in humans with HIV, the number of CD8 T cells specific for a CMV epitope was found to correlate with the number of CMV-responsive CD4 T cells (149), consistent with a need for CD4 cells to maintain CD8 cells in humans. Recent experiments with mice have established that interleukin-21 (IL-21) is the major mediator produced by CD4 T cells that promotes optimal CD8 T cell functions during chronic viral infection (84, 101, 272). Circulating IL-21 concentrations are reduced in HIV-infected subjects and correlate with CD4 T cell counts (130, 131), indicating that CD4 T cells are an important source of IL-21 in humans as well as in mice. Taken together, there is evidence that HIV infection can indirectly lead to the depletion of CD8 T cells that recognize microbial antigens, and this may contribute to susceptibility to opportunistic infections, including TB. With the identification of a large number of specific T cell epitopes from M. tuberculosis (23) and multiple methods of identifying and characterizing the functional activity of antigen-specific CD4 and CD8 T cells, testing the hypothesis that HIV infection depletes M. tuberculosis-specific CD8 in addition to CD4 T cells can be readily accomplished.
HIV Effects on TB Immunity: Effects of Antiretroviral Therapy on Restoration or Recovery of Immunity to TB
As noted previously, there is strong evidence that ART reduces the incidence of TB but that ART does not reduce the incidence of TB to that found for HIV-uninfected populations (156) (Fig. 5). This is compatible with several possible explanations: (i) ART and the suppression of viremia do not restore CD4 cells to the quantitative level needed for normal immunity to TB, (ii) there is a qualitative defect in CD4 T cell effector functions that persists and causes increased susceptibility to TB, (iii) CD4 T cells with antigen receptors specific for M. tuberculosis epitopes are deleted from the overall population of T cells or are rendered tolerant, (iv) other essential functional components of adaptive immunity to TB (such as M. tuberculosis antigen-specific CD8 T cells) are not reconstituted by ART, or (v) some combination of these. Little evidence to support or refute any of these possibilities is currently available, although one study has revealed evidence of a specific impairment of IFN-γ secretion by M. tuberculosis antigen-specific T cells that persisted after ART and the control of HIV (241). Whether this contributes to ongoing susceptibility to TB after ART will require further study.
In addition to its importance for the design of optimal approaches to prevent TB in ART-treated individuals, the observation that immunity to M. tuberculosis is not restored to baseline after ART treatment provides a unique opportunity to discover and validate immunological correlates of immunity to TB. Prospective immune profiling of ART-treated individuals in areas with a high TB prevalence, correlated with the development of TB or not, may provide strong evidence for responses associated with protective immunity. Such discoveries would have great value in guiding the design and testing of the next generation of vaccines against TB.
TB Effects on HIV: the Other Side of the Cellular and Molecular Syndemic
As noted above, epidemiological and clinical studies have provided evidence that active TB increases the likelihood of death with HIV and also increases viral loads and viral heterogeneity, especially at sites of TB infection. Modeling this phenomenon in vitro, several studies have revealed that M. tuberculosis infection can increase HIV replication in CD4 T cells and macrophages and have provided insight into multiple underlying mechanisms.
Early after the recognition that TB and HIV were converging to amplify the problems that each of the epidemics posed alone (231), peripheral blood monocytes from patients with active TB were found to be more susceptible to productive infection by HIV in vitro (247). Moreover, levels of HIV have been found to be higher in M. tuberculosis-infected regions of the lungs than in the uninvolved regions of the same patient (274). Several subsequent studies have provided mechanistic insights into this effect of TB. M. tuberculosis activates proinflammatory responses of macrophages by signaling through Toll-like receptor 2 (TLR2) and TLR4 (179) and through nucleotide-binding oligomerization domain 2 (NOD2) (77, 93) and by inducing the secretion of the cytokine TNF. TLR2, TLR4, NOD2, and TNF all signal through the host transcription factor NF-κB (179), which directly activates the transcription of HIV (79, 201, 205). Therefore, it is not surprising that M. tuberculosis activates the production of HIV in macrophages.
Since the quantitative contribution of HIV production by infected macrophages to the overall viral burden is uncertain, it is important to consider that TB also increases the production of HIV by T lymphocytes. In vitro stimulation with M. tuberculosis antigens markedly increases viral production by CD4 T cells from HIV-infected individuals, and this increase is dependent on the prior sensitization of the T cell donor to M. tuberculosis (116). Therefore, by providing a chronic source of antigenic stimulation for M. tuberculosis-specific CD4 T cells, active TB can dramatically increase the production of HIV. Antigen activation can amplify the production of HIV in CD4 T cells by at least three mechanisms. First, T cell antigen receptor (TCR) signaling activates the transcription factor NFATc (nuclear factor of activated T cells-c), which activates the reverse transcription of HIV (147). Second, TCR stimulation, together with CD28 engagement, activates NF-κB (132), which increases the transcription of HIV. Third, the activation of T cells through the TCR increases the expression of the HIV coreceptor CCR5 (271), thereby increasing the susceptibility of CD4 T cells to infection by HIV.
While the effects of M. tuberculosis infection noted above provide strong evidence for multiple mechanisms in which active TB can amplify the production of HIV in vivo and in vitro, at least one mechanism that can actually inhibit HIV production has been found. The chemokine ligands that use CCR5 (MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5) effectively compete with HIV for CCR5 and inhibit the infection of CCR5-expressing cells (148). M. tuberculosis is a potent inducer of these chemokines in primary macrophages (221, 224), indicating that at least in certain local environments, there may be inhibitory effects of M. tuberculosis that can counter the effects that increase the production of HIV. While polymorphisms in the genes encoding CCL3 and CCL5 and higher levels of secretion of these chemokines are associated with slower progression to AIDS (107, 193, 273), it is likely that for patients with active TB, the HIV-promoting effects of TB overcome this potential inhibitory mechanism, thereby worsening the course of HIV infection.
In addition to the known mechanisms for TB worsening HIV, at least one additional mechanism should be considered. TB is characterized histopathologically by the formation of granulomas, which are organized aggregates in which macrophages, dendritic cells, T cells, and B cells are closely apposed (215). Since the macrophages and dendritic cells in granulomas include cells containing bacteria, some of which are also infected with HIV, it is likely that M. tuberculosis-infected macrophages are activated to produce HIV that is then available to infect adjacent macrophages and CD4 T cells in the granuloma. Likewise, HIV-infected CD4 T cells that are activated by the recognition of M. tuberculosis antigens presented by granuloma macrophages and dendritic cells likely produce large amounts of HIV, followed by the efficient infection of adjacent CD4 T cells and macrophages in the granuloma. Since recent studies of the early stages of HIV infection have revealed that the local recruitment of CD4 T cells accelerates the early spread and progression of HIV (118, 119), it is also possible that in TB, granulomas as aggregates of M. tuberculosis- and HIV-infected cells provide a highly efficient site for the amplification of HIV. This hypothesis should be readily testable in nonhuman primate systems and may provide an incentive to modulate cell trafficking to granulomas as an adjunct to the treatment of tuberculosis in HIV-coinfected individuals.
In summary, there is strong evidence that at the cellular and molecular levels, HIV and M. tuberculosis interact to amplify the effects of both pathogens, resulting in a cellular and molecular syndemic that is manifested by accelerated disease in individuals and in a massive global public health catastrophe.
SUMMARY
The emergence of the HIV pandemic in human populations already having a significant burden of TB has markedly worsened the global TB epidemic, with more cases of TB in 2008 than in any year in history. Likewise, TB is a major cause of death in people infected with HIV. The epidemiological, clinical, immunological, and molecular biological studies done to date have revealed that HIV and TB synergize with one another at the population, individual, cellular, and molecular levels. Without an adequate control of the TB-HIV syndemic, the long-term TB elimination target set for 2050 will not be reached (163). There is an urgent need for additional resources and novel approaches for the diagnosis, treatment, and prevention of both HIV and TB. Multidisciplinary approaches that consider HIV and TB together, rather than as separate problems and diseases, will be necessary to prevent the further worsening of the HIV-TB syndemic. Some of the key topics that we think warrant intensified investigation in order to better control the TB-HIV syndemic are discussed here.
Prevention of TB Infection in HIV-Infected Persons
Understanding the reservoir of tuberculosis.
In populations with a high prevalence of HIV and TB, do most people with HIV acquire M. tuberculosis from HIV-infected or from HIV-uninfected individuals? If HIV-uninfected people are the source of a disproportionately high fraction of TB transmission, then intensified case-finding and treatment efforts should also be directed toward this group. On the other hand, are there differences in genetic characteristics of TB strains that infect HIV-infected individuals compared with those that infect HIV-uninfected individuals?
Understanding factors affecting infectiousness and transmission of TB.
How can high-TB transmitters be identified so that their contacts can be targeted for diagnostic and prevention efforts? Are there correlates of high transmission capability beyond the presence of pulmonary cavities, frequent coughing, and smear positivity? Considerable evidence indicates that individuals with active pulmonary TB vary greatly in their efficiency of transmitting TB, but there are few reliable tools to distinguish them. The discovery and validation of bacterial strain characteristics and/or specific host innate or adaptive immune responses that correlate with efficient transmission would allow intensified prevention efforts to be directed at these individuals and their contacts.
Tailoring infection control and ventilation practices to resource-poor settings.
How can we adapt standard infection control and ventilation practices to the realities of resource-limited ambulatory clinics, hospitals, and prisons? Are there cheaper and lower-maintenance methods for ensuring adequate ventilation in waiting rooms, clinics, hospitals, and clinics? Can we standardize and scale up infection control and ventilation practices in resource-limited settings to prevent the nosocomial spread of TB to HIV-infected patients?
Understanding susceptibility to TB infection.
Does HIV predispose an individual to TB by mechanisms beyond the depletion of CD4 T cells? Since immune reconstitution with ART reduces the risk of TB in HIV-infected people but does not reduce the risk to that of people never infected with HIV, it seems clear that there remain specific immune defects despite the restoration of CD4 T cell counts. Well-planned prospective studies of the course of immune reconstitution assayed by high-resolution immunological assays may reveal specific deficits that persist in those people treated with ART who subsequently develop TB. Results of these studies would not only inform future investigations of TB in HIV-infected people but may also reveal unique mechanisms and correlates of immunity to TB that will be valuable in designing TB vaccines and their trials. If an efficacious TB vaccine can be developed, the immunization of immunocompetent individuals may decrease the incidence of TB in HIV-infected people.
Preventing Progression to Active TB in HIV-Infected Persons with LTBI
Understanding the cellular and molecular pathophysiology of TB infection in HIV-infected persons.
Does HIV affect the trafficking of M. tuberculosis in macrophages and dendritic cells? Does HIV affect the survival and/or replication of M. tuberculosis in macrophages and dendritic cells? Studies by multiple investigators have revealed considerable data on the trafficking of M. tuberculosis phagosomes and their interactions with other vesicular organelles, and it is believed that interference with phagosome maturation is an important determinant of M. tuberculosis survival and persistence. However, there is little, if any, information on whether or how the infection of macrophages with HIV affects the maturation of M. tuberculosis-containing phagosomes. An intriguing combination of recent findings suggests a potential mechanism whereby HIV could alter phagosome maturation and promote the intracellular survival of M. tuberculosis. A genome-wide RNA interference (RNAi) screen identified the endosomal sorting complex required for transport (ESCRT) machinery, including the protein tsg101, as being essential for restricting the growth of avirulent or attenuated mycobacteria (213), suggesting that virulent M. tuberculosis may target this apparatus in order to enhance its intracellular survival. Since HIV is known to utilize tsg101 in the process of viral budding (106), it is reasonable to hypothesize that HIV may divert tsg101 and the ESCRT apparatus from mycobacterial phagosomes and thereby promote the intracellular survival and replication of M. tuberculosis.
Developing algorithms to increase the sensitivity of detection of latent TB infection in HIV-infected persons.
The latest U.S. guidelines for the use of IGRAs in HIV-infected persons suggest that both TSTs and IGRAs may be used in combination to diagnose LTBI in a person with HIV when the initial screening test is negative. Developing and standardizing a multistep testing algorithm for the screening of LTBI in HIV-infected person will help improve the ability to diagnose and treat LTBI in HIV-infected persons to prevent progression to active disease.
Discovering biomarkers associated with increased risk for progression to active TB.
Of HIV-infected persons with LTBI, what are the correlates of progression to active TB besides low CD4 counts? Being able to identify markers associated with progression to active TB will help better target more frequent screening and treatment for LTBI.
Continued scale-up and support for antiretroviral treatment.
Treatment with ART decreases the incidence of TB, and therefore, access to ART is an integral part of efforts to control the TB-HIV syndemic.
Timely and Appropriate Treatment for HIV-Infected Persons with Active TB
Improved and faster diagnostics for resource-limited settings.
Current diagnostic tests for TB are outdated, and over the past decade, multiple organizations such as the Stop TB Partnership, the Foundation for Innovative New Diagnostics, the Global Laboratory Initiative, the World Health Organization, and the Bill and Melinda Gates Foundation have invested in and advocated for the development of new diagnostic tools for TB. Additionally, the ability to obtain nearly full genome sequences of bacterial isolates is rapidly improving, and costs are progressively decreasing with new technological developments. The assiduous application of whole-genome sequencing may reveal a much deeper understanding of the diversity and complexity of M. tuberculosis strains and their adaptation to specific host populations. An understanding of these factors is likely to guide efforts to develop novel approaches to the diagnosis, prevention, and treatment of TB in HIV-infected individuals.
Understanding the relationship between drug-resistant TB and HIV.
Are HIV-infected patients more likely to be infected with multiple strains of M. tuberculosis? What are the biological mechanisms that contribute to the association of HIV and drug-resistant TB? While the association of HIV and drug-resistant TB can partially be attributed to the exposure of HIV-infected people to others with drug-resistant TB in poorly ventilated areas, it is plausible to hypothesize that biological factors also play a role in driving this association. Are there strains of M. tuberculosis that disproportionately affect HIV-infected people that are especially prone to becoming drug resistant? Are there host factors in HIV-infected people that contribute to the development of primary drug resistance, for example, a higher bacterial burden with a larger number of spontaneously resistant bacteria than present in immunocompetent people? Is the fitness cost of certain drug resistance mutations overcome by the immunodeficiency associated with HIV infection so that the transmission of drug-resistant strains to HIV-infected people is more efficient than that to immunocompetent hosts?
Understanding drug-drug interactions between antiretroviral and TB medications.
With the introduction of new protease inhibitors, integrase inhibitors, and entry inhibitors for the treatment of HIV, investigations into the pharmacokinetic and pharmacodynamic drug interactions between HIV medications and TB medications are critically needed in order to minimize drug toxicities, the development of HIV drug resistance, and the development of TB drug resistance. Better data on the drug-drug interactions between ARTs and second-line TB drugs are also needed to minimize drug toxicities for the treatment of MDR-TB and HIV.
CONCLUSIONS
The HIV-TB syndemic has had a major impact on human health and disproportionately affects people in Africa. However, a high prevalence of TB anywhere in the world poses risks to the health of people elsewhere, since TB is transmitted by the aerosol route. While the scale of the HIV-TB syndemic seems daunting, the application of existing knowledge and techniques for the diagnosis, treatment, and prevention of TB can make an impact. Further progress will require advances in our understanding of the dual biology of HIV and TB coinfection as well as a better understanding of the interactions of M. tuberculosis with HIV-infected and -uninfected humans and their innate and adaptive immune systems.
ACKNOWLEDGMENTS
We thank Judith Aberg, Bouke deJong, and Ned Landau for their insightful comments on early drafts of the manuscript.
This work was supported in part by grants from the National Institutes of Health (grants R01 AI051242, R01 AI084041, and R01 AI090928).
Biographies
Candice K. Kwan graduated from Harvard College in 1996 and Harvard Medical School in 2002 and completed her residency in internal medicine at Columbia University Medical Center in 2005. After residency, she served as a senior technical officer at Family Health International's China office and then as a program officer in the China office of the Bill and Melinda Gates Foundation. She subsequently completed her infectious diseases fellowship at New York University in 2010 and is currently an officer in the Epidemic Intelligence Service at the CDC. Her interest in HIV and tuberculosis started as a medical student, when she worked on a CDC-sponsored project to evaluate the spectrum of opportunistic diseases in patients with HIV infection in Hanoi, Vietnam. Since then, her research interests have focused on HIV prevention, the epidemiology of HIV infection, and opportunistic infections.
Joel D. Ernst received his medical education and training at the University of Nebraska and the University of California, San Francisco (UCSF). He was on the UCSF faculty for 16 years prior to moving to New York University in 2003 as the Director of Infectious Diseases. He has cared for people with HIV/AIDS since the beginning of the epidemic in San Francisco and has been studying host-pathogen interactions in tuberculosis since 1993. His TB research interests were kindled by his clinical observations as a student and resident and have focused on the mechanisms used by M. tuberculosis to evade and exploit innate and adaptive immune responses in order to persist, cause disease, and be transmitted.
REFERENCES
- 1. Aabye M. G., et al. 2009. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS One 4:e4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdool Karim S. S., Churchyard G. J., Abdool Karim Q., Lawn S. D. 2009. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 374:921–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdool Karim S. S., et al. 2010. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 362:697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aberg J. A., et al. 2009. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 49:651–681 [DOI] [PubMed] [Google Scholar]
- 5. Abgrall S., Del Giudice P., Melica G., Costagliola D. 2010. HIV-associated tuberculosis and immigration in a high-income country: incidence trends and risk factors in recent years. AIDS 24:763–771 [DOI] [PubMed] [Google Scholar]
- 6. Abouya L., et al. 1995. Radiologic manifestations of pulmonary tuberculosis in HIV-1 and HIV-2-infected patients in Abidjan, Côte d'Ivoire. Tuber. Lung Dis. 76:436–440 [DOI] [PubMed] [Google Scholar]
- 7. Ackah A. N., et al. 1995. Response to treatment, mortality, and CD4 lymphocyte counts in HIV-infected persons with tuberculosis in Abidjan, Côte d'Ivoire. Lancet 345:607–610 [DOI] [PubMed] [Google Scholar]
- 8. Ahmed A. B., et al. 2007. The growing impact of HIV infection on the epidemiology of tuberculosis in England and Wales: 1999-2003. Thorax 62:672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aichelburg M. C., et al. 2009. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clin.Infect. Dis. 48:954–962 [DOI] [PubMed] [Google Scholar]
- 10. Aiken C., Konner J., Landau N. R., Lenburg M. E., Trono D. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853–864 [DOI] [PubMed] [Google Scholar]
- 11. Alonso Rodríguez N., et al. 2009. Transmission permeability of tuberculosis involving immigrants, revealed by a multicentre analysis of clusters. Clin. Microbiol. Infect. 15:435–442 [DOI] [PubMed] [Google Scholar]
- 12. Andrews J. R., et al. 2008. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J. Infect. Dis. 198:1582–1589 [DOI] [PubMed] [Google Scholar]
- 13. Badri M., Ehrlich R., Wood R., Pulerwitz T., Maartens G. 2001. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int. J. Tuberc. Lung Dis. 5:225–232 [PubMed] [Google Scholar]
- 14. Badri M., Wilson D., Wood R. 2002. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359:2059–2064 [DOI] [PubMed] [Google Scholar]
- 15. Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E. 1991. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 324:1644–1650 [DOI] [PubMed] [Google Scholar]
- 16. Bartlett J. A., Hornberger J., Shewade A., Bhor M., Rajagopalan R. 2009. Obstacles and proposed solutions to effective antiretroviral therapy in resource-limited settings. J. Int. Assoc. Physicians AIDS Care 8:253–268 [DOI] [PubMed] [Google Scholar]
- 17. Bartlett J. G., Auwaerter P. G., Pham P. 2010. Johns Hopkins POC-IT Center HIV guide. Johns Hopkins University, Baltimore, MD: http://www.hopkins-hivguide.org [Google Scholar]
- 17a. Bartlett J. G., Auwaerter P. G., Pham P. A. 2010. Johns Hopkins POC-IT Center ABX guide: diagnosis & treatment of infectious diseases, 2nd ed Jones & Bartlett, Sudbury, MA: http://hopkins-abxguide.org/ [Google Scholar]
- 18. Basu S., et al. 2007. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet 370:1500–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Batungwanayo J., et al. 1992. Pulmonary tuberculosis in Kigali, Rwanda. Impact of human immunodeficiency virus infection on clinical and radiographic presentation. Am. Rev. Respir. Dis. 146:53–56 [DOI] [PubMed] [Google Scholar]
- 20. Bekker L. G., Wood R. 2010. The changing natural history of tuberculosis and HIV coinfection in an urban area of hyperendemicity. Clin. Infect. Dis. 50:S208–S214 [DOI] [PubMed] [Google Scholar]
- 21. Blaak H., et al. 2000. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc. Natl. Acad. Sci. U. S. A. 97:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blanc F.-X., et al. 2010. Significant enhancement in survival with early (2 weeks) vs. late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis, abstr. THLBB0106. International AIDS Conference, Vienna, Austria [Google Scholar]
- 23. Blythe M. J., et al. 2007. An analysis of the epitope knowledge related to mycobacteria. Immunome Res. 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bocchino M., Bellofiore B., Matarese A., Galati D., Sanduzzi A. 2009. IFN-gamma release assays in tuberculosis management in selected high-risk populations. Expert Rev. Mol. Diagn. 9:165–177 [DOI] [PubMed] [Google Scholar]
- 25. Boehme C. C., et al. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borgdorff M. W., Behr M. A., Nagelkerke N. J., Hopewell P. C., Small P. M. 2000. Transmission of tuberculosis in San Francisco and its association with immigration and ethnicity. Int. J. Tuberc. Lung Dis. 4:287–294 [PubMed] [Google Scholar]
- 27. Borgdorff M. W., et al. 2010. Progress towards tuberculosis elimination: secular trend, immigration and transmission. Eur. Respir. J. 36:339–347 [DOI] [PubMed] [Google Scholar]
- 28. Boulanger C., et al. 2009. Pharmacokinetic evaluation of rifabutin in combination with lopinavir-ritonavir in patients with HIV infection and active tuberculosis. Clin. Infect. Dis. 49:1305–1311 [DOI] [PubMed] [Google Scholar]
- 29. Boulle A., et al. 2008. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA 300:530–539 [DOI] [PubMed] [Google Scholar]
- 30. Brainard D. M., et al. Lack of a clinically meaningful pharmacokinetic effect of rifabutin on raltegravir: in vitro/in vivo correlation. J. Clin. Pharmacol., in press [DOI] [PubMed] [Google Scholar]
- 31. Brenchley J. M., et al. 2008. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112:2826–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruns H., et al. 2009. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Invest. 119:1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burman W. J., Jones B. E. 2003. Clinical and radiographic features of HIV-related tuberculosis. Semin. Respir. Infect. 18:263–271 [DOI] [PubMed] [Google Scholar]
- 34. Busi Rizzi E., Schininà V., Palmieri F., Girardi E., Bibbolino C. 2004. Cavitary pulmonary tuberculosis HIV-related. Eur. J. Radiol. 52:170–174 [DOI] [PubMed] [Google Scholar]
- 35. Cain K. P., et al. 2010. An algorithm for tuberculosis screening and diagnosis in people with HIV. N. Engl. J. Med. 362:707–716 [DOI] [PubMed] [Google Scholar]
- 36. Carvalho A. C., et al. 2001. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am. J. Respir. Crit. Care Med. 164:2166–2171 [DOI] [PubMed] [Google Scholar]
- 37. Cattamanchi A., et al. 2008. Detailed analysis of the radiographic presentation of Mycobacterium kansasii lung disease in patients with HIV infection. Chest 133:875–880 [DOI] [PubMed] [Google Scholar]
- 38. CDC 1991. Epidemiologic notes and reports of nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons—Florida and New York, 1988-1991. MMWR Morb. Mortal. Wkly. Rep. 40:585–591 [PubMed] [Google Scholar]
- 39. CDC 2005. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:1–141 [PubMed] [Google Scholar]
- 40. CDC 2007. Managing drug interactions in the treatment of HIV-related tuberculosis. CDC, Atlanta, GA: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm [Google Scholar]
- 41. CDC 1993. Outbreak of multidrug-resistant tuberculosis at a hospital—New York City, 1991. MMWR Morb. Mortal. Wkly. Rep. 42:427–433 [PubMed] [Google Scholar]
- 42. CDC 2006. Prevention and control of tuberculosis in correctional and detention facilities: recommendations from CDC. Endorsed by the Advisory Council for the Elimination of Tuberculosis, the National Commission on Correctional Health Care, and the American Correctional Association. MMWR Recommend. Rep. 55(RR-9):1–44 [PubMed] [Google Scholar]
- 43. CDC 1992. Transmission of multidrug-resistant tuberculosis among immunocompromised persons in a correctional system—New York, 1991. MMWR Morb. Mortal. Wkly. Rep. 41:507–509 [PubMed] [Google Scholar]
- 44. CDC 1993. Tuberculosis morbidity-United States, 1992. MMWR Morb. Mortal. Wkly. Rep. 42:696–697,703–704 [PubMed] [Google Scholar]
- 45. Charalambous S., et al. 2008. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int. J. Tuberc. Lung Dis. 12:942–948 [PubMed] [Google Scholar]
- 46. Chaudhry A., et al. 2005. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 175:4566–4574 [DOI] [PubMed] [Google Scholar]
- 47. Chaudhry A., et al. 2009. HIV-1 Nef promotes endocytosis of cell surface MHC class II molecules via a constitutive pathway. J. Immunol. 183:2415–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen C. Y., et al. 2009. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 5:e1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho S., et al. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 97:12210–12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clark M., Riben P., Nowgesic E. 2002. The association of housing density, isolation and tuberculosis in Canadian First Nations communities. Int. J. Epidemiol. 31:940–945 [DOI] [PubMed] [Google Scholar]
- 51. Clerici M., et al. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Invest. 84:1892–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen K., Meintjes G. 2010. Management of individuals requiring antiretroviral therapy and TB treatment. Curr. Opin. HIV AIDS 5:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Colebunders R., Bastian I. 2000. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 4:97–107 [PubMed] [Google Scholar]
- 54. Colebunders R., et al. 2006. Tuberculosis immune reconstitution inflammatory syndrome in countries with limited resources. Int. J. Tuberc. Lung Dis. 10:946–953 [PubMed] [Google Scholar]
- 55. Collins K. R., Quiñones-Mateu M. E., Toossi Z., Arts E. J. 2002. Impact of tuberculosis on HIV-1 replication, diversity, and disease progression. AIDS Rev. 4:165–176 [PubMed] [Google Scholar]
- 56. Cooper A. M., et al. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corbett E. L., et al. 2009. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int. J. Tuberc. Lung Dis. 13:1231–1237 [PMC free article] [PubMed] [Google Scholar]
- 58. Corbett E. L., et al. 2007. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 4:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Corbett E. L., et al. 2004. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am. J. Respir. Crit. Care Med. 170:673–679 [DOI] [PubMed] [Google Scholar]
- 60. Corbett E. L., De Cock K. M. 2001. The clinical significance of interactions between HIV and TB: more questions than answers. Int. J. Tuberc. Lung Dis. 5:205–207 [PubMed] [Google Scholar]
- 61. Corbett E. L., Marston B., Churchyard G. J., De Cock K. M. 2006. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 367:926–937 [DOI] [PubMed] [Google Scholar]
- 62. Corbett E. L., et al. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009–1021 [DOI] [PubMed] [Google Scholar]
- 63. Corbett E. L., et al. 2010. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull. World Health Organ. 88:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cruciani M., Malena M., Bosco O., Gatti G., Serpelloni G. 2001. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin. Infect. Dis. 33:1922–1930 [DOI] [PubMed] [Google Scholar]
- 65. Cunningham R., Sabin F., Sugiyama S., Kindwald J. 1925. Role of the monocyte in tuberculosis. Bull. Johns Hopkins Hosp. xxxvii:231–278 [Google Scholar]
- 66. Daley C. L., Hahn J. A., Moss A. R., Hopewell P. C., Schecter G. F. 1998. Incidence of tuberculosis in injection drug users in San Francisco: impact of anergy. Am. J. Respir. Crit. Care Med. 157:19–22 [DOI] [PubMed] [Google Scholar]
- 67. Daley C. L., et al. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N. Engl. J. Med. 326:231–235 [DOI] [PubMed] [Google Scholar]
- 68. Da Silva R. M., da Rosa L., Lemos R. N. 2006. Radiographic alterations in patients presenting human immunodeficiency virus/tuberculosis coinfection: correlation with CD4+ T cell counts. J. Bras. Pneumol. 32:228–233(In Portugese.) [PubMed] [Google Scholar]
- 69. Day J. H., et al. 2004. Does tuberculosis increase HIV load? J. Infect. Dis. 190:1677–1684 [DOI] [PubMed] [Google Scholar]
- 70. DeRiemer K., Kawamura L. M., Hopewell P. C., Daley C. L. 2007. Quantitative impact of human immunodeficiency virus infection on tuberculosis dynamics. Am. J. Respir. Crit. Care Med. 176:936–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Desalu O., et al. 2009. Impact of immunosuppression on radiographic features of HIV related pulmonary tuberculosis among Nigerians. Turk. Thorac. J. 11:112–116 [Google Scholar]
- 72. Desvignes L., Ernst J. D. 2009. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31:974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dheda K., Lampe F. C., Johnson M. A., Lipman M. C. 2004. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J. Infect. Dis. 190:1670–1676 [DOI] [PubMed] [Google Scholar]
- 74. Dheda K., Smit R. V. Z., Badri M., Pai M. 2009. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr. Opin. Pulm. Med. 15:188–200 [DOI] [PubMed] [Google Scholar]
- 75. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC [Google Scholar]
- 76. Diedrich C. R., et al. 2010. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS One 5:e9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Divangahi M., et al. 2008. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J. Immunol. 181:7157–7165 [DOI] [PubMed] [Google Scholar]
- 78. Doitsh G., et al. 2010. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. U. S. A. 86:5974–5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dye C., Williams B. G. 2010. The population dynamics and control of tuberculosis. Science 328:856–861 [DOI] [PubMed] [Google Scholar]
- 81. Egwaga S. M., et al. 2006. The impact of the HIV epidemic on tuberculosis transmission in Tanzania. AIDS 20:915–921 [DOI] [PubMed] [Google Scholar]
- 82. Elliott J. H., et al. 2009. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J. Infect. Dis. 200:1736–1745 [DOI] [PubMed] [Google Scholar]
- 83. El-Sadr W. M., Tsiouris S. J. 2008. HIV-associated tuberculosis: diagnostic and treatment challenges. Semin. Respir. Crit. Care Med. 29:525–531 [DOI] [PubMed] [Google Scholar]
- 84. Elsaesser H., Sauer K., Brooks D. G. 2009. IL-21 is required to control chronic viral infection. Science 324:1569–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Emmelkamp J. M., Rockstroh J. K. 2007. CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions—review of the literature. Eur. J. Med. Res. 12:409–417 [PubMed] [Google Scholar]
- 86. Escombe A. R., et al. 2008. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 5:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Escombe A. R., et al. 2007. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin. Infect. Dis. 44:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Escombe A. R., et al. 2007. Natural ventilation for the prevention of airborne contagion. PLoS Med. 4:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Espinal M. A., et al. 2000. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet 355:275–280 [DOI] [PubMed] [Google Scholar]
- 90. Espinal M. A., Reingold A. L., Koenig E., Lavandera M., Sanchez S. 1995. Screening for active tuberculosis in HIV testing centre. Lancet 345:890–893 [DOI] [PubMed] [Google Scholar]
- 91. Fennelly K. P. 2007. Variability of airborne transmission of Mycobacterium tuberculosis: implications for control of tuberculosis in the HIV era. Clin. Infect. Dis. 44:1358–1360 [DOI] [PubMed] [Google Scholar]
- 92. Fennelly K. P., et al. 2004. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 169:604–609 [DOI] [PubMed] [Google Scholar]
- 93. Ferwerda G., et al. 2005. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 1:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Flynn J. L., et al. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. French M. A., French M. A. 2009. HIV/AIDS. Immune reconstitution inflammatory syndrome: a reappraisal. Clin. Infect. Dis. 48:101–107 [DOI] [PubMed] [Google Scholar]
- 96. French M. A., Price P., Stone S. F. 2004. Immune restoration disease after antiretroviral therapy. AIDS 18:1615–1627 [DOI] [PubMed] [Google Scholar]
- 97. Frieden T. R., Fujiwara P. I., Washko R. M., Hamburg M. A. 1995. Tuberculosis in New York City—turning the tide. N. Engl. J. Med. 333:229–233 [DOI] [PubMed] [Google Scholar]
- 98. Frieden T. R., et al. 1996. A multi-institutional outbreak of highly drug-resistant tuberculosis: epidemiology and clinical outcomes. JAMA 276:1229–1235 [PubMed] [Google Scholar]
- 99. Frieden T. R., Sterling T. R., Munsiff S. S., Watt C. J., Dye C. 2003. Tuberculosis. Lancet 362:887–899 [DOI] [PubMed] [Google Scholar]
- 100. Friedman L. N., Williams M. T., Singh T. P., Frieden T. R. 1996. Tuberculosis, AIDS, and death among substance abusers on welfare in New York City. N. Engl. J. Med. 334:828–833 [DOI] [PubMed] [Google Scholar]
- 101. Frohlich A., et al. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580 [DOI] [PubMed] [Google Scholar]
- 102. Gallant J. E., Ko A. H. 1996. Cavitary pulmonary lesions in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 22:671–682 [DOI] [PubMed] [Google Scholar]
- 103. Gandhi N. R., et al. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580 [DOI] [PubMed] [Google Scholar]
- 104. Gandhi N. R., et al. 2010. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am. J. Respir. Crit. Care Med. 181:80–86 [DOI] [PubMed] [Google Scholar]
- 105. Garcia G. F., Moura A. S., Ferreira C. S., da Costa Rocha M. O. 2007. Clinical and radiographic features of HIV-related pulmonary tuberculosis according to the level of immunosuppression. Rev. Soc. Bras. Med. Trop. 40:622–626 [DOI] [PubMed] [Google Scholar]
- 106. Garrus J. E., et al. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55–65 [DOI] [PubMed] [Google Scholar]
- 107. Garzino-Demo A., et al. 1999. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc. Natl. Acad. Sci. U. S. A. 96:11986–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Geldmacher C., et al. 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J. Exp. Med. 207:2869–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Geldmacher C., et al. 2008. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 198:1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Geng E., Kreiswirth B., Burzynski J., Schluger N. W. 2005. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA 293:2740–2745 [DOI] [PubMed] [Google Scholar]
- 111. Getahun H., Gunneberg C., Granich R., Nunn P. 2010. HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 50:S201–S207 [DOI] [PubMed] [Google Scholar]
- 112. Girardi E., et al. 2004. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur. Respir. J. 24:11–17 [DOI] [PubMed] [Google Scholar]
- 113. Girardi E., et al. 2005. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin. Infect. Dis. 41:1772–1782 [DOI] [PubMed] [Google Scholar]
- 114. Glocker E. O., et al. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361:1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Glynn J. R., et al. 2010. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J. Infect. Dis. 201:704–711 [DOI] [PubMed] [Google Scholar]
- 116. Goletti D., et al. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. (Baltimore) 157:1271–1278 [PubMed] [Google Scholar]
- 117. Greenberg S. D., et al. 1994. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance). Radiology 193:115–119 [DOI] [PubMed] [Google Scholar]
- 118. Haase A. T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625–656 [DOI] [PubMed] [Google Scholar]
- 119. Haase A. T. 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464:217–223 [DOI] [PubMed] [Google Scholar]
- 120. Haddow L. J., Moosa M.-Y. S., Easterbrook P. J. 2010. Validation of a published case definition for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 24:103–108 [DOI] [PubMed] [Google Scholar]
- 121. Hammond A. S., et al. 2008. Mycobacterial T cell responses in HIV-infected patients with advanced immunosuppression. J. Infect. Dis. 197:295–299 [DOI] [PubMed] [Google Scholar]
- 122. Harries A. D., et al. 2001. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet 357:1519–1523 [DOI] [PubMed] [Google Scholar]
- 123. Harries A. D., Zachariah R., Lawn S. D. 2009. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int. J. Tuberc. Lung Dis. 13:6–16 [PubMed] [Google Scholar]
- 124. Hazenberg M. D., Hamann D., Schuitemaker H., Miedema F. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285–289 [DOI] [PubMed] [Google Scholar]
- 125. Hellerstein M., et al. 1999. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat. Med. 5:83–89 [DOI] [PubMed] [Google Scholar]
- 126. Hellerstein M. K., et al. 2003. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J. Clin. Invest. 112:956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Holtz T. H. 2007. XDR-TB in South Africa: revised definition. PLoS Med. 4:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Horsburgh C. R. 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350:2060–2067 [DOI] [PubMed] [Google Scholar]
- 129. Humphreys I. R., et al. 2006. A role for dendritic cells in the dissemination of mycobacterial infection. Microbes Infect. 8:1339–1346 [DOI] [PubMed] [Google Scholar]
- 130. Iannello A., et al. 2010. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J. Immunol. 184:114–126 [DOI] [PubMed] [Google Scholar]
- 131. Iannello A., et al. 2008. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 21:385–388 [DOI] [PubMed] [Google Scholar]
- 132. Jamieson C., McCaffrey P. G., Rao A., Sen R. 1991. Physiologic activation of T cells via the T cell receptor induces NF-kappa B. J. Immunol. 147:416–420 [PubMed] [Google Scholar]
- 133. Janssens J. P., Rieder H. L. 2008. An ecological analysis of incidence of tuberculosis and per capita gross domestic product. Eur. Respir. J. 32:1415–1416 [DOI] [PubMed] [Google Scholar]
- 134. Jenny-Avital E. R., Joseph K. 2009. Rifamycin-resistant Mycobacterium tuberculosis in the highly active antiretroviral therapy era: a report of 3 relapses with acquired rifampin resistance following alternate-day rifabutin and boosted protease inhibitor therapy. Clin. Infect. Dis. 48:1471–1474 [DOI] [PubMed] [Google Scholar]
- 135. Jindani A., Nunn A. J., Enarson D. A. 2004. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 364:1244–1251 [DOI] [PubMed] [Google Scholar]
- 136. Jones B. E., et al. 1993. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. Rev. Respir. Dis. 148:1292–1297 [DOI] [PubMed] [Google Scholar]
- 137. Joosten S. A., et al. 2010. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog. 6:e1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Joseph P., et al. 2006. Multidrug-resistant tuberculosis at an HIV testing center in Haiti. AIDS 20:415–418 [DOI] [PubMed] [Google Scholar]
- 139. Jouanguy E., et al. 2000. In a novel form of IFN-gamma receptor 1 deficiency, cell surface receptors fail to bind IFN-gamma. J. Clin. Invest. 105:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jouanguy E., et al. 1997. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Invest. 100:2658–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kalsdorf B., et al. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 180:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kaplan J. E., et al. 2009. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommend. Rep. 58:1–207 [PubMed] [Google Scholar]
- 143. Kawooya V. K., Kawooya M., Okwera A. 2000. Radiographic appearances of pulmonary tuberculosis in HIV-1 seropositive and seronegative adult patients. East Afr. Med. J. 77:303–307 [PubMed] [Google Scholar]
- 144. Keiper M. D., Beumont M., Elshami A., Langlotz C. P., Miller W. T. 1995. CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis. A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest 107:74–80 [DOI] [PubMed] [Google Scholar]
- 145. Kenyon T. A., et al. 2002. Risk factors for transmission of Mycobacterium tuberculosis from HIV-infected tuberculosis patients, Botswana. Int. J. Tuberc. Lung Dis. 6:843–850 [PubMed] [Google Scholar]
- 146. Khan F. A., et al. 2010. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin. Infect. Dis. 50:1288–1299 [DOI] [PubMed] [Google Scholar]
- 147. Kinoshita S., Chen B. K., Kaneshima H., Nolan G. P. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595–604 [DOI] [PubMed] [Google Scholar]
- 148. Kinter A. L., et al. 1996. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous beta-chemokines and the viral inductive effects of other endogenous cytokines. Proc. Natl. Acad. Sci. U. S. A. 93:14076–14081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Komanduri K. V., et al. 2001. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 279:459–470 [DOI] [PubMed] [Google Scholar]
- 150. Korenromp E. L., Scano F., Williams B. G., Dye C., Nunn P. 2003. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin. Infect. Dis. 37:101–112 [DOI] [PubMed] [Google Scholar]
- 151. Lawn S. D., Badri M., Wood R. 2005. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS 19:2109–2116 [DOI] [PubMed] [Google Scholar]
- 152. Lawn S. D., Bekker L.-G., Miller R. F. 2005. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect. Dis. 5:361–373 [DOI] [PubMed] [Google Scholar]
- 153. Lawn S. D., Bekker L. G. 2009. Co-pathogenesis of tuberculosis and HIV, p. 96–106In Schaaf H. S., et al. (ed.), Tuberculosis: a comprehensive clinical reference. Saunders-Elsevier, Oxford, United Kingdom [Google Scholar]
- 154. Lawn S. D., et al. 2006. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin. Infect. Dis. 42:1040–1047 [DOI] [PubMed] [Google Scholar]
- 155. Lawn S. D., Churchyard G. 2009. Epidemiology of HIV-associated tuberculosis. Curr. Opin. HIV AIDS 4:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lawn S. D., Myer L., Edwards D., Bekker L.-G., Wood R. 2009. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lawn S. D., Wood R. 2007. When should antiretroviral treatment be started in patients with HIV-associated tuberculosis in South Africa? S. Afr. Med. J. 97:412, 414–415 [PubMed] [Google Scholar]
- 158. Leidl L., et al. 2010. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur. Respir. J. 35:619–626 [DOI] [PubMed] [Google Scholar]
- 159. Leung A. N., et al. 1996. Pulmonary tuberculosis: comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Radiology 198:687–691 [DOI] [PubMed] [Google Scholar]
- 160. Li J., Munsiff S. S., Driver C. R., Sackoff J. 2005. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997–2000. Clin. Infect. Dis. 41:83–91 [DOI] [PubMed] [Google Scholar]
- 161. Lin C. Y., et al. 2009. Aetiology of cavitary lung lesions in patients with HIV infection. HIV Med. 10:191–198 [DOI] [PubMed] [Google Scholar]
- 162. Reference deleted.
- 163. Lonnroth K., et al. 2010. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet 375:1814–1829 [DOI] [PubMed] [Google Scholar]
- 164. Lönnroth K., Jaramillo E., Williams B. G., Dye C., Raviglione M. 2009. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc. Sci. Med. 68:2240–2246 [DOI] [PubMed] [Google Scholar]
- 165. Loudon R. G., Spohn S. K. 1969. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 99:109–111 [DOI] [PubMed] [Google Scholar]
- 166. Ma C. S., et al. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. MacPherson D. W., et al. 2009. Population mobility, globalization, and antimicrobial drug resistance. Emerg. Infect. Dis. 15:1727–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Manas E., et al. 2004. Impact of tuberculosis on the course of HIV-infected patients with a high initial CD4 lymphocyte count. Int. J. Tuberc. Lung Dis. 8:451–457 [PubMed] [Google Scholar]
- 169. Manosuthi W., Chottanapand S., Thongyen S., Chaovavanich A., Sungkanuparph S. 2006. Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 43:42–46 [DOI] [PubMed] [Google Scholar]
- 170. Manosuthi W., Mankatitham W., Lueangniyomkul A., Chimsuntorn S., Sungkanuparph S. 2008. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 9:294–299 [DOI] [PubMed] [Google Scholar]
- 171. Manosuthi W., et al. 2009. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin. Infect. Dis. 48:1752–1759 [DOI] [PubMed] [Google Scholar]
- 172. Manosuthi W., et al. 2009. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 23:2467–2471 [DOI] [PubMed] [Google Scholar]
- 173. Marais S., Wilkinson R. J., Pepper D. J., Meintjes G. 2009. Management of patients with the immune reconstitution inflammatory syndrome. Curr. HIV/AIDS Rep. 6:162–171 [DOI] [PubMed] [Google Scholar]
- 174. Martínez-Lirola M., et al. 2008. Advanced survey of tuberculosis transmission in a complex socioepidemiologic scenario with a high proportion of cases in immigrants. Clin. Infect. Dis. 47:8–14 [DOI] [PubMed] [Google Scholar]
- 175. Mase S. R., et al. 2007. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int. J. Tuberc. Lung Dis. 11:485–495 [PubMed] [Google Scholar]
- 176. Matloubian M., Concepcion R. J., Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mazurek M., et al. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recommend. Rep. 59:1–25 [PubMed] [Google Scholar]
- 178. McIlleron H., Meintjes G., Burman W. J., Maartens G. 2007. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J. Infect. Dis. 196(Suppl. 1):S63–S75 [DOI] [PubMed] [Google Scholar]
- 179. Means T. K., et al. 1999. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163:3920–3927 [PubMed] [Google Scholar]
- 180. Mehandru S., et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Meintjes G., et al. 2008. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect. Dis. 8:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Meintjes G., Rabie H., Wilkinson R. J., Cotton M. F. 2009. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin. Chest Med. 30:797–810 [DOI] [PubMed] [Google Scholar]
- 183. Meintjes G., et al. 2009. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin. Infect. Dis. 48:667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Meintjes G., et al. 2008. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am. J. Respir. Crit. Care Med. 178:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Meintjes G., et al. 2009. Randomized placebo-controlled trial of prednisone for the TB immune reconstitution inflammatory syndrome, abstr. 34. Abstr. Conf. Retrovir. Oppor. Infect. 2009, Montreal, Canada [Google Scholar]
- 186. Menzies D., et al. 2009. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 6:e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Middelkoop K., et al. 2008. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin. Infect. Dis. 47:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Milburn H. J. 2001. Primary tuberculosis. Curr. Opin. Pulm. Med. 7:133–141 [DOI] [PubMed] [Google Scholar]
- 189. Miller E. A., Ernst J. D. 2009. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J. Clin. Invest. 119:1079–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Milner J. D., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Minion J., Leung E., Menzies D., Pai M. 2010. Microscopic-observation drug susceptibility and thin layer agar assays for the detection of drug resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 10:688–698 [DOI] [PubMed] [Google Scholar]
- 192. Minion J., Pai M. 2010. Expanding the role of the microscopic observation drug susceptibility assay in tuberculosis and HIV management. Clin. Infect. Dis. 50:997–999 [DOI] [PubMed] [Google Scholar]
- 193. Modi W. S., et al. 2006. Genetic variation in the CCL18-CCL3-CCL4 chemokine gene cluster influences HIV type 1 transmission and AIDS disease progression. Am. J. Hum. Genet. 79:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mogues T., Goodrich M. E., Ryan L., LaCourse R., North R. J. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Monkongdee P., et al. 2009. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am. J. Respir. Crit. Care Med. 180:903–908 [DOI] [PubMed] [Google Scholar]
- 196. Moore D. A., et al. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 355:1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Morris L., et al. 2003. Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patients. J. Infect. Dis. 187:1967–1971 [DOI] [PubMed] [Google Scholar]
- 198. Mukadi Y. D., Maher D., Harries A. 2001. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS 15:143–152 [DOI] [PubMed] [Google Scholar]
- 199. Mukadi Y. D., et al. 1997. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Côte d'Ivoire. AIDS 11:1151–1158 [DOI] [PubMed] [Google Scholar]
- 200. Mwandumba H. C., et al. 2004. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J. Immunol. 172:4592–4598 [DOI] [PubMed] [Google Scholar]
- 201. Nabel G., Baltimore D. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711–713 [DOI] [PubMed] [Google Scholar]
- 202. Odhiambo J. A., et al. 1999. Tuberculosis and the HIV epidemic: increasing annual risk of tuberculous infection in Kenya, 1986-1996. Am. J. Public Health 89:1078–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Okwera A., et al. 1994. Randomised trial of thiacetazone and rifampicin-containing regimens for pulmonary tuberculosis in HIV-infected Ugandans. Lancet 344:1323–1328 [DOI] [PubMed] [Google Scholar]
- 204. Okwera A., et al. 2006. Comparison of intermittent ethambutol with rifampicin-based regimens in HIV-infected adults with PTB, Kampala. Int. J. Tuberc. Lung Dis. 10:39–44 [PMC free article] [PubMed] [Google Scholar]
- 205. Osborn L., Kunkel S., Nabel G. J. 1989. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. U. S. A. 86:2336–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Palaci M., et al. 2007. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J. Clin. Microbiol. 45:4064–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Paolo W. F., Nosanchuk J. D. 2004. Tuberculosis in New York city: recent lessons and a look ahead. Lancet Infect. Dis. 4:287–293 [DOI] [PubMed] [Google Scholar]
- 208. Pastores S. M., Naidich D. P., Aranda C. P., McGuinnes G., Rom W. N. 1993. Intrathoracic adenopathy associated with pulmonary tuberculosis in patients with human immunodeficiency virus infection. Chest 103:1433–1437 [DOI] [PubMed] [Google Scholar]
- 209. Patel R. B., Burke T. F. 2009. Urbanization—an emerging humanitarian disaster. N. Engl. J. Med. 361:741–743 [DOI] [PubMed] [Google Scholar]
- 210. Perlman D. C., et al. 1997. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG). Clin. Infect. Dis. 25:242–246 [DOI] [PubMed] [Google Scholar]
- 211. Perlman D. C., Leung C. C., Yew W. W. 2009. Diagnosing tuberculosis in patients with HIV: do we know enough? Am. J. Respir. Crit. Care Med. 180:800–801 [DOI] [PubMed] [Google Scholar]
- 212. Peto H. M., Pratt R. H., Harrington T. A., LoBue P. A., Armstrong L. R. 2009. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin. Infect. Dis. 49:1350–1357 [DOI] [PubMed] [Google Scholar]
- 213. Philips J. A., Porto M. C., Wang H., Rubin E. J., Perrimon N. 2008. ESCRT factors restrict mycobacterial growth. Proc. Natl. Acad. Sci. U. S. A. 105:3070–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Post F. A., Wood R., Pillay G. P. 1995. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+ T-lymphocyte count. Tuber. Lung Dis. 76:518–521 [DOI] [PubMed] [Google Scholar]
- 215. Randhawa P. S. 1990. Lymphocyte subsets in granulomas of human tuberculosis: an in situ immunofluorescence study using monoclonal antibodies. Pathology 22:153–155 [DOI] [PubMed] [Google Scholar]
- 216. Reddy K. P., et al. 2010. Microscopic observation drug susceptibility assay for tuberculosis screening before isoniazid preventive therapy in HIV-infected persons. Clin. Infect. Dis. 50:988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Reid M. J. A., Shah N. S. 2009. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect. Dis. 9:173–184 [DOI] [PubMed] [Google Scholar]
- 218. Rieder H. 2005. Annual risk of infection with Mycobacterium tuberculosis. Eur. Respir. J. 25:181–185 [DOI] [PubMed] [Google Scholar]
- 219. Rieder H. L., Cauthen G. M., Comstock G. W., Snider D. E. 1989. Epidemiology of tuberculosis in the United States. Epidemiol. Rev. 11:79–98 [DOI] [PubMed] [Google Scholar]
- 220. Sabin F. R., Doan C. A. 1927. The relation of monocytes and clasmatocytes to early infection in rabbits with bovine tubercle bacilli. J. Exp. Med. 46:627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sadek M. I., Sada E., Toossi Z., Schwander S. K., Rich E. A. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513–521 [DOI] [PubMed] [Google Scholar]
- 222. Sanguanwongse N., et al. 2008. Antiretroviral therapy for HIV-infected tuberculosis patients saves lives but needs to be used more frequently in Thailand. J. Acquir. Immune Defic. Syndr. 48:181–189 [DOI] [PubMed] [Google Scholar]
- 223. Santoro-Lopes G., de Pinho A. M., Harrison L. H., Schechter M. 2002. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin. Infect. Dis. 34:543–546 [DOI] [PubMed] [Google Scholar]
- 224. Saukkonen J. J., et al. 2002. Beta-chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect. Immun. 70:1684–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Schiffer J. T., Sterling T. R. 2007. Timing of antiretroviral therapy initiation in tuberculosis patients with AIDS: a decision analysis. J. Acquir. Immune Defic. Syndr. 44:229–234 [DOI] [PubMed] [Google Scholar]
- 226. Scriba T. J., et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 180:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Selwyn P. A., et al. 1989. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320:545–550 [DOI] [PubMed] [Google Scholar]
- 228. Shafer R. W., Edlin B. R. 1996. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin. Infect. Dis. 22:683–704 [DOI] [PubMed] [Google Scholar]
- 229. Shafer R. W., Kim D. S., Weiss J. P., Quale J. M. 1991. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine 70:384–397 [DOI] [PubMed] [Google Scholar]
- 230. Shelburne S. A., Montes M., Hamill R. J. 2006. Immune reconstitution inflammatory syndrome: more answers, more questions. J. Antimicrob. Chemother. 57:167–170 [DOI] [PubMed] [Google Scholar]
- 231. Slutkin G., Leowski J., Mann J. 1988. The effects of the AIDS epidemic on the tuberculosis problem and tuberculosis programmes. Bull. Int. Union Tuberc. Lung Dis. 63:21–24 [PubMed] [Google Scholar]
- 232. Small P. M., Pai M. 2010. Tuberculosis diagnosis—time for a game change. N. Engl. J. Med. 363:1070–1071 [DOI] [PubMed] [Google Scholar]
- 233. Smith R. L., Yew K., Berkowitz K. A., Aranda C. P. 1994. Factors affecting the yield of acid-fast sputum smears in patients with HIV and tuberculosis. Chest 106:684–686 [DOI] [PubMed] [Google Scholar]
- 234. Sonnenberg P., et al. 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191:150–158 [DOI] [PubMed] [Google Scholar]
- 235. Sonnenberg P., et al. 2001. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 358:1687–1693 [DOI] [PubMed] [Google Scholar]
- 236. Steingart K. R., et al. 2006. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:664–674 [DOI] [PubMed] [Google Scholar]
- 237. Stenger S., et al. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121–125 [DOI] [PubMed] [Google Scholar]
- 238. Stenger S., et al. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684–1687 [DOI] [PubMed] [Google Scholar]
- 239. Sterling T. R., Pham P. A., Chaisson R. E. 2010. HIV infection-related tuberculosis: clinical manifestations and treatment. Clin. Infect. Dis. 50:S223–S230 [DOI] [PubMed] [Google Scholar]
- 240. Stumptner-Cuvelette P., et al. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. U. S. A. 98:12144–12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sutherland R., et al. 2006. Impaired IFN-gamma-secreting capacity in mycobacterial antigen-specific CD4 T cells during chronic HIV-1 infection despite long-term HAART. AIDS 20:821–829 [DOI] [PubMed] [Google Scholar]
- 242. Syed Ahamed Kabeer B., Raman B., Thomas A., Perumal V., Raja A. 2010. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS One 5:e9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Tabarsi P., et al. 2009. Early initiation of antiretroviral therapy results in decreased morbidity and mortality among patients with TB and HIV. J. Int. AIDS Soc. 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Tailleux L., et al. 2003. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Toossi Z. 2003. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J. Infect. Dis. 188:1146–1155 [DOI] [PubMed] [Google Scholar]
- 246. Toossi Z., et al. 2001. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin. Exp. Immunol. 123:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Toossi Z., Sierra-Madero J. G., Blinkhorn R. A., Mettler M. A., Rich E. A. 1993. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J. Exp. Med. 177:1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Reference deleted.
- 249. UNAIDS 2009. AIDS epidemic update: November 2009. UNAIDS, Geneva, Switzerland [Google Scholar]
- 250. UNAIDS 2008. UNAIDS report on the global AIDS epidemic 2008. UNAIDS, Geneva, Switzerland: http://data.unaids.org/pub/GlobalReport/2008/JC1511_GR08_ExecutiveSummary_en.pdf [Google Scholar]
- 251. UNFPA 2007. State of the world population 2007: unleashing the potential of urban growth. United Nations Population Fund, Geneva, Switzerland: [PubMed] [Google Scholar]
- 252. van der Wel N., et al. 2007. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298 [DOI] [PubMed] [Google Scholar]
- 253. Velasco M., et al. 2009. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J. Acquir. Immune Defic. Syndr. 50:148–152 [DOI] [PubMed] [Google Scholar]
- 254. Veldsman C., et al. 2009. QuantiFERON-TB GOLD ELISA assay for the detection of Mycobacterium tuberculosis-specific antigens in blood specimens of HIV-positive patients in a high-burden country. FEMS Immunol. Med. Microbiol. 57:269–273 [DOI] [PubMed] [Google Scholar]
- 255. Vinnard C., Macgregor R. R. 2009. Tuberculous meningitis in HIV-infected individuals. Curr. HIV/AIDS Rep. 6:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Wallis R. S., et al. 2010. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375:1920–1937 [DOI] [PubMed] [Google Scholar]
- 257. Weiner M., et al. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 40:1481–1491 [DOI] [PubMed] [Google Scholar]
- 258. Wenning L. A., et al. 2009. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob. Agents Chemother. 53:2852–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Whalen C., et al. 1995. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am. J. Respir. Crit. Care Med. 151:129–135 [DOI] [PubMed] [Google Scholar]
- 260. Whalen C. C., et al. 2000. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 14:1219–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. WHO 2008. Essential prevention and care interventions for adults and adolescents living with HIV in resource-limited settings. World Health Organization, Geneva, Switzerland [Google Scholar]
- 262. WHO 2009. Global tuberculosis control: a short update to the 2009 report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 263. WHO 2009. Global tuberculosis control: epidemiology, strategy, financing. World Health Organization report 2009. World Health Organization, Geneva, Switzerland [Google Scholar]
- 264. WHO 2007. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf [Google Scholar]
- 265. WHO 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland [Google Scholar]
- 266. WHO 2009. Treatment of tuberculosis: guidelines for national programs, 4th ed World Health Organization, Geneva, Switzerland [Google Scholar]
- 267. WHO 2007. Tuberculosis care with TB-HIV co-management—integrated management of adolescent and adult illness. World Health Organization, Geneva, Switzerland [Google Scholar]
- 268. Wilson P. 2007. Is natural ventilation a useful tool to prevent the airborne spread of TB? PLoS Med. 4:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Wolf A. J., et al. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179:2509–2519 [DOI] [PubMed] [Google Scholar]
- 270. Wood R., et al. 2007. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am. J. Respir. Crit. Care Med. 175:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Yang Y. F., et al. 2001. IL-12 as well as IL-2 upregulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. J. Clin. Immunol. 21:116–125 [DOI] [PubMed] [Google Scholar]
- 272. Yi J. S., Du M., Zajac A. J. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science 324:1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Zagury D., et al. 1998. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 95:3857–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Zhang Y., Nakata K., Weiden M., Rom W. N. 1995. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J. Clin. Invest. 95:2324–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]