Abstract
The coat protein of the RNA bacteriophage MS2 is a translational repressor and interacts with a specific RNA stem-loop to inhibit translation of the viral replicase gene. As part of an effort to dissect genetically its RNA binding function, mutations were identified in the coat protein sequence that suppress mutational defects in the translational operator. Each of the mutants displayed a super-repressor phenotype, repressing translation from the wild-type and a variety of mutant operators better than did the wild-type coat protein. At least one mutant probably binds RNA more tightly than wild-type. The other mutants, however, were defective for assembly of virus-like particles, and self-associated predominantly as dimers. It is proposed that this assembly defect accounts for their super-repressor characteristics, since failure to assemble into virus-like particles elevates the effective concentration of repressor dimers. This hypothesis is supported by the observation that deletion of thirteen amino acids known to be important for assembly of dimers into capsids also resulted in the same assembly defect and in super-repressor activity. A second class of assembly defects is also described. Deletion of two amino acids from the C-terminus of coat protein resulted in failure to form capsids, most of the coat protein having the apparent molecular weight expected of trimers. This mutant (dl-8) was completely defective for repressor activity, probably because of an inability to form dimers. These results point out the inter-dependence of the structural and regulatory functions of coat protein.
Full text
PDF


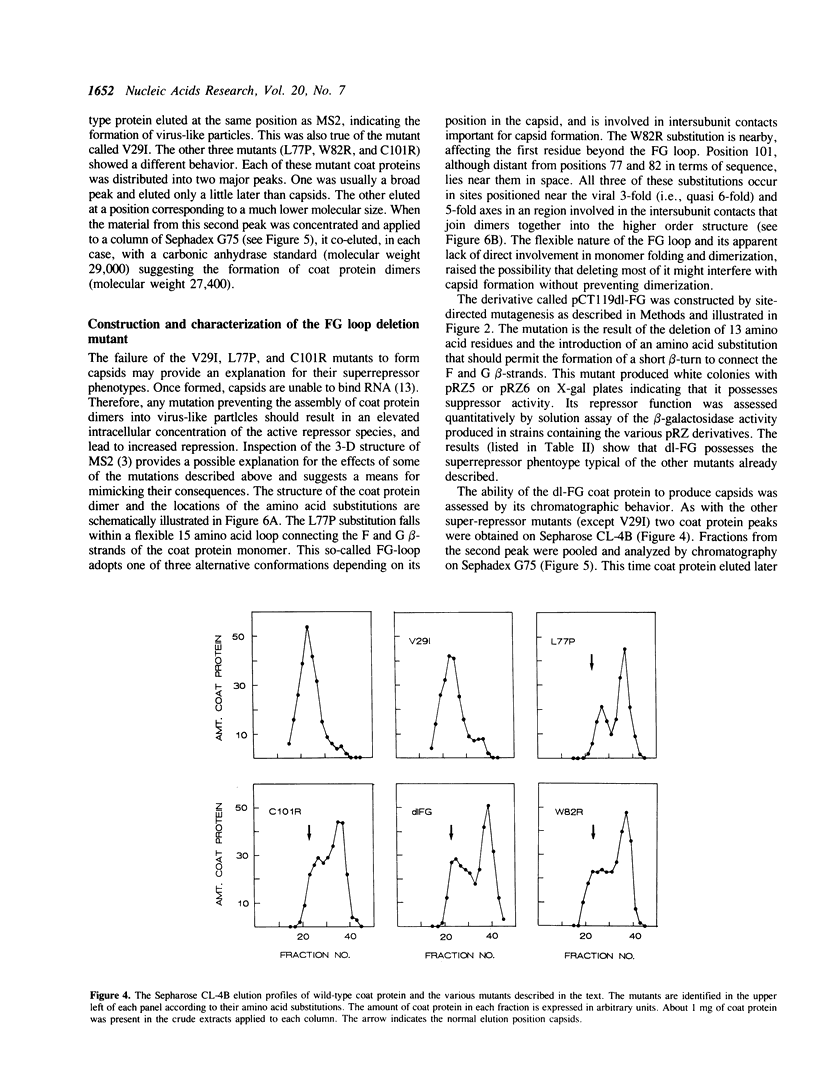
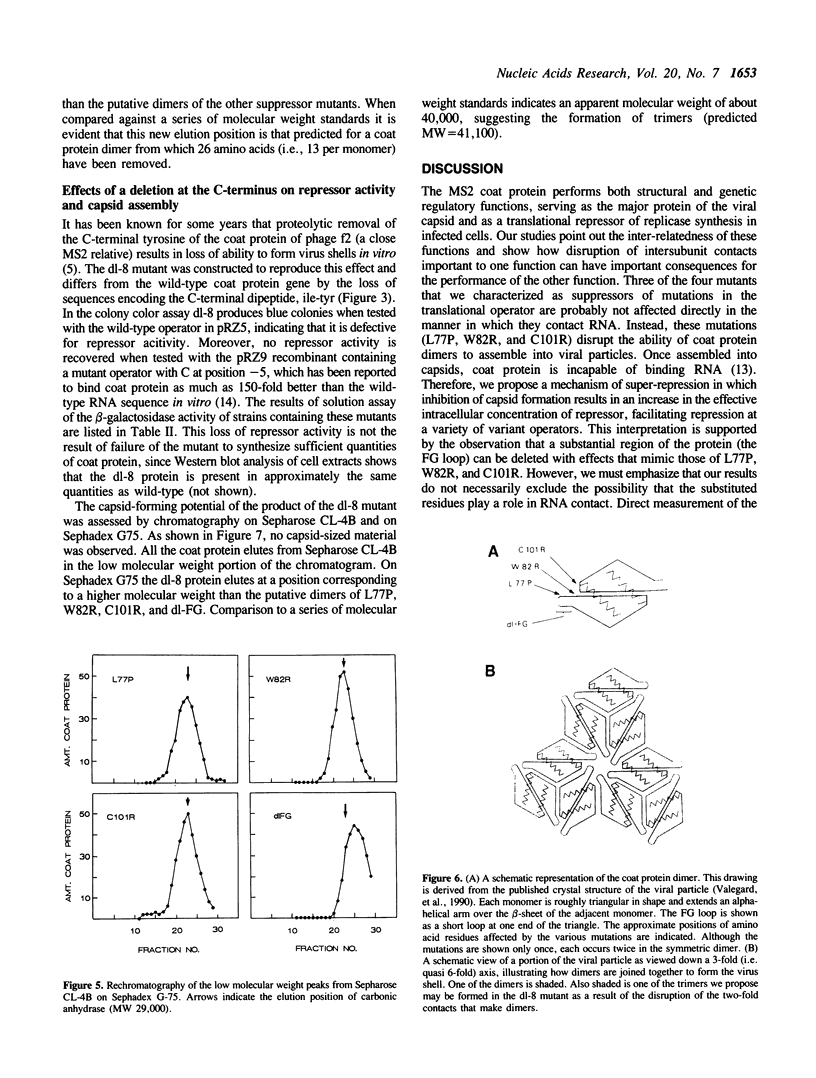
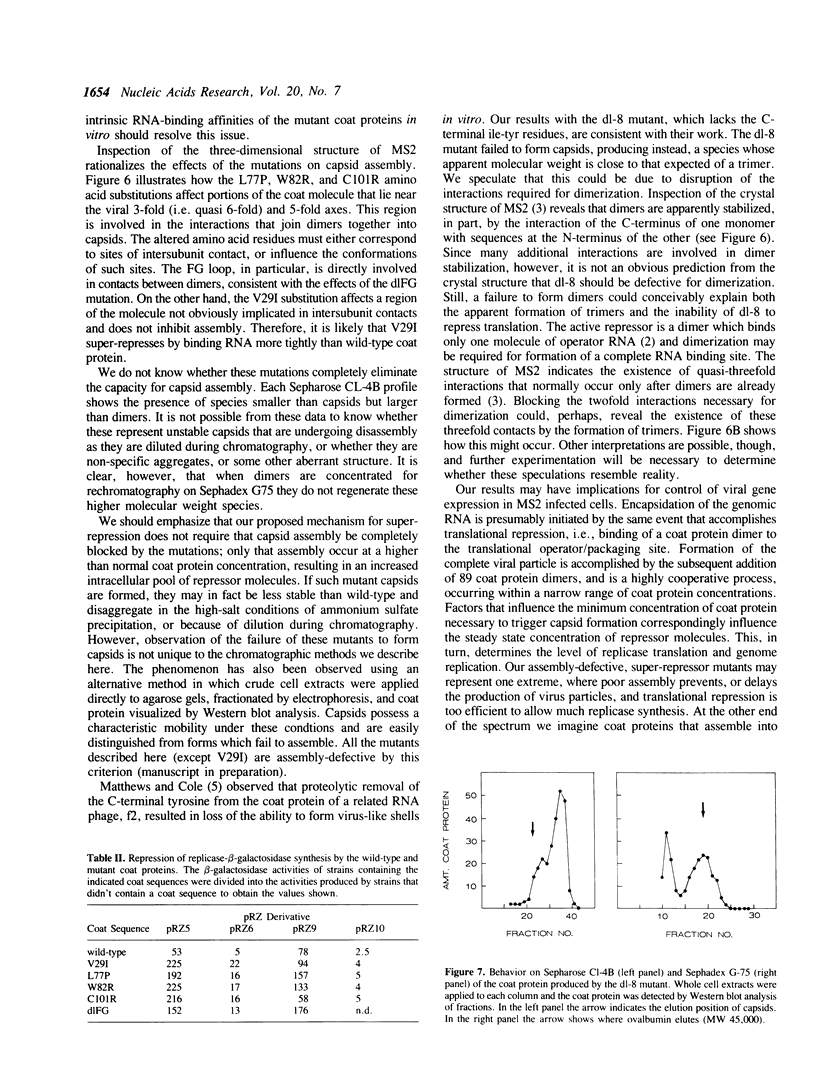

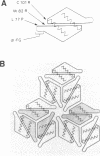
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckett D., Uhlenbeck O. C. Ribonucleoprotein complexes of R17 coat protein and a translational operator analog. J Mol Biol. 1988 Dec 20;204(4):927–938. doi: 10.1016/0022-2836(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Spahr P. F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3033–3037. doi: 10.1073/pnas.69.10.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carey J., Cameron V., de Haseth P. L., Uhlenbeck O. C. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 1983 May 24;22(11):2601–2610. doi: 10.1021/bi00280a002. [DOI] [PubMed] [Google Scholar]
- Carey J., Lowary P. T., Uhlenbeck O. C. Interaction of R17 coat protein with synthetic variants of its ribonucleic acid binding site. Biochemistry. 1983 Sep 27;22(20):4723–4730. doi: 10.1021/bi00289a017. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Matthews K. S., Cole R. D. Effects of chemical modification of f2 viral coat protein on its shell-forming activity. J Mol Biol. 1972 Mar 14;65(1):17–23. doi: 10.1016/0022-2836(72)90488-3. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Peabody D. S. Translational repression by bacteriophage MS2 coat protein does not require cysteine residues. Nucleic Acids Res. 1989 Aug 11;17(15):6017–6027. doi: 10.1093/nar/17.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translational repression by bacteriophage MS2 coat protein expressed from a plasmid. A system for genetic analysis of a protein-RNA interaction. J Biol Chem. 1990 Apr 5;265(10):5684–5689. [PubMed] [Google Scholar]
- Uhlenbeck O. C., Carey J., Romaniuk P. J., Lowary P. T., Beckett D. Interaction of R17 coat protein with its RNA binding site for translational repression. J Biomol Struct Dyn. 1983 Oct;1(2):539–552. doi: 10.1080/07391102.1983.10507460. [DOI] [PubMed] [Google Scholar]
- Valegård K., Liljas L., Fridborg K., Unge T. The three-dimensional structure of the bacterial virus MS2. Nature. 1990 May 3;345(6270):36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]