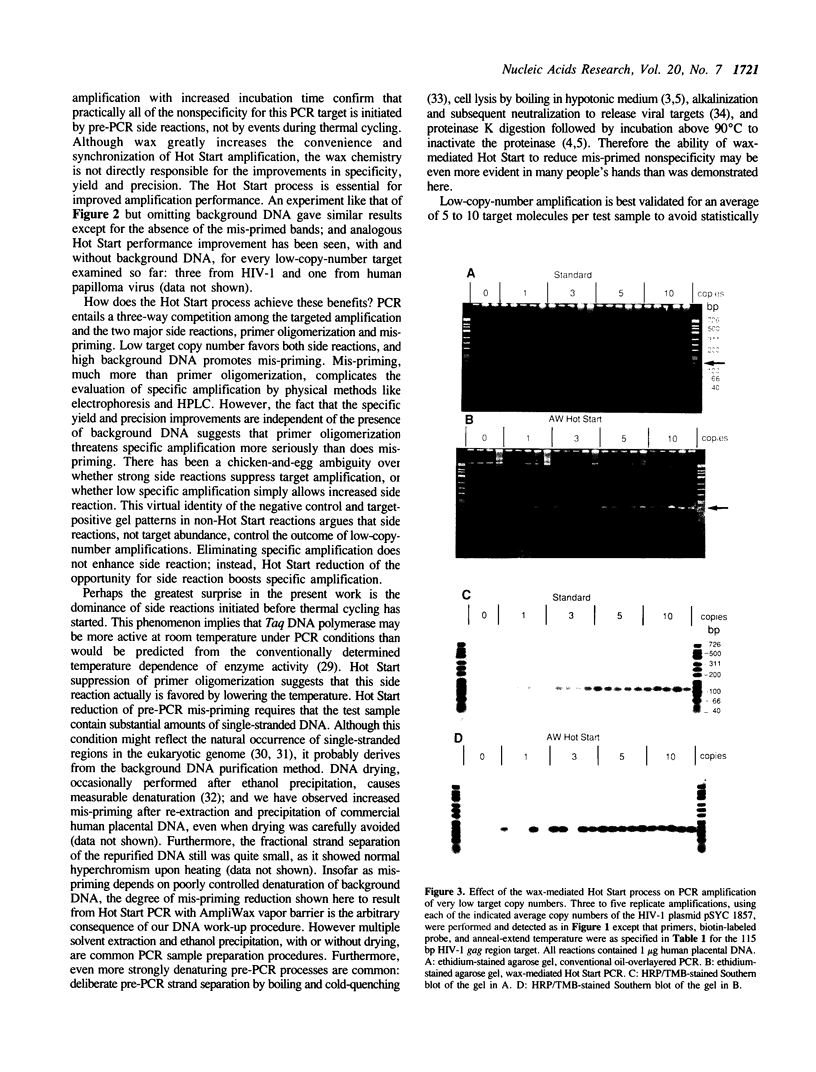
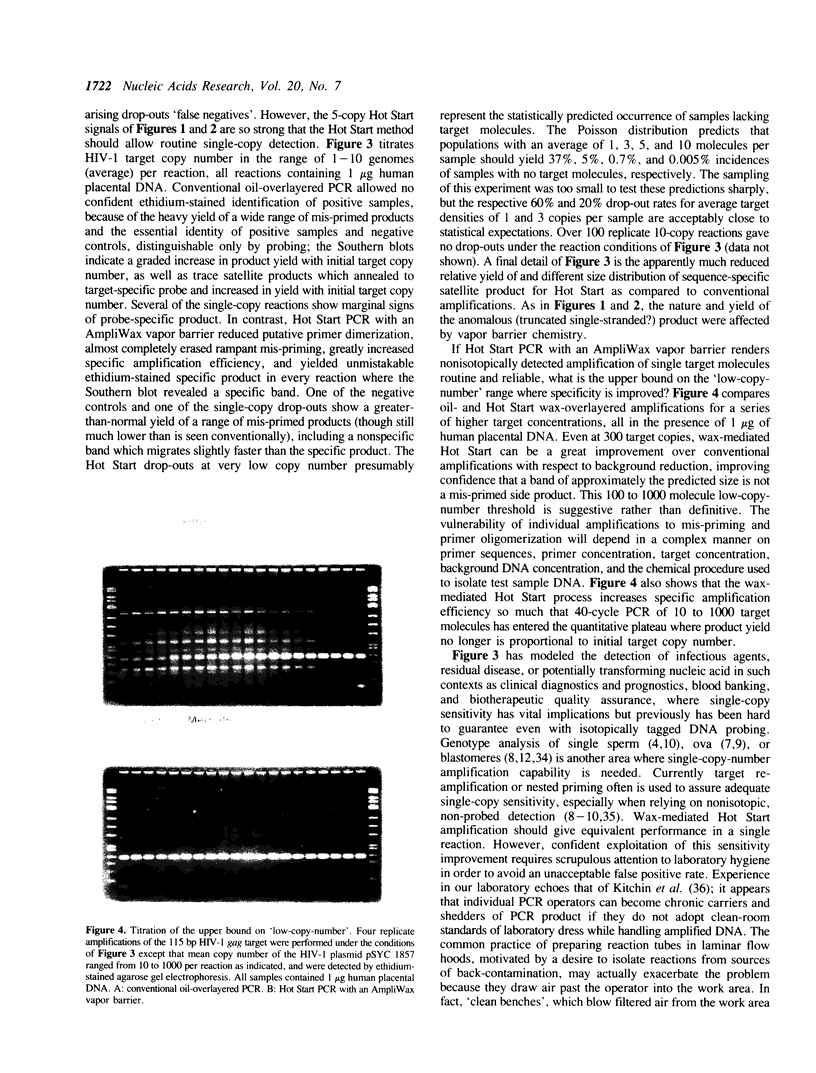
Abstract
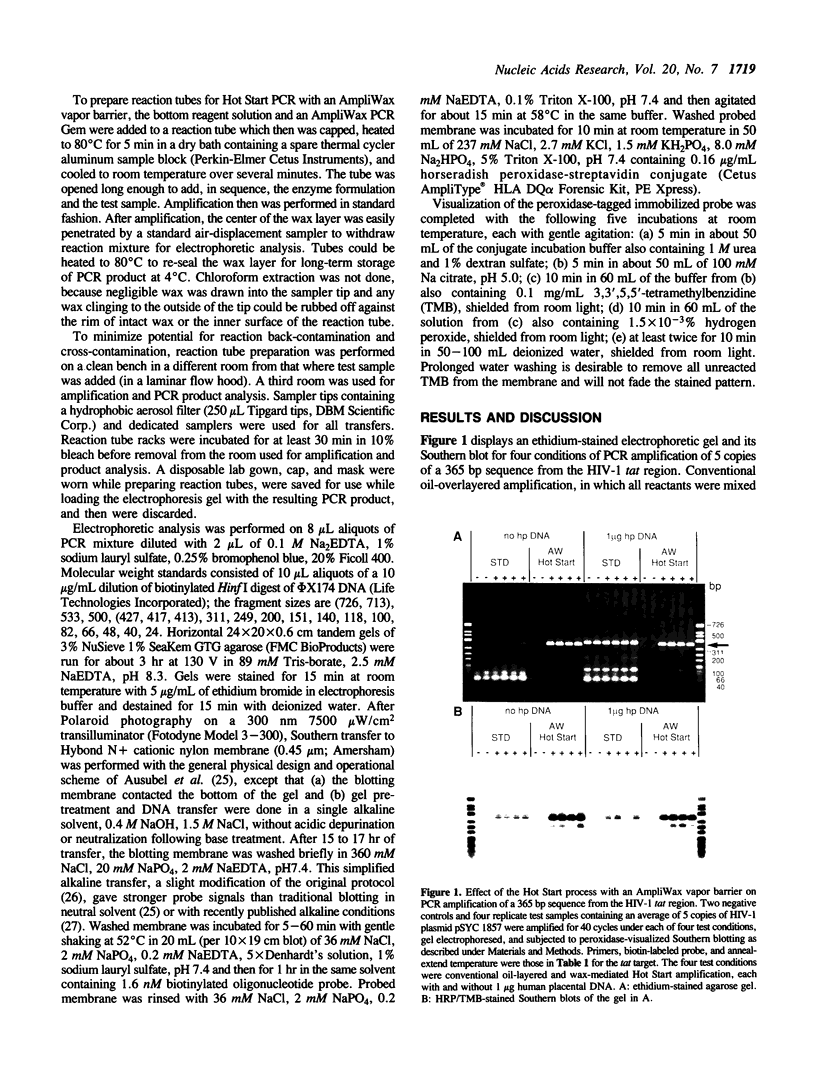
A Hot Start Polymerase Chain Reaction (PCR) entails the withholding of at least one reagent from the reaction mixture until the reaction tube temperature has reached 60-80 degrees C. Hot Start amplification with an AmpliWax vapor barrier uses a layer of solid wax to separate the retained reagent(s) and the test sample from the bulk of the reagents until the first heating step of automated thermal cycling melts the wax and convectively mixes the two aqueous layers. Wax-mediated Hot Start PCR greatly increases the specificity, yield, and precision of amplifying low copy numbers of three HIV targets. In the presence of 1 microgram of human placental DNA (1.6 x 10(5) diploid genomes) the specificity improvement entails considerable to complete reduction in the amplification of mis-primed sequences and putative primer oligomers. When mis-priming is negligible, the procedural improvement still suppresses putative primer oligomerization. Hot Start PCR with an AmpliWax vapor barrier permits routine amplification of a single target molecule with detection by ethidium stained gel electrophoresis; nonisotopically visualized probing suffices for confirmation. The improved amplification performance is evident for target copy numbers below approximately 10(3).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- Coutelle C., Williams C., Handyside A., Hardy K., Winston R., Williamson R. Genetic analysis of DNA from single human oocytes: a model for preimplantation diagnosis of cystic fibrosis. BMJ. 1989 Jul 1;299(6690):22–24. doi: 10.1136/bmj.299.6690.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Mayur K., Yolken R. H., Viscidi R. P. Immunodetection of DNA with biotinylated RNA probes: a study of reactivity of a monoclonal antibody to DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):96–105. doi: 10.1016/0003-2697(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis S. I., Alevizaki M., Denny P., Ferrier G. J., Legon S. Generation of DNA probes for peptides with highly degenerate codons using mixed primer PCR. Nucleic Acids Res. 1988 Nov 11;16(21):10371–10371. doi: 10.1093/nar/16.21.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakes D. J., Berezney R. DNA binding properties of the nuclear matrix and individual nuclear matrix proteins. Evidence for salt-resistant DNA binding sites. J Biol Chem. 1991 Jun 15;266(17):11131–11140. [PubMed] [Google Scholar]
- Handyside A. H., Kontogianni E. H., Hardy K., Winston R. M. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990 Apr 19;344(6268):768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- Handyside A. H., Pattinson J. K., Penketh R. J., Delhanty J. D., Winston R. M., Tuddenham E. G. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989 Feb 18;1(8634):347–349. doi: 10.1016/s0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- Hart C., Chang S. Y., Kwok S., Sninsky J., Ou C. Y., Schochetman G. A replication-deficient HIV-1 DNA used for quantitation of the polymerase chain reaction (PCR). Nucleic Acids Res. 1990 Jul 11;18(13):4029–4030. doi: 10.1093/nar/18.13.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher S. L., Teplitz R. L., Elrod S. L. Rapid alkaline transfer of low molecular weight DNA from NuSieve GTG agarose gels. Biotechniques. 1990 Sep;9(3):260–262. [PubMed] [Google Scholar]
- Holding C., Monk M. Diagnosis of beta-thalassaemia by DNA amplification in single blastomeres from mouse preimplantation embryos. Lancet. 1989 Sep 2;2(8662):532–535. doi: 10.1016/s0140-6736(89)90655-7. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Neumann R., Keyte J. Amplification of human minisatellites by the polymerase chain reaction: towards DNA fingerprinting of single cells. Nucleic Acids Res. 1988 Dec 9;16(23):10953–10971. doi: 10.1093/nar/16.23.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Feinstone S. M., Miller R. H. Rapid and sensitive method for the detection of serum hepatitis B virus DNA using the polymerase chain reaction technique. J Clin Microbiol. 1989 Sep;27(9):1930–1933. doi: 10.1128/jcm.27.9.1930-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E. D., Dong M. W. Rapid analysis and purification of polymerase chain reaction products by high-performance liquid chromatography. Biotechniques. 1990 May;8(5):546–555. [PubMed] [Google Scholar]
- Keller G. H., Huang D. P., Manak M. M. A sensitive nonisotopic hybridization assay for HIV-1 DNA. Anal Biochem. 1989 Feb 15;177(1):27–32. doi: 10.1016/0003-2697(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin P. A., Szotyori Z., Fromholc C., Almond N. Avoidance of PCR false positives [corrected]. Nature. 1990 Mar 15;344(6263):201–201. doi: 10.1038/344201a0. [DOI] [PubMed] [Google Scholar]
- Kumar R., Barbacid M. Oncogene detection at the single cell level. Oncogene. 1988 Dec;3(6):647–651. [PubMed] [Google Scholar]
- Larzul D., Chevrier D., Guesdon J. L. A non-radioactive diagnostic test for the detection of HBV DNA sequences in serum at the single molecule level. Mol Cell Probes. 1989 Mar;3(1):45–57. doi: 10.1016/0890-8508(89)90036-4. [DOI] [PubMed] [Google Scholar]
- Larzul D., Guigue F., Sninsky J. J., Mack D. H., Bréchot C., Guesdon J. L. Detection of hepatitis B virus sequences in serum by using in vitro enzymatic amplification. J Virol Methods. 1988 Jul;20(3):227–237. doi: 10.1016/0166-0934(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Li H., Cui X., Arnheim N. Direct electrophoretic detection of the allelic state of single DNA molecules in human sperm by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4580–4584. doi: 10.1073/pnas.87.12.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Monk M., Holding C. Amplification of a beta-haemoglobin sequence in individual human oocytes and polar bodies. Lancet. 1990 Apr 28;335(8696):985–988. doi: 10.1016/0140-6736(90)91060-n. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Neubauer A., Neubauer B., Liu E. Polymerase chain reaction based assay to detect allelic loss in human DNA: loss of beta-interferon gene in chronic myelogenous leukemia. Nucleic Acids Res. 1990 Feb 25;18(4):993–998. doi: 10.1093/nar/18.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Kalsheker N., Graham A., Powell S., Gammack A., Riley J., Markham A. F. Diagnosis of alpha 1-antitrypsin deficiency by enzymatic amplification of human genomic DNA and direct sequencing of polymerase chain reaction products. Nucleic Acids Res. 1988 Sep 12;16(17):8233–8243. doi: 10.1093/nar/16.17.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen D. B., Eckstein F. Incomplete primer extension during in vitro DNA amplification catalyzed by Taq polymerase; exploitation for DNA sequencing. Nucleic Acids Res. 1989 Dec 11;17(23):9613–9620. doi: 10.1093/nar/17.23.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Probst H., Herzog R. DNA regions associated with the nuclear matrix of Ehrlich ascites cells expose single-stranded sites after deproteinization. Eur J Biochem. 1985 Jan 2;146(1):167–171. doi: 10.1111/j.1432-1033.1985.tb08634.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Robinson M. O., Bellvé A. R., Simon M. I., Riggs A. D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 1990 Mar 11;18(5):1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J., Inagami S., Lovegren E., Chalkley R. DNA denatures upon drying after ethanol precipitation. Nucleic Acids Res. 1987 Nov 11;15(21):8739–8754. doi: 10.1093/nar/15.21.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirasophon W., Ponglikitmongkol M., Wilairat P., Boonsaeng V., Panyim S. A novel detection of a single Plasmodium falciparum in infected blood. Biochem Biophys Res Commun. 1991 Feb 28;175(1):179–184. doi: 10.1016/s0006-291x(05)81217-3. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]