Abstract
The first case in Europe of Klebsiella pneumoniae carbapenemase (KPC) 2 transfer from K. pneumoniae to Escherichia coli in the same patient is described. KPC-positive plasmids from the two species were identical, indicating horizontal plasmid transfer. Selection of the KPC-producing E. coli strain was triggered by therapy with meropenem.
CASE REPORT
In April 2010, a 55-year-old male Italian patient with a history of epilepsy, alcoholism, and hypertension was admitted to the intensive care unit of the Saint Antony Hospital (ICU-SAH) of Padua after suffering a stroke. The patient had extensive left frontoparietal hemorrhage, which required evacuative craniotomy and a transitory tracheotomy. Microbiological analysis isolated Klebsiella pneumoniae and Escherichia coli strains from a blood sample. MIC values were measured by the Vitek 2 automated system (bioMérieux, Hazelwood, MO) and are reported in Table 1. According to the CLSI standards (4), the K. pneumoniae isolate was fully resistant to carbapenems (imipenem and meropenem MICs of ≥16 mg/liter, 26 April, Table 1), while the E. coli isolate was fully susceptible (imipenem MIC of ≤1 mg/liter, 26 April, Table 1). The patient, who had not undergone any previous antimicrobial therapy, was treated with gentamicin (240 mg/day for 13 days). After 2 days of therapy, the patient's hemoculture yielded no microbial growth.
Table 1.
Antimicrobial susceptibilities of bacterial strains found from April to October 2010 in the indicated samples from the case patient
| Date of isolation | Species | Sample | MIC (mg/liter)a |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | MER | AMP | AMX | FEP | CFZ | CTX | CAZ | PIP | TZP | OXA | TGC | GEN | AMK | SXT | NIT | CIP | LVX | NOR | ERY | CLI | |||
| 26 Apr | K. pneumoniae | Blood | ≥16 | ≥16 | ≥32 | ≥32 | ≥64 | ≥64 | ≥64 | ≥128 | ≥128 | 2 | 4 | ≥64 | ≥320 | ≥8 | |||||||
| 26 Apr | E. coli | Blood | ≤1 | ≤0.25 | ≥32 | 8 | ≤4 | ≥128 | ≤4 | ≤1 | ≥320 | 1 | |||||||||||
| 23 Aug | S. aureus | BAL fluid | ≥4 | ≤0.5 | ≤10 | ≥8 | ≤0.25 | ≤0.25 | |||||||||||||||
| 23 Aug | E. coli | BAL fluid | ≤1 | ≤0.25 | ≥32 | ≥32 | ≤1 | ≥128 | ≥128 | ≤1 | ≥320 | ||||||||||||
| 2 Sep | S. epidermidis | Blood | ≤2 | ≤0.25 | ≤2 | ≤10 | ≤1 | ≤0.25 | ≤0.5 | ||||||||||||||
| 2 Sep | E. coli | Urine | ≤1 | ≤0.25 | ≥32 | ≥32 | 8 | ≥64 | ≥64 | ≥128 | ≥128 | ≥16 | 16 | ≤20 | ≤16 | ≥16 | |||||||
| 8 Sep | P. aeruginosa | Skin | ≥8 | 8 | 8 | 4 | 16 | 8 | ≤1 | ≤2 | 160 | 1 | |||||||||||
| 8 Sep | E. coli | Skin | 4 | 1 | ≥32 | ≥32 | 4 | ≥64 | 4 | 4 | ≥128 | ≥128 | ≤0.5 | ≤1 | 4 | 160 | ≤16 | 1 | 2 | ||||
| 27 Sep | P. aeruginosa | BAL fluid | ≥8 | 8 | 8 | 4 | 16 | 8 | ≤1 | ≤2 | 160 | 2 | |||||||||||
| 27 Sep | E. coli | BAL fluid | 4 | 1 | ≥32 | ≥32 | 4 | ≥64 | 4 | 4 | ≥128 | ≥128 | ≤0.5 | ≤1 | 4 | ≥320 | ≤16 | 1 | 2 | ||||
| 6 Oct | E. coli | BAL fluid | ≥16 | 1 | ≥32 | ≥32 | 4 | ≥64 | 4 | 4 | ≥128 | ≥128 | ≥0.5 | ≤1 | 4 | ≥320 | ≥16 | 1 | 1 | ||||
IPM, imipenem; MER, meropenem; AMP, ampicillin; AMX, amoxicillin-clavulanic acid; FEP, cefepime; CFZ, cefazolin; CTX, cefotaxime; CAZ, ceftazidime; PIP, piperacillin; TZP, piperacillin-tazobactam; OXA, oxacillin; TGC, tigecycline; GEN, gentamicin; AMK, amikacin; SXT, sulfamethoxazole-trimethoprim; NIT, nitrofurantoin; CIP, ciprofloxacin; LVX, levofloxacin; NOR, norfloxacin; ERY, erythromycin; CLI, clindamycin.
In August 2010, the patient was transferred to the intensive care unit of the Teaching Hospital (ICU-TO) of Padua for respiratory arrest, which required endotracheal intubation. At that time, microbiology tests revealed the presence of Staphylococcus aureus and carbapenem-susceptible E. coli in bronchoalveolar lavage (BAL) fluid and nasal swab samples, respectively (23 August, Table 1). At the beginning of September, the patient developed hyperthermia (>38°C), and blood and urine cultures grew Staphylococcus epidermidis and carbapenem-susceptible E. coli (imipenem MIC of ≤1 mg/liter), respectively (2 September, Table 1). To cure these infections, the patient initially received ceftriaxone (2 g/day for 3 days), which was replaced with meropenem (3 g/day for 7 days) and teicoplanin (400 mg on the first day and 200 mg/day for the next 4 days). Six days later, surveillance microbiological tests of a skin sample were positive for both Pseudomonas aeruginosa and E. coli (8 September, Table 1). The latter isolate had an increased imipenem MIC (4 mg/liter) that, interpreted according to CLSI criteria (4), indicated resistance to the drug. At that time, the presence of K. pneumoniae carbapenemase (KPC) was phenotypically confirmed by the modified Hodge test (5). The patient was isolated and treated with a wide-spectrum empirical therapy of daptomycin and fluconazole (500 and 200 mg/day, respectively, for 10 days). In addition, the bladder and central venous (right subclavian) catheters were replaced.
At the end of September, a BAL fluid culture still grew P. aeruginosa and E. coli. They were both resistant to imipenem (MICs of ≥8 and 4 mg/liter, respectively, 27 September, Table 1). Nine days later, the E. coli isolate in the BAL fluid showed further increased resistance to imipenem (MIC of ≥16 mg/liter, 6 October, Table 1). The patient was treated with levofloxacin (750 mg/day for 10 days) and up to December 2010, clinical samples showed no clinically relevant microbial growth.
The first isolate of K. pneumoniae (26 April) and the last isolates of E. coli and P. aeruginosa (6 October and 27 September, respectively) were genotypically analyzed using specific primers (12) to check for the presence of carbapenemase-mediated resistance; amplicon sequencing confirmed the presence of the blaKPC-2 gene in both the K. pneumoniae and E. coli strains but not in the P. aeruginosa strain. The three strains were further analyzed for the presence of additional mechanisms of resistance to β-lactams. In particular, the bla genes for TEM-, SHV-, CTX-, IMP-, VIM-, NMC/IMI-, SME-, SPM-, and OXA-type carbapenemases and β-lactamases were tested by PCR amplification and sequencing (21). The blaTEM-1 and blaOXA-9 genes of class A and D β-lactamases, respectively (2), were found in both K. pneumoniae and E. coli; the former also contained the blaVIM-1 gene, while P. aeruginosa did not present any of the β-lactamases tested for.
Multilocus sequence typing analysis was performed according to reported guidelines (11) on K. pneumoniae and E. coli, which were shown to belong to sequence type 147 (ST147) and ST9, respectively. The former was circulating in the ICU-SAH, where the patient had been initially hospitalized (unpublished data), and many of these strains were KPC positive. No information about the presence of E. coli ST9 in our hospital was available, but no other phenotypically imipenem-resistant strains were isolated during that period of time in the two hospitals.
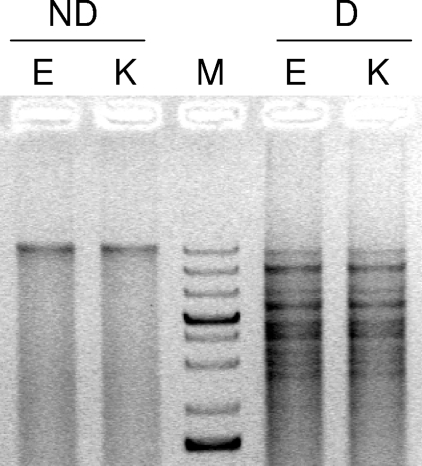
Purified plasmids from K. pneumoniae and E. coli were transformed into an E. coli Top10 (Invitrogen) recipient strain and selected on plates containing 100 mg/liter ampicillin; screening for blaKPC-positive colonies showed that, in both cases, E. coli Top10 was transformed with a 20-kb plasmid, which encoded KPC-2, blaTEM-1, and blaOXA-9, and not blaVIM-1, which was located on a different plasmid. Plasmids from the transformed colonies were purified and digested with the HindIII and EcoRI restriction enzymes (Fermentas Life Sciences, Milan, Italy). The restriction patterns were identical, indicating that the K. pneumoniae and E. coli clinical isolates contained the same plasmid that bore the KPC-2 resistance gene (Fig. 1).
Fig. 1.
Plasmid analysis of KPC-2-positive K. pneumoniae and E. coli isolates. Plasmids from clinical strains were purified and transformed into E. coli Top10 cells. After plasmid purification of the transformed KPC-2-positive colonies, plasmids were loaded onto 1% agarose gel before (nondigested, ND lanes) or after (digested, D lanes) digestion with EcoRI and HindIII. K stands for K. pneumoniae, and E stands for E. coli. M is a molecular size marker of 75 to 20,000 bp (GeneRuler 1 kb Plus DNA Ladder; Fermentas).
The presence of this plasmid increased the MICs of imipenem and meropenem, measured by Etest (AB Biodisk, Solna, Sweden), from 0.25 and 0.32 mg/liter to 3 and 1 mg/liter, respectively. In addition, transformed Top10 gained resistance to all β-lactam antibiotics.
Bacteria producing KPCs are rapidly emerging as a cause of multidrug-resistant infections worldwide. Bacterial isolates harboring these enzymes are capable of hydrolyzing a broad spectrum of beta-lactams, including penicillins, cephalosporins, carbapenems, and monobactams (19).
In the last few years, KPCs have been detected in nosocomial Enterobacteriaceae (mainly K. pneumoniae) and P. aeruginosa. Epidemiological studies have highlighted that the great majority of KPC-producing isolates of K. pneumoniae are clonally related (17). However, blaKPC has been reported to usually reside on a transmissible element (Tn4401) (15) and has been recovered in other genera of bacteria (20). In E. coli, KPCs have been described in only a few cases, especially in the United States, where they were reported first and subsequently, (1, 7, 10, 13), in Israel (9, 16), in China (3, 14), and very recently in Brazil (6).
To our knowledge, this is the second report of a KPC-positive E. coli in Europe; the first one was reported in France from a patient initially hospitalized in Israel (18). In addition, K. pneumoniae belonging to ST147 has been shown for the first time here to bear KPC-mediated resistance. Transfer of KPC-3 from K. pneumoniae to E. coli within the same patient has been reported only once and very recently in Israel (8). Here we show the transmission of KPC-2 from K. pneumoniae to E. coli within the same patient. Interestingly, resistance of the E. coli strain to imipenem could be found only 5 months after the initial detection of a blood infection by the KPC-positive K. pneumoniae strain. The patient was transferred from ICU-SAH to ICU-TO, which is located in a different hospital complex within the city of Padua. K. pneumoniae with KPC-2 has been isolated in ICU-SAH (unpublished data), while no such strains have been found in ICU-TO so far. Hence, we argue that the horizontal transfer of a KPC-2 plasmid between K. pneumoniae and E. coli happened in April, while the two strains coexisted in the blood of the patient. They were subsequently both cleared from the blood, but E. coli probably colonized other organs, where it was later found. E. coli initially retained susceptibility to carbapenems, but subsequent therapy with meropenem selected those E. coli cells that contained the KPC-2 plasmid (found 6 days after the beginning of carbapenem therapy).
Finally, although an increasing number of reports have detected the acquisition of KPC-mediated resistance by the nonenterobacterial species P. aeruginosa (17), no sign of horizontal transfer of a KPC plasmid from E. coli was found in a patient coinfected with these two bacterial species.
Treatment of infection caused by KPC-positive bacteria is particularly worrisome, as the carbapenems are often regarded as agents of last resort for resistant Gram-negative infections; optimal treatment of infections caused by KPC-positive bacteria is not well established yet, and clinical outcome data remain scarce. In addition, the phenotypic detection of carbapenemase production remains difficult, a fact that has undoubtedly contributed to KPC dissemination. So far, only molecular techniques can positively detect the presence of blaKPC genes in clinical isolates. Rapid routine detection of KPCs is not only particularly needed for K. pneumoniae but should be promptly extended (in an infected patient, as well as in patients hospitalized in the same care unit) to all bacterial species reported to bear this resistance mechanism in order to optimize antibiotic therapy, limit KPC-mediated resistance spread worldwide, and therefore increase patient survival.
Acknowledgments
This work was supported by MIUR, grant FIRB-Ideas RBID082ATK.
Footnotes
Published ahead of print on 16 March 2011.
REFERENCES
- 1. Bratu S., et al. 2007. Detection and spread of Escherichia coli possessing the plasmid-borne carbapenemase KPC-2 in Brooklyn, New York. Clin. Infect. Dis. 44:972–975 [DOI] [PubMed] [Google Scholar]
- 2. Bush K., Jacoby G. A. 2010. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 54:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai J. C., Zhou H. W., Zhang R., Chen G. X. 2008. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob. Agents Chemother. 52:2014–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; update. CLSI document M100-S20 June 2010 update. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; update, 20th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. D'Alincourt Carvalho-Assef A. P., et al. 2010. Escherichia coli producing KPC-2 carbapenemase: first report in Brazil. Diagn. Microbiol. Infect. Dis. 68:337–338 [DOI] [PubMed] [Google Scholar]
- 7. Deshpande L. M., Jones R. N., Fritsche T. R., Sader H. S. 2006. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000-2004). Microb. Drug Resist. 12:223–230 [DOI] [PubMed] [Google Scholar]
- 8. Goren M. G., et al. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16:1014–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goren M. G., Navon-Venezia S., Chmelnitsky I., Carmeli Y. 2010. Carbapenem-resistant KPC-2-producing Escherichia coli in a Tel Aviv medical center, 2005 to 2008. Antimicrob. Agents Chemother. 54:2687–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong T., et al. 2005. Escherichia coli: development of carbapenem resistance during therapy. Clin. Infect. Dis. 40:e84–e86 [DOI] [PubMed] [Google Scholar]
- 11. Jolley K. A., Chan M. S., Maiden M. C. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaczmarek F. M., Dib-Hajj F., Shang W., Gootz T. D. 2006. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) beta-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin PhoE. Antimicrob. Agents Chemother. 50:3396–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landman D., et al. 2010. Susceptibility profiles, molecular epidemiology, and detection of KPC-producing Escherichia coli isolates from the New York City vicinity. J. Clin. Microbiol. 48:4604–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li G., et al. 2011. Novel genetic environment of the plasmid-mediated KPC-3 gene detected in Escherichia coli and Citrobacter freundii isolates from China. Eur. J. Clin. Microbiol. Infect. Dis. 30:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naas T., et al. 2008. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob. Agents Chemother. 52:1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navon-Venezia S., et al. 2006. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob. Agents Chemother. 50:3098–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 18. Petrella S., et al. 2008. Genetic and structural insights into the dissemination potential of the extremely broad-spectrum class A beta-lactamase KPC-2 identified in an Escherichia coli strain and an Enterobacter cloacae strain isolated from the same patient in France. Antimicrob. Agents Chemother. 52:3725–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poirel L., Pitout J. D., Nordmann P. 2007. Carbapenemases: molecular diversity and clinical consequences. Future Microbiol. 2:501–512 [DOI] [PubMed] [Google Scholar]
- 20. Queenan A. M., Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richter S. N., et al. 2010. Characterisation of qnr plasmid-mediated quinolone resistance in Enterobacteriaceae from Italy: association of the qnrB19 allele with the integron element ISCR1 in Escherichia coli. Int. J. Antimicrob. Agents 35:578–583 [DOI] [PubMed] [Google Scholar]