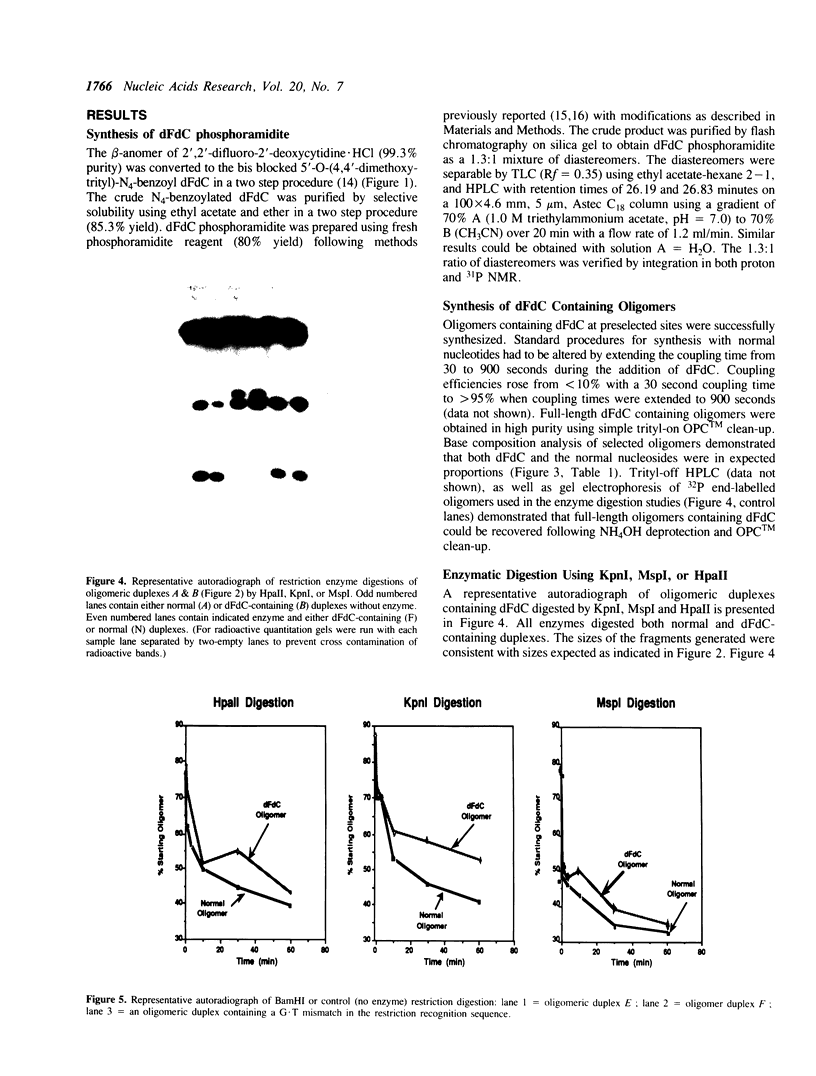
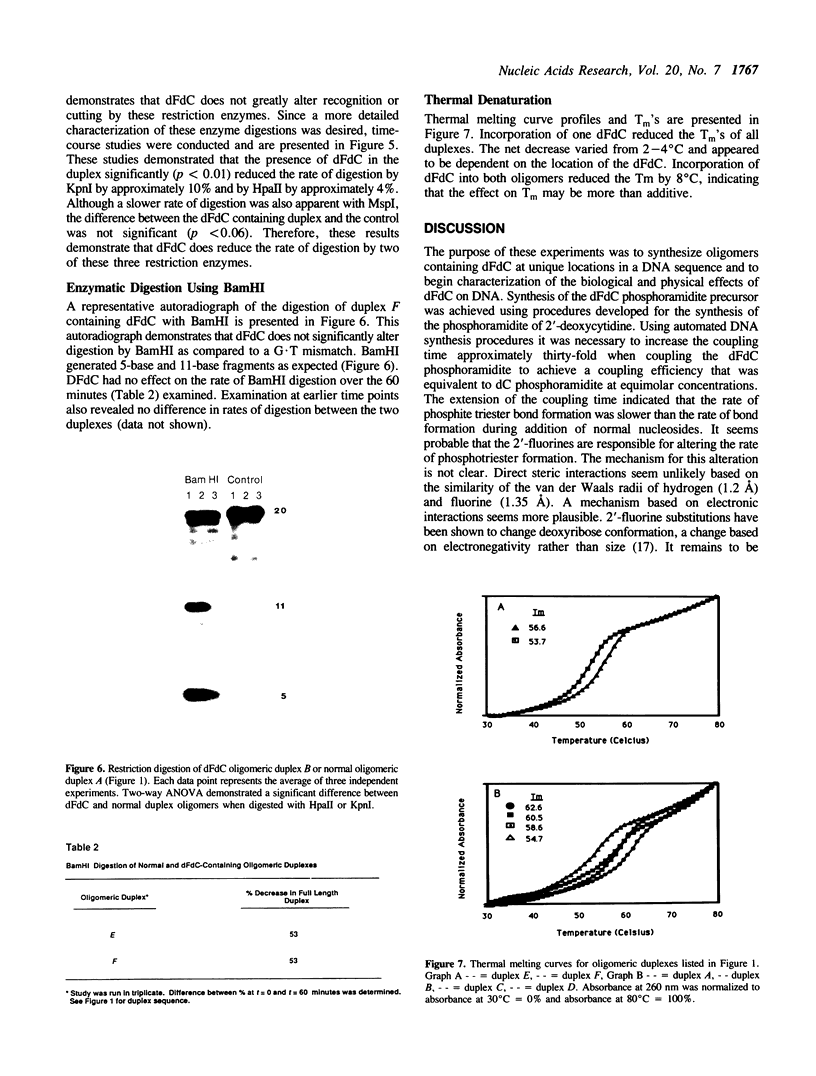
Abstract
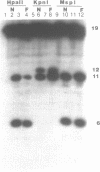
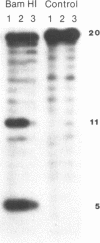
The anti-cancer drug 2',2'-difluoro-2'-deoxycytidine (dFdC) is internally incorporated into DNA in vitro. To determine the effects of this incorporation on DNA structure and function, the beta-cyanoethyl phosphoramidite of dFdC was synthesized and oligodeoxyribonucleotides containing dFdC were made using automated solid phase DNA synthesis techniques. Extension of the coupling time was required to achieve high coupling efficiency, suggesting a significant reduction in the rate of phosphotriester formation. Insertion of dFdC 5' into the recognition sequence of restriction enzymes HpaII and KpnI reduced the rate of cutting by 4% and 14% over 60 minutes. This reduction is similar to the effects seen with arabinofuranosylcytidine (ara-C) but small compared to the reductions caused by base analogues and phosphothioates. Insertion of dFdC into the BamHI recognition sequence, but not 5' to the cut site, did not alter the rate of cutting/recognition. The presence of a single dFdC reduced the Tm's of oligomers by 2-4 degrees C, depending on sequence and location. These results demonstrate that, once incorporated into DNA, dFdC does not greatly alter recognition between DNA and restriction enzymes; however, it does significantly alter duplex stability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbruzzese J. L., Grunewald R., Weeks E. A., Gravel D., Adams T., Nowak B., Mineishi S., Tarassoff P., Satterlee W., Raber M. N. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991 Mar;9(3):491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley G. P., Mikita T., Klaus M. M., Nussbaum A. L. Chemical synthesis of DNA oligomers containing cytosine arabinoside. Nucleic Acids Res. 1988 Oct 11;16(19):9165–9176. doi: 10.1093/nar/16.19.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar J. W., Zempsky W., Warder D., Bergson C., Ward D. C. Effect of nucleotide analogs on the cleavage of DNA by the restriction enzymes AluI, DdeI, HinfI, RsaI, and TaqI. J Biol Chem. 1983 Dec 25;258(24):15206–15213. [PubMed] [Google Scholar]
- Braakhuis B. J., van Dongen G. A., Vermorken J. B., Snow G. B. Preclinical in vivo activity of 2',2'-difluorodeoxycytidine (Gemcitabine) against human head and neck cancer. Cancer Res. 1991 Jan 1;51(1):211–214. [PubMed] [Google Scholar]
- Eadie J. S., McBride L. J., Efcavitch J. W., Hoff L. B., Cathcart R. High-performance liquid chromatographic analysis of oligodeoxyribonucleotide base composition. Anal Biochem. 1987 Sep;165(2):442–447. doi: 10.1016/0003-2697(87)90294-6. [DOI] [PubMed] [Google Scholar]
- Eritja R., Kaplan B. E., Mhaskar D., Sowers L. C., Petruska J., Goodman M. F. Synthesis and properties of defined DNA oligomers containing base mispairs involving 2-aminopurine. Nucleic Acids Res. 1986 Jul 25;14(14):5869–5884. doi: 10.1093/nar/14.14.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindey G. B., Hertel L. W., Plunkett W. Cytotoxicity and antitumor activity of 2',2'-difluorodeoxycytidine (Gemcitabine). Cancer Invest. 1990;8(2):313–313. doi: 10.3109/07357909009017602. [DOI] [PubMed] [Google Scholar]
- Heinemann V., Hertel L. W., Grindey G. B., Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2',2'-difluorodeoxycytidine and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1988 Jul 15;48(14):4024–4031. [PubMed] [Google Scholar]
- Heinemann V., Xu Y. Z., Chubb S., Sen A., Hertel L. W., Grindey G. B., Plunkett W. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2',2'-difluorodeoxycytidine. Mol Pharmacol. 1990 Oct;38(4):567–572. [PubMed] [Google Scholar]
- Hertel L. W., Boder G. B., Kroin J. S., Rinzel S. M., Poore G. A., Todd G. C., Grindey G. B. Evaluation of the antitumor activity of gemcitabine (2',2'-difluoro-2'-deoxycytidine). Cancer Res. 1990 Jul 15;50(14):4417–4422. [PubMed] [Google Scholar]
- Huang P., Chubb S., Hertel L. W., Grindey G. B., Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991 Nov 15;51(22):6110–6117. [PubMed] [Google Scholar]
- McClelland M., Nelson M. The effect of site-specific DNA methylation on restriction endonucleases and DNA modification methyltransferases--a review. Gene. 1988 Dec 25;74(1):291–304. doi: 10.1016/0378-1119(88)90305-8. [DOI] [PubMed] [Google Scholar]
- Ono A., Ueda T. Synthesis of decadeoxyribonucleotides containing N6-methyladenine, N4-methylcytosine, and 5-methylcytosine: recognition and cleavage by restriction endonucleases (nucleosides and nucleotides part 74). Nucleic Acids Res. 1987 Jan 12;15(1):219–232. doi: 10.1093/nar/15.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters J. M., de Vroom E., van der Marel G. A., van Boom J. H., Altona C. Conformational consequences of the incorporation of arabinofuranosylcytidine in DNA. An NMR study of the DNA fragments d(CGCTAGCG) and d(CGaCTAGCG) in solution. Eur J Biochem. 1989 Sep 15;184(2):415–425. doi: 10.1111/j.1432-1033.1989.tb15033.x. [DOI] [PubMed] [Google Scholar]
- Pieters J. M., de Vroom E., van der Marel G. A., van Boom J. H., Koning T. M., Kaptein R., Altona C. Hairpin structures in DNA containing arabinofuranosylcytosine. A combination of nuclear magnetic resonance and molecular dynamics. Biochemistry. 1990 Jan 23;29(3):788–799. doi: 10.1021/bi00455a029. [DOI] [PubMed] [Google Scholar]
- Richardson F. C., Richardson K. K. Alterations in DNA-restriction enzyme interactions by O4-alkyldeoxythymidines. Mol Carcinog. 1991;4(2):162–168. doi: 10.1002/mc.2940040212. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt J. M., Topal M. D. O6-methylguanine in place of guanine causes asymmetric single-strand cleavage of DNA by some restriction enzymes. Biochemistry. 1990 Feb 13;29(6):1632–1637. doi: 10.1021/bi00458a039. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Eckstein F. Effect of deoxynucleoside phosphorothioates incorporated in DNA on cleavage by restriction enzymes. J Biol Chem. 1982 Jun 10;257(11):6595–6599. [PubMed] [Google Scholar]