Abstract
A real-time PCR targeting IS6110 was employed for the detection of Mycobacterium tuberculosis DNA in specimens collected from 10 patients treated with intravesical M. bovis bacillus Galmette-Guérin (BCG) immunotherapy for bladder malignancy. BCG DNA was detected in all urine specimens taken 24 h after the instillations, as well as in 24% of the specimens collected 7 days after the instillations; it was also detected in a single specimen taken 6 weeks after the last instillation. BCG DNA was detected in 8.3% of the blood specimens taken 1 day after instillation, and its amplification was associated with cases of self-limiting fever. These findings give indications that this real-time PCR is helpful to recognize BCG bacteremic cases, which may lead to mycobacterial infection.
INTRODUCTION
Mycobacterium bovis bacillus Calmette-Guérin (BCG), a member of the Mycobacterium tuberculosis complex, has been extensively studied as an immunotherapeutic agent for use in treatment of bladder malignancy. Treatment with BCG instillations has been associated with significant reductions in disease progression and mortality (5, 10). Although the precise mechanism of action has not been fully elucidated, it has been suggested that the interaction of BCG with the urothelium cells results in cytokine secretion, which leads to an enhanced Th1-type immune response and cell-mediated immunity (2). Local side effects, such as hematuria, cystitis, and granulomatous prostatitis, are commonly described (5, 9). In contrast, definite data regarding systematic complications, such as granulomatous hepatitis, pneumonitis, and azotemia, are scarce, whereas follow-up studies have shown that BCG bacteremia and BCG-related infections may occur in up to 7 and 15% of the case patients, respectively (9, 11). It should be noted that some of these systematic adverse effects might be related to immunoallergic reactions, with delayed onset up to several months after BCG therapy completion. In either case, rapid detection of a potential BCG bacteremia may allow the clinician to decide on the appropriate therapy. Such rapid detection can be performed only using molecular techniques (7, 16), as culture methods have up to a 40-day turnaround time.
In a recent report (14), we have described a highly sensitive and specific real-time PCR assay, based on molecular beacon chemistry, which rapidly detects M. tuberculosis complex isolates among pulmonary and extrapulmonary specimens. In the present study, we employed this assay in order to prospectively investigate the persistence of BCG in urine and bladder tissue as well as in blood samples of patients receiving BCG immunotherapy.
MATERIALS AND METHODS
Study patients.
Patients with superficial bladder tumors grade II and III (stage T1) receiving intravesical BCG immunotherapy were enrolled. Therapy was started 3 weeks after complete transurethral tumor resection and was administered once weekly for a total of 6 weeks. Each therapy consisted of a single dose of 50 ml of 0.9% NaCl containing 3 × 106 CFU/ml of BCG (Immunocyst Connaught, Ihringen, Germany), which was instilled into the bladder for a period of 2 h. Only patients who completed the immunotherapy schedule and provided written consent were included in the study. Follow-up cystoscopy with cold cup biopsy was performed 6 weeks after the last instillation and, following that, every 3 months during the first year and every 6 months thereafter, until 30 months after the last instillation.
Collection of specimens.
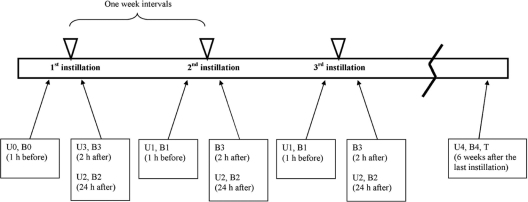
The following samples were collected from each patient and submitted to the laboratory (Fig. 1): (i) urine (U0) and blood (B0) samples 1 h prior to the first instillation, (ii) urine (U1) and blood (B1) samples 1 h prior to the remaining five instillations (from the second to the sixth ones), (iii) urine (U2) and blood (B2) samples 24 h after all six instillations, (iv) a urine sample (U3) 2 h after the first instillation only, (v) blood samples (B3) 2 h after all six instillations, and (vi) blood (B4), urine (U4), and bladder tissue biopsy specimen (T) samples 6 weeks after the last instillation. All urine samples were centrifuged at 5,000 × g for 20 min, the supernatant was removed, and the pellet was used for DNA extraction. A QIAamp DNA minikit (Qiagen GmbH, Hilden, Germany) was used for DNA extraction from the pellet of urine samples as well as the tissue specimens, whereas a QIAamp DNA blood midikit (Qiagen) was used for blood specimens. Both kits were used according to the manufacturer's instructions. The proteinase K pretreatment was performed overnight for tissue specimens.
Fig. 1.
Timetable of specimen collection.
Molecular testing.
A real-time amplification protocol targeting the IS6110 sequence was performed as previously described (14), using an iCycler thermal cycler (Bio-Rad Laboratories, Milan, Italy). For the positive control, DNA was extracted from the M. tuberculosis reference strain H37Rv with the standard phenol-chloroform-isoamyl alcohol method. All samples were tested neat and diluted 10−1 in distilled H2O.
RESULTS AND DISCUSSION
Patients' characteristics.
A total of 10 patients were enrolled, and 8 of these were male. The median age was 72 years (range, 57 to 80 years), and all were diagnosed with a stage T1 tumor (grade II for three patients and grade III for seven patients). The BCG therapy schedule was completed by all patients, and no major side effects were reported. A total of 140 urine, 190 blood, and 10 tissue specimens were examined, comprising 10 U0 and 10 B0 specimens, 50 U1 and 50 B1 specimens, 60 U2 and 60 B2 specimens, 10 U3 and 60 B3 specimens, and, finally, 10 each of the U4, B4, and T specimens (Fig. 1). The detection of BCG DNA was based on a real-time PCR assay, which has been proven more sensitive and specific than culture or conventional PCR, with extrapulmonary specimens (14).
BCG DNA in urine samples.
BCG DNA or DNA of other members of the M. tuberculosis complex was not detected in the urine samples collected before the onset of the therapy (U0 specimens). In addition, none of the patients had signs or symptoms of urogenital tuberculosis. As shown in Table 1, BCG DNA was detected 24 h after the instillations in all patients (all 60 U2 specimens), as well as 1 week after the instillations in half of the patients (12 out of the total 50 U1 specimens, 24%). Finally, 1 out of the 10 U4 specimens (10%), taken 6 weeks after the last instillation, was positive and belonged to patient 4. These data indicate persistence of BCG in the bladder, which has also previously been described by mycobacterial culture and/or conventional PCR assays (3, 7). It has been suggested that BCG DNA may be detected in bladder biopsy specimens even a year after the instillation (7).
Table 1.
Specimen positivity and clinical presentation of the 10 patients
| Patient | Specimen type |
Clinical presentationc |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U0 (10)a | B0 (10) | U1 (50) | B1 (50) | U2 (60) | B2 (60) | U3 (10) | B3 (60) | U4 (10) | B4 (10) | T (10) | Dysuria | Hematuria | Fever | |
| 1 | 0/1b | 0/1 | 0/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 2 | 0/1 | 0/1 | 0/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 3 | 0/1 | 0/1 | 2/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 4 | 0/1 | 0/1 | 2/5 | 0/5 | 6/6 | 2/6 | 1/1 | 0/6 | 1/1 | 0/1 | 0/1 | + | + | + |
| 5 | 0/1 | 0/1 | 2/5 | 0/5 | 6/6 | 3/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | + | + |
| 6 | 0/1 | 0/1 | 0/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 7 | 0/1 | 0/1 | 3/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 8 | 0/1 | 0/1 | 0/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
| 9 | 0/1 | 0/1 | 0/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | + | − |
| 10 | 0/1 | 0/1 | 3/5 | 0/5 | 6/6 | 0/6 | 1/1 | 0/6 | 0/1 | 0/1 | 0/1 | + | − | − |
Numbers in parentheses indicate the total number of specimens collected from each specimen type.
Number of positive specimens/total number of specimens collected from each patient.
+ and −, presence and absence of a clinical symptom, respectively.
BCG DNA in blood samples.
BCG was detected in 5 of the 60 B2 samples (8.3%) taken 24 h after each instillation. These samples belonged to two patients (two blood samples collected after the second and third instillations from patient 4 and three blood samples collected after the first, third, and fifth instillations from patient 5). The respective DNA amounts detected for patient 4 were 6.18 and 6.38 pg/ml, and those for patient 5 were 7.44, 65.0, and 13.81 pg/ml. These figures correspond to approximately 1,250 bacterial cells per ml for both specimens of patient 4 and to approximately 1,500, 13,000, and 2,750 bacterial cells per ml for patient 5 specimens. Definite data in the literature are limited regarding the incidence and/or risk ratio of bacteremia following BCG instillations and especially whether bacteremia is self-limiting or may actually lead to infection. A single study has reported that 9 out of 126 intravesical BCG instillations (7.1%) were associated with mycobacteremia (16), although no data regarding the following BCG-related infections were provided. In other studies, rates of BCG-related infections after instillation of up to 15% have been described (12, 13), but an association with the total number of instillations, as well as the number of instillations leading to bacteremia, was not found. In the present study, all the remaining blood samples (B0, B1, B3, B4) as well as T specimens were negative for BCG DNA. A 30-month follow up showed no recurrence of the tumor or BCG-related infections, local or disseminated.
Quantification of the mycobacterial DNA showed that the positive blood specimens of patient 4 had comparable DNA loads but loads considerably lower than those in two of the three positive specimens of patient 5. It should be noted that the two patients differed only in the scale of the transurethral surgical excision of the tumor, which was greater in patient 5. Thus, a definite conclusion regarding the differences in the mycobacterial DNA loads cannot be made. Quantitative PCR studies have previously been performed in cases of M. tuberculosis infection (4, 6, 15). These studies have shown controversial results regarding the potential advantage of bacterial load quantification and its correlation with patient response during treatment follow-up. In specimens obtained before the initiation of antituberculosis therapy, the DNA load may correlate well with culture results and clinical condition. However, the decline of cultivable bacilli during antimicrobial chemotherapy may not correspond to the DNA load detected (6), and even after 12 months, M. tuberculosis DNA may be detected in culture-negative sputum specimens, particularly from patients with severe disease (15). In that respect, PCR-based follow-up of the patients, especially using quantitative assays, is not recommended in the current guidelines for mycobacterial infections (1, 8).
Local and systematic complications of intravesical BCG immunotherapy.
All 10 patients reported local side effects that included dysuria (pain and/or difficulty upon urination) and urinary frequency, whereas 3 patients also reported macroscopic hematuria (patients 4, 5, and 9; Table 1). These local side effects are expected as a consequence of the inflammatory response and generally increase with successive treatments (9). More specifically, dysuria has been reported to be the most frequent adverse reaction, followed by hematuria (in approximately 90 and 30% of the cases, respectively) (9).
Systematic complications (self-limiting fever and fatigue) were detected in two of the three patients who reported hematuria (patients 4 and 5; Table 1). These two patients were the ones found to be positive for BCG bacteremia. No fever or other systematic signs were recorded among the remaining patients, who were negative for BCG bacteremia. The presence of hematuria may explain the BCG dissemination in these two patients, although hematuria was also observed in patient 9, but no fatigue or fever was reported and the respective blood samples were negative for mycobacterial DNA. Symptoms such as fatigue, joint pain, and muscle ache may develop following hypersensitivity reactions but may also occur as a result of BCG bacteremia, which can eventually lead to BCG-related infection. In that respect, rapid differentiation is critical for appropriate therapy decision.
Conclusions.
The present study showed that 8.3% of the instillations were followed by mycobacteremia and these cases were associated with self-limiting fever, although a BCG-related infection was not detected. However, it should be mentioned that identification of mycobacterial DNA in blood samples cannot definitely rule out the possibility that the general side effects may be the result of a hypersensitivity reaction to BCG and not a true mycobacterial infection (2, 16). In either case, early detection of mycobacterial DNA in the blood, using a sensitive and specific real-time PCR protocol, may provide the attending physician with the opportunity to consider a possible disseminated infection, if the symptoms persist after the expected time frame of a hypersensitivity reaction.
Footnotes
Published ahead of print on 26 January 2011.
REFERENCES
- 1. Anonymous 2005. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: controlling tuberculosis in the United States. Am. J. Respir. Crit. Care Med. 172:1169–1227 [DOI] [PubMed] [Google Scholar]
- 2. Bohle A., Brandau S. 2003. Immune mechanisms in bacillus Calmette-Guérin immunotherapy for superficial bladder cancer. J. Urol. 170:964–969 [DOI] [PubMed] [Google Scholar]
- 3. Bowyer L., Hall R. R., Reading J., March M. M. 1995. The persistence of bacille Calmette-Guérin in the bladder after intravesical treatment for bladder cancer. Br. J. Urol. 75:188–192 [DOI] [PubMed] [Google Scholar]
- 4. Broccolo F., et al. 2003. Rapid diagnosis of mycobacterial infections and quantitation of Mycobacterium tuberculosis load by two real-time calibrated PCR assays. J. Clin. Microbiol. 41:4565–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brosman S. A. 1982. Experience with bacillus Calmette-Guérin in patients with superficial bladder carcinoma. J. Urol. 128:27–30 [DOI] [PubMed] [Google Scholar]
- 6. Desjardin L. E., et al. 1998. Comparison of the ABI 7700 system (TaqMan) and competitive PCR for quantification of IS6110 DNA in sputum during treatment of tuberculosis. J. Clin. Microbiol. 36:1964–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durek C., et al. 2001. The fate of bacillus Calmette-Guérin after intravesical instillation. J. Urol. 165:1765–1768 [PubMed] [Google Scholar]
- 8. Griffith D. E., et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 9. Lamm D. L. 1992. Complications of bacillus Calmette-Guérin immunotherapy. Urol. Clin. North Am. 19:565–572 [PubMed] [Google Scholar]
- 10. Lamm D. L. 1992. Long-term results of intravesical therapy for superficial bladder cancer. Urol. Clin. North Am. 19:573–580 [PubMed] [Google Scholar]
- 11. Lamm D. L. 2000. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin. Infect. Dis. 31(Suppl. 3):S86–S90 [DOI] [PubMed] [Google Scholar]
- 12. Lamm D. L., Stogdill V. D., Stogdill B. J., Crispen R. G. 1986. Complications of bacillus Calmette-Guérin immunotherapy in 1,278 patients with bladder cancer. J. Urol. 135:272–274 [DOI] [PubMed] [Google Scholar]
- 13. Lamm D. L., et al. 1992. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J. Urol. 147:596–600 [DOI] [PubMed] [Google Scholar]
- 14. Papaparaskevas J., Houhoula D. P., Siatelis A., Tsakris A. 2008. Molecular-beacon-based real-time PCR for detection and quantification of Mycobacterium tuberculosis DNA in clinical samples. J. Clin. Microbiol. 46:3177–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomsen V. Ø., Kok-Jensen A., Buser M., Philppi-Schulz S., Burkardt H. J. 1999. Monitoring treatment of patients with pulmonary tuberculosis: can PCR be applied? J. Clin. Microbiol. 37:3601–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuncer S., et al. 1997. Detection of bacillus Calmette-Guérin in the blood by the polymerase chain reaction method of treated bladder cancer patients. J. Urol. 158:2109–2112 [DOI] [PubMed] [Google Scholar]