Abstract
Vitamin E (α-tocopherol) is the major lipid-soluble antioxidant in many species. Niemann-Pick type C (NPC) disease is a lysosomal storage disorder caused by mutations in the NPC1 or NPC2 gene, which regulates lipid transport through the endocytic pathway. NPC disease is characterized by massive intracellular accumulation of unesterified cholesterol and other lipids in lysosomal vesicles. We examined the roles that NPC1/2 proteins play in the intracellular trafficking of tocopherol. Reduction of NPC1 or NPC2 expression or function in cultured cells caused a marked lysosomal accumulation of vitamin E in cultured cells. In vivo, tocopherol significantly accumulated in murine Npc1-null and Npc2-null livers, Npc2-null cerebella, and Npc1-null cerebral cortices. Plasma tocopherol levels were within the normal range in Npc1-null and Npc2-null mice, and in plasma samples from human NPC patients. The binding affinity of tocopherol to the purified sterol-binding domain of NPC1 and to purified NPC2 was significantly weaker than that of cholesterol (measurements kindly performed by R. Infante, University of Texas Southwestern Medical Center, Dallas, TX). Taken together, our observations indicate that functionality of NPC1/2 proteins is necessary for proper bioavailability of vitamin E and that the NPC pathology might involve tissue-specific perturbations of vitamin E status.
Keywords: nutrition, oxidized lipids, Niemann-Pick disease
Niemann-Pick type C (NPC) disease is a heritable lysosomal storage disorder, in which the intracellular transport of lipids is perturbed (1). The cellular phenotype of NPC disease is massive accumulation of cholesterol and other lipids in membranous organelles derived from late endosomes and lysosomes (2–5). Since the “trapped” cholesterol is not metabolically available, various regulatory pathways sense an apparent shortage, and paradoxically, denovo synthesis is increased, further exacerbating the situation (6, 7). Although dysregulated lipid processing occurs in most organ systems of NPC patients, the primary pathology they present is localized to the central nervous system, in the form of progressive neurodegeneration. Specifically, NPC patients suffer from motor and coordination dysfunctions, seizures, and cognitive impairments that typically present during the first decade of life. NPC disease is fatal, and most patients succumb to it before reaching teen age (e.g., Refs. 1, 8, 9). Intensive investigations in the recent two decades have led to the development of diverse therapeutic intervention strategies, most of which aim to repair the imbalance in specific lipids or metabolites (10). To date, however, only limited clinical benefit has been achieved, at best leading to stabilization of clinical symptoms (e.g., Ref. 11). The molecular culprits underlying NPC disease have been shown to be loss-of-function mutations in either NPC1 or NPC2 proteins, which reside in the lysosomal limiting membrane or lumen, respectively (12–15). Although the precise mechanisms of action of these proteins are not fully understood, it is generally accepted that NPC1 and NPC2 function in sequence in removing free cholesterol from the lysosomal lumen to the cytosol (16–21). While NPC-affected lysosomes accumulate large amounts of free cholesterol, intracellular transport of glycosphingolipids, sphingomyelin, and sphingosine is also severely perturbed (22). Which of these trapped molecules (or their metabolites) is the metabolic root for the NPC pathology is presently unknown (22).
It is interesting to note that common pathological and biochemical hallmarks are shared by NPC disease and deficiency in the dietary antioxidant vitamin E. First, in both cases, the major site of dysfunction is the central nervous system (CNS), and the major clinical presentation is cerebellar ataxia (23–25), accompanied by specific injury to cerebellar Purkinje neurons (26, 27). Second, axonal spheroids (focal swellings) are frequently observed in both NPC disease (28) and in vitamin E deficiency (29–31). Similarly, pronounced hypomyelination is characteristic of advanced-stage disease in both cases (32, 33). Finally, modest supplementation with vitamin E has been reported to result in a mild improvement in motor performance in a mouse model of NPC disease (34). On the cellular level, it has been established that uptake of vitamin E occurs via endocytosis (35, 36) and that a significant portion of the vitamin is found in lysosomes (37). In light of these observations, we hypothesized that proper intracellular trafficking of vitamin E (and in turn, adequate antioxidant protection) depends on timely egress from the lysosome and, therefore, on the functionality of NPC1/2. We describe here our findings regarding α-tocopherol status in cells that express defective alleles or reduced expression of NPC1/2, in various tissues from mice in which expression of NPC1/2 is disrupted and in plasma from human NPC patients.
MATERIALS AND METHODS
Cell culture
Human fibroblasts harboring the p.P237S and p.I1061T missense mutations in the NPC1 gene were obtained from Coriell Cell Repository (GM03123; Camden, NJ) and grown in Eagle's minimum essential medium with Earle's salts, 2 mM L-glutamine and 15% fetal bovine serum at 37°C and 5% CO2 (38). Control human fibroblasts (CRL-2076) were obtained from American Type Culture Collection (Manassas, VA). Immortalized human hepatocytes (IHH) (39, 40) were a generous gift from R. Ray (Saint Louis University, St. Louis, MO) and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% calf serum. Lentiviral short hairpin RNA (shRNA) constructs targeted against human NPC1, human NPC2, and a control shRNA in the pLKO vector (Open Biosystems, Huntsville, AL) were transfected into HEK293T cells using Lipofectamine-Plus (Invitrogen, Carlsbad, CA). Culture media were harvested 24 and 48 h posttransfection, pooled, and pelleted by centrifugation at 100,000 g for 1.5 h. The pellet was resuspended in PBS and used for polybrene-mediated (4 μg/ml) transduction of IHH cells using standard protocols. Stable knockdown clones were selected in media supplemented with puromycin (10 μg/ml; Sigma Chemical Co., St. Louis, MO) 48 h after transduction. Knockdown efficiency was evaluated by immunoblotting using antibodies raised against NPC1 (Abcam, Cambridge, MA) or NPC2 (generous gift of Peter Lobel, Rutgers University, Rutgers, NJ). For evaluating the endogenous expression levels of the α-tocopherol transfer protein (TTP), samples were immunoblotted using the A8E5 anti-TTP antibody (H. Arai).
Disease models
The Npc1−/− mice (BABLc/NPCnih) were originally described in Ref. 12, and the Npc2−/− mice were described in Ref. 41. Human serum samples were collected from NPC1 patients and healthy age-appropriate unaffected subjects under a clinical protocol (06-CH-0186) approved by the NICHD Institutional Review Board of the National Institute of Child Health and Human Development. Both consent and assent, if appropriate, were obtained. Serum samples were de-identified and maintained at −80 degrees centigrade.
Fluorescence microscopy
Cells were plated on poly-L-lysine coated glass coverslips in 24-well tissue culture plates. NBD-cholesterol (Invitrogen) and NBD-tocopherol (42, 43) were complexed to serum lipoprotein as described earlier (35, 44) and added to the culture media to a final concentration of 20 μM and incubated for 17 h at 37°C. The fluorescent lipid was “chased” by incubation in normal media for 3 h more. Cells were fixed for 20 min in 3.7% paraformaldehyde and mounted in SlowFade Gold antifade reagent (Invitrogen) prior to imaging on a confocal or inverted fluorescence microscope (Zeiss LSM 510 and Leica DMI 4000B, respectively). For quantitation of accumulated fluorophores, ten microscopic images were captured under identical conditions, each containing 30-60 cells. Fluorescence intensities were quantitated using Image J software (http://rsbweb.nih.gov/ij/index.html). The RGB images were converted to an 8-bit images; a common threshold set for all images. For colocalization studies, LysoTracker Red DND-99 (75 nM, Invitrogen) was added 30 min prior to fixing. For visualization of free cholesterol, fixed and permeabilized cells were incubated with 25 μg/ml filipin (Streptomyces filipinensis; Sigma Chemical) for 1 h at room temperature in the dark, prior to washing in PBS and visualization.
Analytical determinations
Total cholesterol.
Cells were harvested, resuspended in PBS, and lysed by repeated passing through a 22-gauge needle. Total cholesterol was measured using the Amplex Red Cholesterol Assay kit (Invitrogen) according to manufacturer's protocol. Fluorescence was excited at 530 nm and emission was collected at 590 nm on a Tecan GENios Pro plate reader (Tecan, Durham, NC). Total cholesterol was normalized to total protein, as determined by the Bio-Rad protein assay kit.
Tocopherols and free cholesterol.
Serum and appropriate tissues from Npc1−/− and Npc2−/− mice and their wild-type littermates were freshly excised and flash-frozen as described previously (45). Lipids were extracted, silylated, and analyzed by GC-MS on a Hewlett-Packard 6890 gas chromatograph coupled to a Hewlett-Packard 5872 mass selective detector operated in selected ion mode as previously described (46). Deuterated α-tocopherol added prior to extraction served as an internal standard. Monitored masses of trimethylsilyl ethers (TMS) were 511.6 (d9-α-tocopherol-TMS), 502.6 (d0-α-tocopherol-TMS), 488.6 (d0-γ-tocopherol-TMS), and 458.7 (cholesterol-TMS). A previously determined detector response correction factor was applied in quantitation of cholesterol. Tocopherol and unesterified cholesterol concentrations were normalized to tissue wet weight.
Binding of tocopherol to purified NPC1 and NPC2
The affinity of α-tocopherol to the purified NPC1/2 proteins was measured by Rodney Infante and Joseph Goldstein at the University of Texas Southwestern Medical Center (Dallas, TX) using a published assay based on competition with radio-labeled cholesterol (17). Briefly, 4 pmol purified sterol binding domain (NTD) of NPC1, or 8 pmol purified full-length NPC2 were incubated overnight with 130 nmol [3H]cholesterol at 4°C. The proteins were then incubated with 6 μM unlabeled competitor (cholesterol, epicholesterol, 25-hydroxycholesterol, or dl-α tocopherol), and protein-bound radioactivity was measured after affinity chromatography with nickel-agarose and scintillation counting.
Statistical analyses
Statistical significance of data was determined using unpaired Student's t-test. P values < 0.05 were taken as the threshold of significance. Data were analyzed and graphed using the IgorPro software package (Wavemetrics, Inc., Portland, OR).
RESULTS
α-tocopherol accumulates in NPC-affected fibroblasts
The NPC1 and NPC2 proteins are residents of the lysosome that are required for proper transit of cholesterol through the endocytic pathway (15, 47). Given that sphingomyelin, glycosphingolipids, and phospholipids also accumulate in NPC-affected lysosomes (2, 48–51), we hypothesized that NPC1/2 proteins participate in the endocytic processing of the lipid-soluble antioxidant α-tocopherol (vitamin E). To visualize the intracellular trafficking of α-tocopherol, we utilized NBD-tocopherol, a fluorescent analog that we previously characterized in vitro (42, 43, 52, 53) and in vivo (35, 54). Using fluorescence microscopy, we visualized the accumulation of NBD-tocopherol in cultured fibroblasts isolated from an NPC-affected patient (harboring the c.709C>T and c.3182T>C substitutions in the NPC1 gene) and control fibroblasts. As shown in Fig. 1, control fibroblasts retained very little NBD-tocopherol. However, NPC-fibroblasts accumulated much higher (ca. 3-fold) levels of the fluorescent vitamin, appearing in a punctate, perinuclear distribution pattern. These observations indicate that egress of α-tocopherol from the endocytic compartment requires a functional NPC1 protein.
Fig. 1.
NBD-tocopherol accumulates in human NPC1 fibroblasts. Indicated fibroblasts were incubated with serum-complexed NBD-tocopherol overnight and “chased” in normal growth media for 3 h. Fixed cells were imaged by fluorescence microscopy. A: Representative fluorescence micrographs. Magnification: 60×. B: Quantitation of fluorescence intensity of 10 images, each including at least 30 cells. Asterisks denote significant difference (P > 0.05) from control shRNA cells, as determined by Student's t-test.
Generation and characterization of NPC1 and NPC2 knockdown hepatocyte cell lines
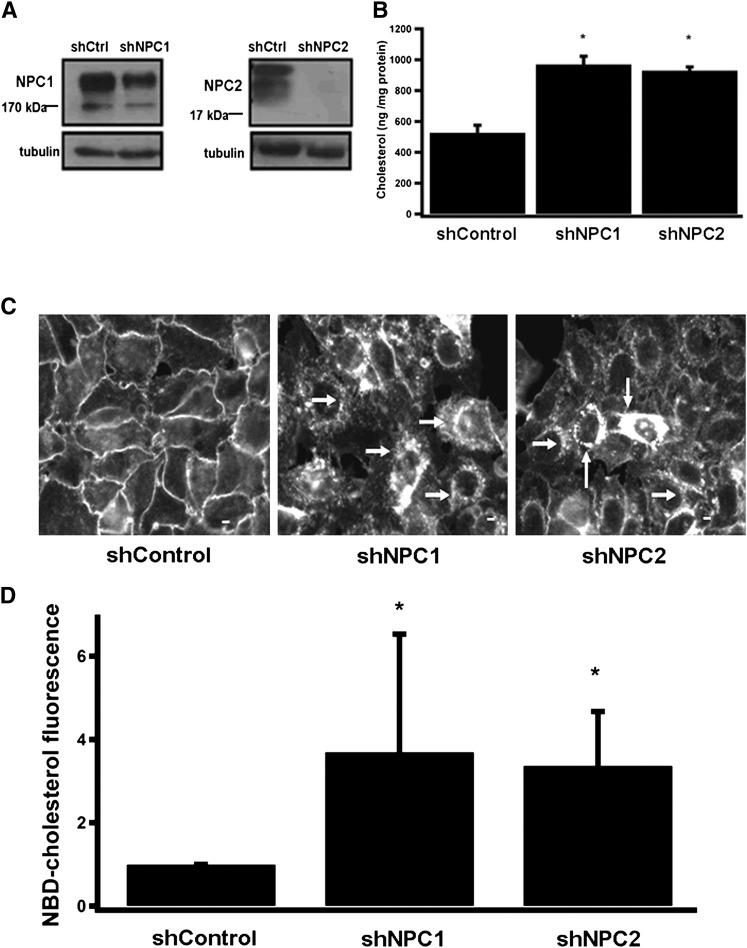
Although genetic defects in NPC1 and NPC2 lead to severe accumulation of cholesterol in the liver (55), no hepatocyte cell culture model is presently available to study the disease. We therefore generated lentiviruses that encode shRNAs against the human NPC1 and NPC2 transcripts, and used these reagents to generate IHH (39) in which the expression of NPC1 or NPC2 is stably disrupted. As shown in Fig. 2A, expression of NPC1 and NPC2 in the stable “knockdown” cell lines was reduced by ∼50% and 90%, respectively, compared with IHH cells which express a control shRNA. Since egress of α-tocopherol from the liver depends on the hepatic TTP (35, 56), we examined whether expression levels of TTP are altered in NPC1/2 knockdown cells. Immunoblotting with anti-TTP antibodies revealed that expression levels of TTP in these cells was comparable to the levels observed in control IHH cells (data not shown). As altered intracellular distribution of cholesterol is the cellular hallmark of NPC disease (55), we examined the levels and intracellular distribution of cholesterol in the NPC1/2 “knockdown” cells. Fig. 2B shows the amount of total cholesterol retained in these cells, as determined by the Amplex Red colorimetric assay kit. In both shNPC1 and shNPC2 cells, total cellular cholesterol was increased by approximately 2-fold compared with control cells. To examine the effects of NPC1/2 on the intracellular distribution pattern of cholesterol, we employed the fluorescent fungal macrolide filipin, which selectively binds to free (unesterified) cholesterol in membranes (57), and is a primary tool for diagnosing NPC disease (2, 58). In control IHH cells, filipin fluorescence outlined free cholesterol exclusively in the cells’ plasma membranes (Fig. 2C, left panel). In shNPC1 and shNPC2 cells, however, the filipin-staining pattern was markedly different: First, intensity of the fluorescence signal was much higher compared with control cells, indicating significant accumulation of free cholesterol. Second, filipin staining was seen primarily within the hepatocytes, in a punctate, perinuclear pattern (arrows in center and right panels of Fig. 2C). This pattern is essentially identical to the lysosomal accumulation of free cholesterol in other NPC1/2 cell types (38, 41). Finally, we examined the intracellular fate of cholesterol that was taken up through endocytosis. Toward this end, we monitored the uptake of the fluorescent analog NBD-cholesterol (59–62) that was precomplexed to serum lipoproteins. The shNPC1 and shNPC2 cells accumulated significantly higher levels (∼3-fold) of NBD-cholesterol compared with control hepatocytes (Fig. 2D).Taken together, these results indicate that hepatocytes with disrupted expression of NPC1 or NPC2 display the established lipid-trafficking defects that characterize NPC disease. Therefore, we conclude that the stable shRNA IHH cell lines are an appropriate model system for investigating the roles of NPC proteins in the intrahepatocyte trafficking of lipids, including vitamin E.
Fig. 2.
Characterization of human hepatocytes stably expressing shRNAs to NPC1 or NPC2. IHH cells expressing the indicated shRNA were generated by lentiviral transduction and antibiotic selection as detailed in Materials and Methods. A: Expression of NPC1 and NPC2 was examined by Western blotting in lysates from the indicated sublines. B: Cellular content of total (esterified plus free) cholesterol in the different sublines was measured using the Amplex Red kit. Shown are averages and standard deviations of three independent experiments. C: Content and distribution of unesterified cholesterol were determined by filipin staining. Note that in control cells, free cholesterol is localized exclusively to the plasma membrane, whereas shNPC sublines exhibit pronounced intracellular accumulation, appearing as perinuclear vesicles (white arrows). Scale bar = 10 μm. D: Accumulation of NBD-cholesterol was examined after overnight loading with serum-complexed NBD-cholesterol as described in Materials and Methods. Ten fluorescent images, each containing 40-60 cells, were digitized and fluorescence intensity determined using Image J software. Asterisks in B and D denote significant difference (P > 0.05) from control shRNA cells, as determined by Student's t-test.
Disrupted expression of NPC1/2 causes lysosomal accumulation of vitamin E in IHH cells
To examine the involvement of NPC proteins in trafficking of α-tocopherol, we “loaded” the different IHH cell lines with serum-complexed NBD-tocopherol and examined accumulation of the vitamin using fluorescence microscopy. As shown in Fig. 3A, NBD-tocopherol was efficiently taken up by the cells and concentrated in a vesicular, perinuclear compartment, reminiscent of our previous observations in human HepG2 and rat McARH-7777 hepatocytes (35, 54). We quantitated fluorescence intensity in images from three independent experiments, and we found that cells with reduced expression of either NPC1 or NPC2 accumulated ∼2-fold more NBD-tocopherol compared with control cells. Next, we utilized confocal fluorescence microscopy to determine the intracellular compartment in which NBD-tocopherol accumulates. As shown in Fig. 3C, the intracellular distribution pattern of NBD-tocopherol colocalized with that of LysoTracker, an established marker of the late endocytic/lysosomal compartment (63, 64). We concluded that functionality of NPC1 and NPC2 is required for the egress of endocytosed vitamin E from the endocytic compartment. Furthermore, under conditions of NPC1/2 impairment, the majority of tocopherol accumulates in lysosomes in a pattern similar to that of NBD-cholesterol.
Fig. 3.
Intracellular accumulation of NBD-tocopherol in shNPC1 and shNPC2 IHH cells. Cells were “loaded” with NBD-tocopherol as described in Materials and Methods, and intracellular distribution of the vitamin was examined using fluorescence microscopy. A: Representative NBD-fluorescence images. B: Accumulation of NBD-tocopherol. Cells were loaded with NBD-tocopherol, and fluorescence intensity was measured as described in Materials and Methods. Asterisks denote significant difference (P > 0.05) from control shRNA cells, as determined by Student's t-test. C: Confocal fluorescence micrographs showing colocalization of NBD-tocopherol (green) with the lysosomal marker LysoTracker (red).
Tocopherol is a poor ligand for NPC1 and NPC2
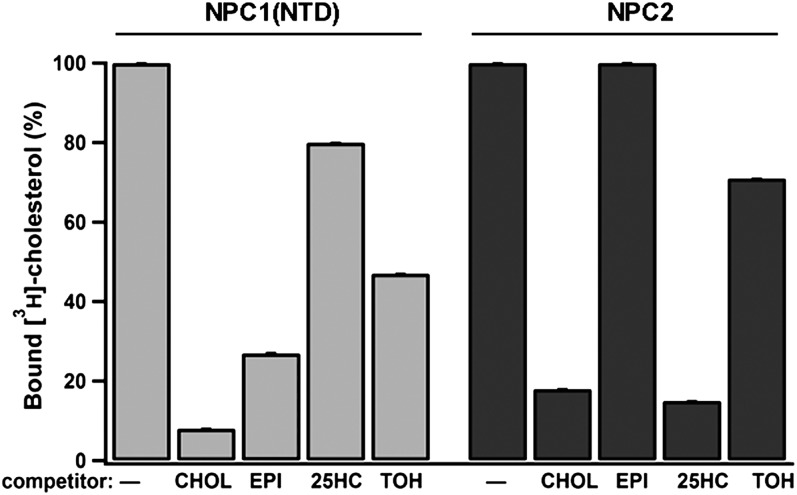
To gain insights into the molecular mechanisms by which NPC1/2 affect tocopherol trafficking, we directly measured the binding affinity of these proteins for α-tocopherol. Toward this end, we examined the efficacy of vitamin E in competing with [3H]cholesterol for binding to purified NPC2 or to purified recombinant sterol binding domain of NPC1 (residues 1-240) (17, 19). Under saturating conditions (tocopherol:binding site molar ratio = 1000), α-tocopherol was able to displace only 30% and 50% of the [3H]cholesterol bound to NPC2 and NPC1, respectively (Fig. 4), whereas unlabeled cholesterol displaced >85% of the bound ligand. These results indicate that the affinity of α-tocopherol to NPC proteins is 2-3 orders of magnitude weaker than that of cholesterol. These in vitro findings are put into physiological perspective when appreciating that, in vivo, concentrations of cholesterol are 100- to 1000-fold higher than those of α-tocopherol (65), and this ratio is likely higher in lysosomes (66).These considerations suggest that α-tocopherol is not likely to occupy a significant fraction of the NPC1/NPC2 binding pockets in lysosomes of intact cells. Thus, we concluded that the accumulation of α-tocopherol observed in the NPC-defective cells is likely an indirect effect, secondary to the significant buildup of lipids and the extensive structural reconfiguration of the late endocytic compartment. This conclusion is consistent with reports regarding other lipids that do not directly bind to NPC1 or NPC2, but that accumulate under NPC1 or NPC2 loss-of-function (21, 67, 68).
Fig. 4.
Binding of vitamin E to NPC1/2 in vitro. Binding of radiolabeled cholesterol to the purified proteins was measured in the presence of the indicated competitors as described in Materials and Methods. CHOL, cholesterol; EPI, epicholesterol; 25HC, 25-hydroxycholesterol; TOH, dl-α-tocopherol. Shown are averages and standard deviations of three experiments. See Ref. 17 for details.
Vitamin E status in NPC-affected mice
To examine the involvement of NPC proteins in the status of vitamin E in vivo, we employed GC-MS to determine the tocopherol content in extracts from plasma, livers, and brains of Npc1−/− and Npc2−/− mice (41). To frame our findings in the context of overall lipid status, we also determined the free cholesterol content of these extracts. In the liver, both cholesterol and tocopherol accumulated in NPC-affected mice to higher levels than in wild-type animals. Specifically, hepatic concentrations of cholesterol increased by 10- and 6-fold in the livers of 12-week old Npc1−/− and Npc2−/− mice, respectively (Fig. 5A). These values are similar to the hepatic values reported earlier for these models (41, 55, 51, 68). Analyses of vitamin E content revealed that hepatic levels of tocopherol also increased in 12-week-old NPC-affected mice, albeit to a lesser degree (Fig. 5A). Since vitamin E shares with cholesterol many common uptake and transport steps, it is also instructive to present the concentration values as tocopherol:cholesterol mole ratios (see Refs. 69 and 70 for detailed discussion). As seen in Fig. 5E, the tocopherol:cholesterol ratio was significantly decreased in NPC-affected livers. Thus, while NPC-affected livers accumulated both lipids, hepatic accumulation of cholesterol exceeded that of tocopherol by >5-fold. As a result, the effective disruption in hepatic vitamin E status caused by NPC is actually more severe than appears at first sight. Mechanistically, such disproportionate accumulation of the two lipids is likely to reflect additional, vitamin E-specific routes of egress from the endocytic pathway that are not shared by cholesterol. The existence of such secretion pathways is supported by the rapid turnover of hepatic tocopherol in plasma (approximately 1 hepatic pool per day) (71) and by our observations that in cultured hepatocytes, some NBD-tocopherol colocalizes with the rapidly recycling, transferrin-positive compartment (J. Qian and D. Manor, unpublished observations).
Fig. 5.
Tocopherol and unesterified cholesterol content in tissue extracts from Npc1−/−, Npc −/−, and wild-type mice. Analytes were measured using GC-MS as described in Materials and Methods. A, E: Liver. B, F: Cerebral cortex. C, G: Cerebellum. Shown are averages and standard deviations (n = 3). Asterisks denote significant difference (P < 0.05) compared with age-matched controls, as determined by Student's t-test. D: Expression levels of the α tocopherol transfer protein in livers of the different mouse models. Expression levels were evaluated by anti-TTP Western blotting of soluble extracts prepared from three animals of the indicated genotypes. WT, wild-type.
We also found that expression levels of TTP did not differ among the wild-type, Npc1−/−, and Npc2−/− mice (Fig. 5D). We concluded that accumulation of tocopherol in NPC-affected mice does not stem from altered TTP expression but, rather, is a consequence of impairment in the function of NPC1/2 proteins. In the cortex, we observed a significant (40-50%) decrease in the content of tocopherol as well as cholesterol in 12-week-old Npc1−/− mice (Fig. 5B). We attributed this decrease to the severe hypomyelination of the cortex that accompanies NPC disease (28, 41, 72). Both cholesterol and tocopherol are important constituents of myelin (73–75), and vitamin E deficiency causes hypomyelination (76–78). In the cerebellum, the only statistically significant difference was observed in 12-week-old Npc2−/− mice, which exhibited a ∼30% increase in the content of tocopherol as well as cholesterol, compared with wild-type animals (Fig. 5C). No significant differences in tocopherol or cholesterol content were observed in Npc1−/− mice, although the fractional lipid content (mole ratio) of tocopherol was slightly increased (Fig. 5G). This could be explained by the fact that lipid accumulation is balanced by lipid loss that accompanies neurodegeneration in this tissue (6, 45, 73, 79).
Plasma tocopherol and cholesterol levels in NPC-affected mice and humans
Figure 6 shows the concentrations of tocopherol and cholesterol in plasma samples from Npc1−/−, Npc2−/−, and wild-type mice. In agreement with published reports (6), plasma cholesterol values of Npc1−/− mice were not significantly different from wild-type animals (Fig. 6A). Similarly, plasma vitamin E levels were unchanged in Npc1−/− mice (Fig. 6B). In 12-week-old Npc2−/− mice, however, plasma levels of both cholesterol and tocopherol were elevated by approximately 30%. Unlike in other tissues, however, the increase in the two lipids was essentially identical, such that the tocopherol:cholesterol ratio did not differ among the different mouse models (Fig. 6C).
Fig. 6.
Plasma tocopherol levels are normal in Npc1−/− and Npc2−/− mice and NPC-affected humans. Tocopherol and cholesterol levels were measured in plasma samples of the indicated mouse models using GC-MS as described in Materials and Methods. Shown are averages and standard deviations (n = 3). Asterisks denote significant difference (P < 0.05) compared with age-matched controls, as determined by Student's t-test.
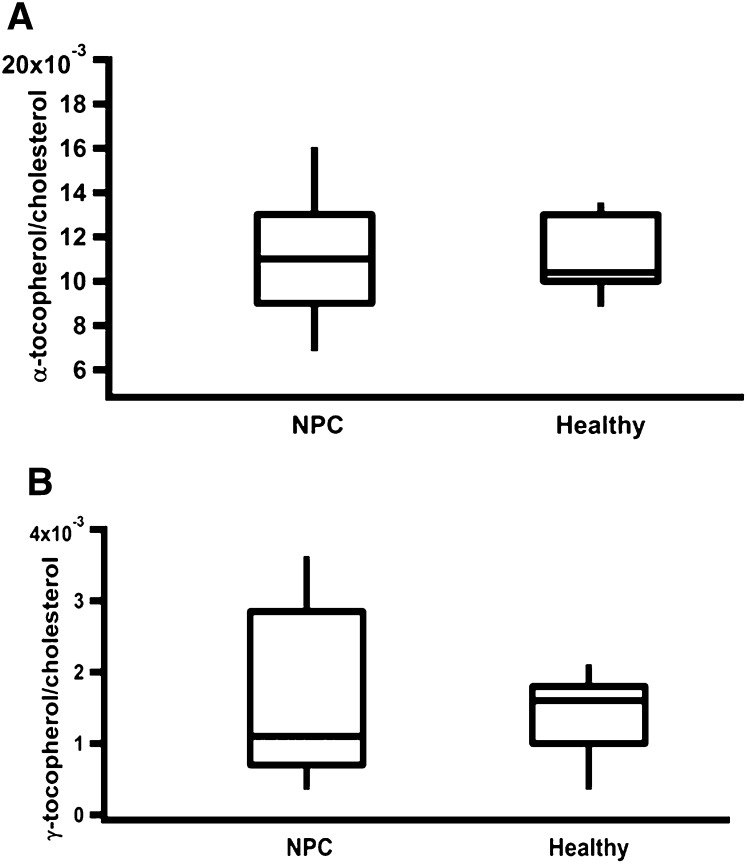
Lastly, we analyzed the plasma levels of tocopherol in a cohort of 45 NPC1 patients and 20 age-appropriate control subjects. Total cholesterol levels in plasma samples from NPC patients were <200 mg/dl; i.e., within the normal range for adults as defined by the American Heart Association (80). These values are similar to those reported previously for NPC1 patients (81). Importantly, plasma levels of α-tocopherol and the most prevalent vitamin E form in the US diet, γ-tocopherol, were within the clinically normal range (12-50 μM) (82). Figure 7A and B show the concentrations of α- and γ-tocopherol, respectively, after normalization to plasma cholesterol levels. Taken together, our data indicate that although NPC-affected cells and tissues showed significant alterations in the status of vitamin E and cholesterol, plasma levels were not affected in NPC-affected mice and humans.
Fig. 7.
Plasma vitamin E:cholesterol ratios are normal in human NPC1 patients. Plasma was collected from 45 NPC1 patients and 20 healthy age-appropriate controls, and concentrations of α-tocopherol (A) and γ-tocopherol (B) as well as unesterified cholesterol were determined using GC-MS as described in Materials and Methods. Data are represented in a box plot, in which the horizontal line designates the median value, separating the upper and lower quartiles. The “whiskers” show the maximum and minimum spread of the data.
DISCUSSION
Niemann-Pick type C disease is a debilitating, fatal disorder in which intracellular lipid transport is impaired due to loss-of-function mutations in the NPC1 or NPC2 protein. The main biochemical phenotype associated with NPC disease is accumulation of unesterified cholesterol and other lipids in a vesicular compartment of an endosomal/lysosomal origin. A number of metabolic scenarios can be envisioned to be at the root of NPC pathology. First, the extensive localized accumulation of lipids may be toxic, thereby compromising cell function and viability. Second, since the affected lipids are “sequestered” away from their proper sites of action, the affected cell may experience a catastrophic deficiency of these metabolites. Lastly, physical disruption of the endocytic compartment may deprive the cell of other molecules that rely on this pathway for cellular transport. Despite intense research efforts in the past 50 years, many questions regarding the etiology of NPC disease remain unanswered. Thus, it is still not known which of scenarios described above is of highest significance during disease progression. Similarly, it has not been conclusively determined which of the lipids sequestered in NPC lysosomes is the primary culprit responsible for NPC pathology (see Ref. 22 for discussion). Moreover, the detailed biochemical mechanisms of action of the NPC1 protein are still enigmatic.
The neurological hallmarks of NPC disease share striking similarity to those presented during vitamin E deficiency. On the clinical level, the primary presentation of both diseases is ataxia, reflecting selective injury of cerebellar Purkinje neurons. On the microscopic level, the two pathologies share the presence of axonal swellings (spheroids) and hypomyelination. These associations raise the possibility that oxidative stress is a significant factor contributing to the etiology of NPC disease. Indeed, NPC-affected cells exhibit mitochondrial dysfunctions (83), increased expression of reactive oxygen species (ROS)-producing and oxidative stress-responsive genes (84), and elevated plasma levels of oxidized cholesterol (85). Recent studies demonstrated that plasma samples from human NPC patients exhibit compromised ex-vivo antioxidant capacity (86). Our findings confirm and extend these observations with regards to the lipophilic antioxidant vitamin E. We showed here that tocopherol is sequestered in vesicles of lysosomal origin in NPC-affected fibroblasts and hepatocytes. Furthermore, we showed that vitamin E status is perturbed in brains and livers of Npc1−/− and Npc2−/− mice. Thus, it is possible that imbalance in vitamin E status contributes to the progression of NPC disease, and conversely, that supplementation with α-tocopherol may benefit those afflicted with this disorder. Although vitamin E supplementation was reported in Npc1−/− mice (34), the measured endpoints were limited, and no study of such supplementation has been reported in human patients.
It is important to note that concentrations of vitamin E in plasma samples from NPC-affected mice and humans were not significantly different from those of healthy controls. The immediate implication of these findings is that plasma tocopherol concentrations do not reflect vitamin E status in tissues and cells and, thus, are of limited clinical use. This is not the first time such a concern has been raised. Sokol et al. studied a small pediatric cholestasis cohort and found that in some cases vitamin E deficiency occurs in the presence of “normal” plasma tocopherol levels (87). On the mechanistic level, these findings may be explained by the presence of homeostatic mechanisms that maintain constant circulating levels of tocopherol, despite severe localized perturbations in specific tissues and cells, similar to the regulation of plasma cholesterol. On the practical level, these observations raise the urgent need for an adequate biomarker that reflects tissue vitamin E status. Since analysis of the NPC-affected tissue is impractical, a sensitive circulating indicator of oxidative stress may be the appropriate biomarker in this case. Circulating unsaturated lipid peroxidation products, such as HETEs and isoprostanes (88, 89), and hydroxylated cholesterol metabolites (85) may serve as the proper biomarker for these purposes. Clearly, there is a dire need to better define and optimize the most suitable plasma marker for oxidation status and to streamline its applicability for routine clinical use.
Our data indicate that CNS tocopherol status is adversely affected in NPC disease. In light of the relative ease, low cost, and lack of ill effects associated with moderate vitamin E supplementation, our observations support the design of a clinical trial in which the clinical benefit of vitamin E supplementation will be assessed in NPC patients.
Acknowledgments
The authors thank T. Y. Chang, Laura Liscum, Peter Lobel, and members of their labs for invaluable advice and reagents. The authors thank the Hadley Hope Fund and Ed Cutler (Phlebotomy Services International) for their assistance in obtaining samples from control subjects. The authors would also like to acknowledge the contribution of the caretakers and patients who participated in this study.
Footnotes
Abbreviations:
- CNS
- central nervous system
- IHH
- immortalized human hepatocytes
- NPC
- Niemann-Pick type C
- shRNA
- short hairpin RNA
- ROS
- reactive oxygen species
- TMS
- trimethylsilyl ether
- TTP
- α-tocopherol transfer protein
This work was supported by National Institutes of Health Grants DK-067494 (D.M.) and HD-045561 (S.U.W.); Bench-to-Bedside Award (F.P.) from the National Institutes of Health, Office of Rare Diseases; and the Intramural Research Program (F.P.) of the National Institutes of Health, National Institute of Child Health and Human Development. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies. N.Y. was supported by the Ara Parseghian Medical Research Foundation (Tucson, AZ) and Dana Angel's Research Trust (Greenwich, CT).
REFERENCES
- 1.Brady R. O., Carstea E. D., Pentchev P. G. 1997. The Niemann-Pick Diseases Group. In The Molecular And Genetic Basis of Neurological Disease Rosenberg R. N., Pruisner S. B., DiMauro S., Barchi R. L., Butterworth-Heinmann, Boston: 387–403. [Google Scholar]
- 2.Pentchev P. G., Comly M. E., Kruth H. S., Vanier M. T., Wenger D. A., Patel S., Brady R. O. 1985. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc. Natl. Acad. Sci. USA. 82: 8247–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchette-Mackie E. J., Dwyer N. K., Amende L. M., Kruth H. S., Butler J. D., Sokol J., Comly M. E., Vanier M. T., August J. T., Brady R. O., et al. 1988. Type-C Niemann-Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc. Natl. Acad. Sci. USA. 85: 8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol J., Blanchette-Mackie J., Kruth H. S., Dwyer N. K., Amende L. M., Butler J. D., Robinson E., Patel S., Brady R. O., Comly M. E., et al. 1988. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J. Biol. Chem. 263: 3411–3417. [PubMed] [Google Scholar]
- 5.Cruz J. C., Sugii S., Yu C., Chang T. Y. 2000. Role of Niemann-Pick type C1 protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J. Biol. Chem. 275: 4013–4021. [DOI] [PubMed] [Google Scholar]
- 6.Xie C., Turley S. D., Pentchev P. G., Dietschy J. M. 1999. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am. J. Physiol. 276: E336–E344. [DOI] [PubMed] [Google Scholar]
- 7.Garver W. S., Jelinek D., Oyarzo J. N., Flynn J., Zuckerman M., Krishnan K., Chung B. H., Heidenreich R. A. 2007. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J. Cell. Biochem. 101: 498–516. [DOI] [PubMed] [Google Scholar]
- 8.Vanier M. T. 1999. Lipid changes in Niemann-Pick disease type C brain: personal experience and review of the literature. Neurochem. Res. 24: 481–489. [DOI] [PubMed] [Google Scholar]
- 9.Wraith J. E., Guffon N., Rohrbach M., Hwu W. L., Korenke G. C., Bembi B., Luzy C., Giorgino R., Sedel F. 2009. Natural history of Niemann-Pick disease type C in a multicentre observational retrospective cohort study. Mol. Genet. Metab. 98: 250–254. [DOI] [PubMed] [Google Scholar]
- 10.Davidson C. D., Walkley S. U. 2010. Niemann-Pick Type C Disease - pathophysiology and future perspectives for treatment. US Neurology. 8: 88–94. [Google Scholar]
- 11.Pineda M., Wraith J. E., Mengel E., Sedel F., Hwu W. L., Rohrbach M., Bembi B., Walterfang M., Korenke G. C., Marquardt T., et al. 2009. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol. Genet. Metab. 98: 243–249. [DOI] [PubMed] [Google Scholar]
- 12.Loftus S. K., Morris J. A., Carstea E. D., Gu J. Z., Cummings C., Brown A., Ellison J., Ohno K., Rosenfeld M. A., Tagle D. A., et al. 1997. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 277: 232–235. [DOI] [PubMed] [Google Scholar]
- 13.Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277: 228–231. [DOI] [PubMed] [Google Scholar]
- 14.Naureckiene S., Sleat D. E., Lackland H., Fensom A., Vanier M. T., Wattiaux R., Jadot M., Lobel P. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 290: 2298–2301. [DOI] [PubMed] [Google Scholar]
- 15.Storch J., Xu Z. 2009. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim. Biophys. Acta. 1791: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M. L., Motamed M., Infante R. E., Abi-Mosleh L., Kwon H. J., Brown M. S., Goldstein J. L. 2010. Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 12: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon H. J., Abi-Mosleh L., Wang M. L., Deisenhofer J., Goldstein J. L., Brown M. S., Infante R. E. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Infante R. E., Wang M. L., Radhakrishnan A., Kwon H. J., Brown M. S., Goldstein J. L. 2008. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA. 105: 15287–15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infante R. E., Abi-Mosleh L., Radhakrishnan A., Dale J. D., Brown M. S., Goldstein J. L. 2008. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J. Biol. Chem. 283: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 20.Infante R. E., Radhakrishnan A., Abi-Mosleh L., Kinch L. N., Wang M. L., Grishin N. V., Goldstein J. L., Brown M. S. 2008. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J. Biol. Chem. 283: 1064–1075. [DOI] [PubMed] [Google Scholar]
- 21.Cheruku S. R., Xu Z., Dutia R., Lobel P., Storch J. 2006. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J. Biol. Chem. 281: 31594–31604. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Evans E., Platt F. M. 2010. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 11: 419–428. [DOI] [PubMed] [Google Scholar]
- 23.Vanier M. T., Rodriguez-Lafrasse C., Rousson R., Duthel S., Harzer K., Pentchev P. G., Revol A., Louisot P. 1991. Type C Niemann-Pick disease: biochemical aspects and phenotypic heterogeneity. Dev. Neurosci. 13: 307–314. [DOI] [PubMed] [Google Scholar]
- 24.Doerflinger N., Linder C., Ouahchi K., Gyapay G., Weissenbach J., Le Paslier D., Rigault P., Belal S., Ben Hamida C., Hentati F., et al. 1995. Ataxia with vitamin E deficiency: refinement of genetic localization and analysis of linkage disequilibrium by using new markers in 14 families. Am. J. Hum. Genet. 56: 1116–1124. [PMC free article] [PubMed] [Google Scholar]
- 25.Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J. L., Koenig M. 1995. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat. Genet. 9: 141–145. [DOI] [PubMed] [Google Scholar]
- 26.Sarna J. R., Larouche M., Marzban H., Sillitoe R. V., Rancourt D. E., Hawkes R. 2003. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J. Comp. Neurol. 456: 279–291. [DOI] [PubMed] [Google Scholar]
- 27.Yokota T., Uchihara T., Kumagai J., Shiojiri T., Pang J. J., Arita M., Arai H., Hayashi M., Kiyosawa M., Okeda R., et al. 2000. Postmortem study of ataxia with retinitis pigmentosa by mutation of the alpha-tocopherol transfer protein gene. J. Neurol. Neurosurg. Psychiatry. 68: 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walkley S. U., Suzuki K. 2004. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim. Biophys. Acta. 1685: 48–62. [DOI] [PubMed] [Google Scholar]
- 29.Larnaout A., Belal S., Zouari M., Fki M., Ben Hamida C., Goebel H. H., Ben Hamida M., Hentati F. 1997. Friedreich's ataxia with isolated vitamin E deficiency: a neuropathological study of a Tunisian patient. Acta Neuropathol. 93: 633–637. [DOI] [PubMed] [Google Scholar]
- 30.Pillai S. R., Traber M. G., Kayden H. J., Cox N. R., Toivio-Kinnucan M., Wright J. C., Braund K. G., Whitley R. D., Gilger B. C., Steiss J. E. 1994. Concomitant brainstem axonal dystrophy and necrotizing myopathy in vitamin E-deficient rats. J. Neurol. Sci. 123: 64–73. [DOI] [PubMed] [Google Scholar]
- 31.Sung J. H., Mastri A. R., Park S. H. 1981. Axonal dystrophy in the gracile nucleus in children and young adults. Reappraisal of the incidence and associated diseases. J. Neuropathol. Exp. Neurol. 40: 37–45. [PubMed] [Google Scholar]
- 32.Landrieu P., Said G. 1984. Peripheral neuropathy in type A Niemann-Pick disease. A morphological study. Acta Neuropathol. 63: 66–71. [DOI] [PubMed] [Google Scholar]
- 33.Hahn A. F., Gilbert J. J., Kwarciak C., Gillett J., Bolton C. F., Rupar C. A., Callahan J. W. 1994. Nerve biopsy findings in Niemann-Pick type II (NPC). Acta Neuropathol. 87: 149–154. [DOI] [PubMed] [Google Scholar]
- 34.Bascunan-Castillo E. C., Erickson R. P., Howison C. M., Hunter R. J., Heidenreich R. H., Hicks C., Trouard T. P., Gillies R. J. 2004. Tamoxifen and vitamin E treatments delay symptoms in the mouse model of Niemann-Pick C. J. Appl. Genet. 45: 461–467. [PubMed] [Google Scholar]
- 35.Qian J., Morley S., Wilson K., Nava P., Atkinson J., Manor D. 2005. Intracellular trafficking of vitamin E in hepatocytes: Role of tocopherol transfer protein. J Lipid Res. 46: 2072–2082. [DOI] [PubMed] [Google Scholar]
- 36.Horiguchi M., Arita M., Kaempf-Rotzoll D. E., Tsujimoto M., Inoue K., Arai H. 2003. pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes. Genes Cells. 8: 789–800. [DOI] [PubMed] [Google Scholar]
- 37.Rupar C. A., Albo S., Whitehall J. D. 1992. Rat liver lysosome membranes are enriched in alpha-tocopherol. Biochem. Cell Biol. 70: 486–488. [DOI] [PubMed] [Google Scholar]
- 38.Manson M. E., Corey D. A., White N. M., Kelley T. J. 2008. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 295: L809–L819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu A., Meyer K., Ray R. B., Ray R. 2002. Hepatitis C virus core protein is necessary for the maintenance of immortalized human hepatocytes. Virology. 298: 53–62. [DOI] [PubMed] [Google Scholar]
- 40.Ray R. B., Meyer K., Ray R. 2000. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology. 271: 197–204. [DOI] [PubMed] [Google Scholar]
- 41.Sleat D. E., Wiseman J. A., El-Banna M., Price S. M., Verot L., Shen M. M., Tint G. S., Vanier M. T., Walkley S. U., Lobel P. 2004. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA. 101: 5886–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morley S., Cross V., Cecchini M., Nava P., Atkinson J., Manor D. 2006. Utility of a fluorescent vitamin E analogue as a probe for tocopherol transfer protein activity. Biochemistry. 45: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nava P., Cecchini M., Chirico S., Gordon H., Morley S., Manor D., Atkinson J. 2006. Preparation of fluorescent tocopherols for use in protein binding and localization with the alpha-tocopherol transfer protein. Bioorg. Med. Chem. 14: 3721–3736. [DOI] [PubMed] [Google Scholar]
- 44.Asmis R. 1997. Physical partitioning is the main mechanism of alpha-tocopherol and cholesterol transfer between lipoproteins and P388D1 macrophage-like cells. Eur. J. Biochem. 250: 600–607. [DOI] [PubMed] [Google Scholar]
- 45.Davidson C. D., Ali N. F., Micsenyi M. C., Stephney G., Renault S., Dobrenis K., Ory D. S., Vanier M. T., Walkley S. U. 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 4: e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sontag T. J., Parker R. S. 2007. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J. Lipid Res. 48: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 47.Liscum L., Sturley S. L. 2004. Intracellular trafficking of Niemann-Pick C proteins 1 and 2: obligate components of subcellular lipid transport. Biochim. Biophys. Acta. 1685: 22–27. [DOI] [PubMed] [Google Scholar]
- 48.Harzer K., Massenkeil G., Frohlich E. 2003. Concurrent increase of cholesterol, sphingomyelin and glucosylceramide in the spleen from non-neurologic Niemann-Pick type C patients but also patients possibly affected with other lipid trafficking disorders. FEBS Lett. 537: 177–181. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi T., Beuchat M. H., Lindsay M., Frias S., Palmiter R. D., Sakuraba H., Parton R. G., Gruenberg J. 1999. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1: 113–118. [DOI] [PubMed] [Google Scholar]
- 50.Gondre-Lewis M. C., McGlynn R., Walkley S. U. 2003. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr. Biol. 13: 1324–1329. [DOI] [PubMed] [Google Scholar]
- 51.Kulinski A., Vance J. E. 2007. Lipid homeostasis and lipoprotein secretion in Niemann-Pick C1-deficient hepatocytes. J. Biol. Chem. 282: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 52.Morley S., Cacchini M., Zhang W., Virgulti A., Noy N., Atkinson J., Manor D. 2008. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J. Biol. Chem. 283: 17797–17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W. X., Frahm G., Morley S., Manor D., Atkinson J. 2009. Effect of bilayer phospholipid composition and curvature on ligand transfer by the alpha-tocopherol transfer protein. Lipids. 44: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian J., Atkinson J., Manor D. 2006. Biochemical consequences of heritable mutations in the alpha-tocopherol transfer protein. Biochemistry. 45: 8236–8242. [DOI] [PubMed] [Google Scholar]
- 55.Beltroy E. P., Richardson J. A., Horton J. D., Turley S. D., Dietschy J. M. 2005. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 42: 886–893. [DOI] [PubMed] [Google Scholar]
- 56.Arita M., Nomura K., Arai H., Inoue K. 1997. alpha-tocopherol transfer protein stimulates the secretion of alpha- tocopherol from a cultured liver cell line through a brefeldin A- insensitive pathway. Proc. Natl. Acad. Sci. USA. 94: 12437–12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bornig H., Geyer G. 1974. Staining of cholesterol with the fluorescent antibiotic “filipin”. Acta Histochem. 50: 110–115. [PubMed] [Google Scholar]
- 58.Pentchev P. G., Comly M. E., Kruth H. S., Tokoro T., Butler J., Sokol J., Filling-Katz M., Quirk J. M., Marshall D. C., Patel S., et al. 1987. Group C Niemann-Pick disease: faulty regulation of low-density lipoprotein uptake and cholesterol storage in cultured fibroblasts. FASEB J. 1: 40–45. [DOI] [PubMed] [Google Scholar]
- 59.Storey S. M., Atshaves B. P., McIntosh A. L., Landrock K. K., Martin G. G., Huang H., Ross Payne H., Johnson J. D., Macfarlane R. D., Kier A. B., et al. 2010. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from cultured primary mouse hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G244–G254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez D. M., Ogilvie W. W., Johnston L. J. 2010. NBD-cholesterol probes to track cholesterol distribution in model membranes. Biochim. Biophys. Acta. 1798: 558–568. [DOI] [PubMed] [Google Scholar]
- 61.White N. M., Jiang D., Burgess J. D., Bederman I. R., Previs S. F., Kelley T. J. 2007. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 292: L476–L486. [DOI] [PubMed] [Google Scholar]
- 62.Frolov A., Petrescu A., Atshaves B. P., So P. T., Gratton E., Serrero G., Schroeder F. 2000. High density lipoprotein-mediated cholesterol uptake and targeting to lipid droplets in intact L-cell fibroblasts. A single- and multiphoton fluorescence approach. J. Biol. Chem. 275: 12769–12780. [DOI] [PubMed] [Google Scholar]
- 63.Veyrat-Durebex C., Pomerleau L., Langlois D., Gaudreau P. 2005. Internalization and trafficking of the human and rat growth hormone-releasing hormone receptor. J. Cell. Physiol. 203: 335–344. [DOI] [PubMed] [Google Scholar]
- 64.Huang S. N., Phelps M. A., Swaan P. W. 2003. Involvement of endocytic organelles in the subcellular trafficking and localization of riboflavin. J. Pharmacol. Exp. Ther. 306: 681–687. [DOI] [PubMed] [Google Scholar]
- 65.Gruger E. H., Jr., Tappel A. L. 1971. Reactions of biological antioxidants. 3. Composition of biological membranes. Lipids. 6: 147–148. [DOI] [PubMed] [Google Scholar]
- 66.Buttriss J. L., Diplock A. T. 1988. The relationship between alpha-tocopherol and phospholipid fatty acids in rat liver subcellular membrane fractions. Biochim. Biophys. Acta. 962: 81–90. [DOI] [PubMed] [Google Scholar]
- 67.Ohgami N., Ko D. C., Thomas M., Scott M. P., Chang C. C., Chang T. Y. 2004. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc. Natl. Acad. Sci. USA. 101: 12473–12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R., Lu P., Chu J. W., Sharom F. J. 2009. Characterization of fluorescent sterol binding to purified human NPC1. J. Biol. Chem. 284: 1840–1852. [DOI] [PubMed] [Google Scholar]
- 69.Ford L., Farr J., Morris P., Berg J. 2006. The value of measuring serum cholesterol-adjusted vitamin E in routine practice. Ann. Clin. Biochem. 43: 130–134. [DOI] [PubMed] [Google Scholar]
- 70.Brites F., Gambino G., Wikinski R., Evelson P., Travacio M., Llesuy S. 2005. Evaluation of alpha-tocopherol contained in plasma lipoproteins: How should the data be expressed? Nutr. Metab. Cardiovasc. Dis. 15: 234–237. [DOI] [PubMed] [Google Scholar]
- 71.Traber M. G., Ramakrishnan R., Kayden H. J. 1994. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 91: 10005–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka J., Nakamura H., Miyawaki S. 1988. Cerebellar involvement in murine sphingomyelinosis: a new model of Niemann-Pick disease. J. Neuropathol. Exp. Neurol. 47: 291–300. [DOI] [PubMed] [Google Scholar]
- 73.Dietschy J. M., Turley S. D. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12: 105–112. [DOI] [PubMed] [Google Scholar]
- 74.Xie C., Burns D. K., Turley S. D., Dietschy J. M. 2000. Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J. Neuropathol. Exp. Neurol. 59: 1106–1117. [DOI] [PubMed] [Google Scholar]
- 75.German D. C., Quintero E. M., Liang C. L., Ng B., Punia S., Xie C., Dietschy J. M. 2001. Selective neurodegeneration, without neurofibrillary tangles, in a mouse model of Niemann-Pick C disease. J. Comp. Neurol. 433: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Podratz J. L., Rodriguez E. H., Windebank A. J. 2004. Antioxidants are necessary for myelination of dorsal root ganglion neurons, in vitro. Glia. 45: 54–58. [DOI] [PubMed] [Google Scholar]
- 77.Enrione E. B., Weeks O. I., Kranz S., Shen J. 1999. A vitamin E-deficient diet affects nerve regeneration in rats. Nutrition. 15: 140–144. [DOI] [PubMed] [Google Scholar]
- 78.Hyland S., Muller D., Hayton S., Stoecklin E., Barella L. 2006. Cortical gene expression in the vitamin E-deficient rat: possible mechanisms for the electrophysiological abnormalities of visual and neural function. Ann. Nutr. Metab. 50: 433–441. [DOI] [PubMed] [Google Scholar]
- 79.Yamada A., Saji M., Ukita Y., Shinoda Y., Taniguchi M., Higaki K., Ninomiya H., Ohno K. 2001. Progressive neuronal loss in the ventral posterior lateral and medial nuclei of thalamus in Niemann-Pick disease type C mouse brain. Brain Dev. 23: 288–297. [DOI] [PubMed] [Google Scholar]
- 80.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 1988. Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Arch. Intern. Med. 148: 36–69. [PubMed] [Google Scholar]
- 81.Garver W. S., Jelinek D., Meaney F. J., Flynn J., Pettit K. M., Shepherd G., Heidenreich R. A., Vockley C. M., Castro G., Francis G. A. 2010. The National Niemann-Pick Type C1 Disease Database: correlation of lipid profiles, mutations, and biochemical phenotypes. J. Lipid Res. 51: 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ford E. S., Schleicher R. L., Mokdad A. H., Ajani U. A., Liu S. 2006. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am. J. Clin. Nutr. 84: 375–383. [DOI] [PubMed] [Google Scholar]
- 83.Yu W., Gong J. S., Ko M., Garver W. S., Yanagisawa K., Michikawa M. 2005. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J. Biol. Chem. 280: 11731–11739. [DOI] [PubMed] [Google Scholar]
- 84.Reddy J. V., Ganley I. G., Pfeffer S. R. 2006. Clues to neuro-degeneration in Niemann-Pick type C disease from global gene expression profiling. PLoS ONE. 1: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., Wassif C. A., Yanjanin N. M., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu R., Yanjanin N. M., Bianconi S., Pavan W. J., Porter F. D. 2010. Oxidative stress in Niemann-Pick disease, type C. Mol. Genet. Metab. 101: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sokol R. J., Heubi J. E., Iannaccone S. T., Bove K. E., Balistreri W. F. 1984. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N. Engl. J. Med. 310: 1209–1212. [DOI] [PubMed] [Google Scholar]
- 88.Shishehbor M. H., Zhang R., Medina H., Brennan M. L., Brennan D. M., Ellis S. G., Topol E. J., Hazen S. L. 2006. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic. Biol. Med. 41: 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida Y., Itoh N., Hayakawa M., Habuchi Y., Saito Y., Tsukamoto Y., Cynshi O., Jishage K., Arai H., Niki E. 2010. The role of alpha-tocopherol in motor hypofunction with aging in alpha-tocopherol transfer protein knockout mice as assessed by oxidative stress biomarkers. J. Nutr. Biochem. 21: 66–76. [DOI] [PubMed] [Google Scholar]