Abstract
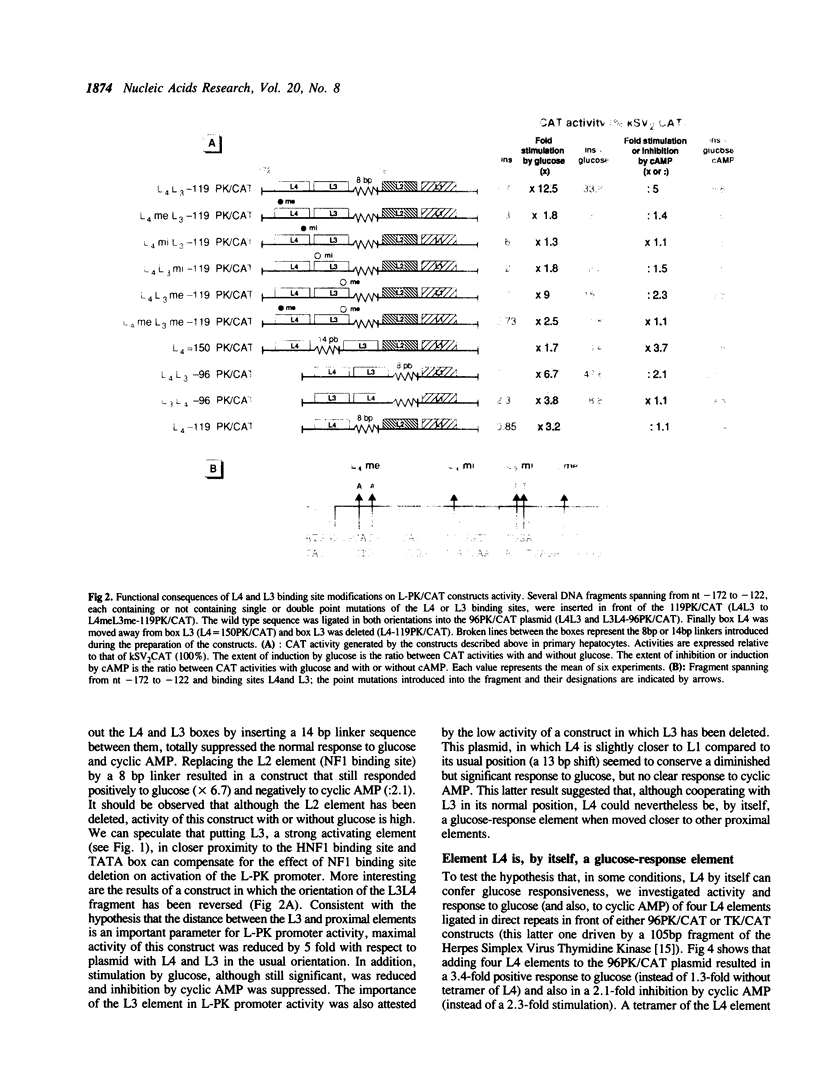
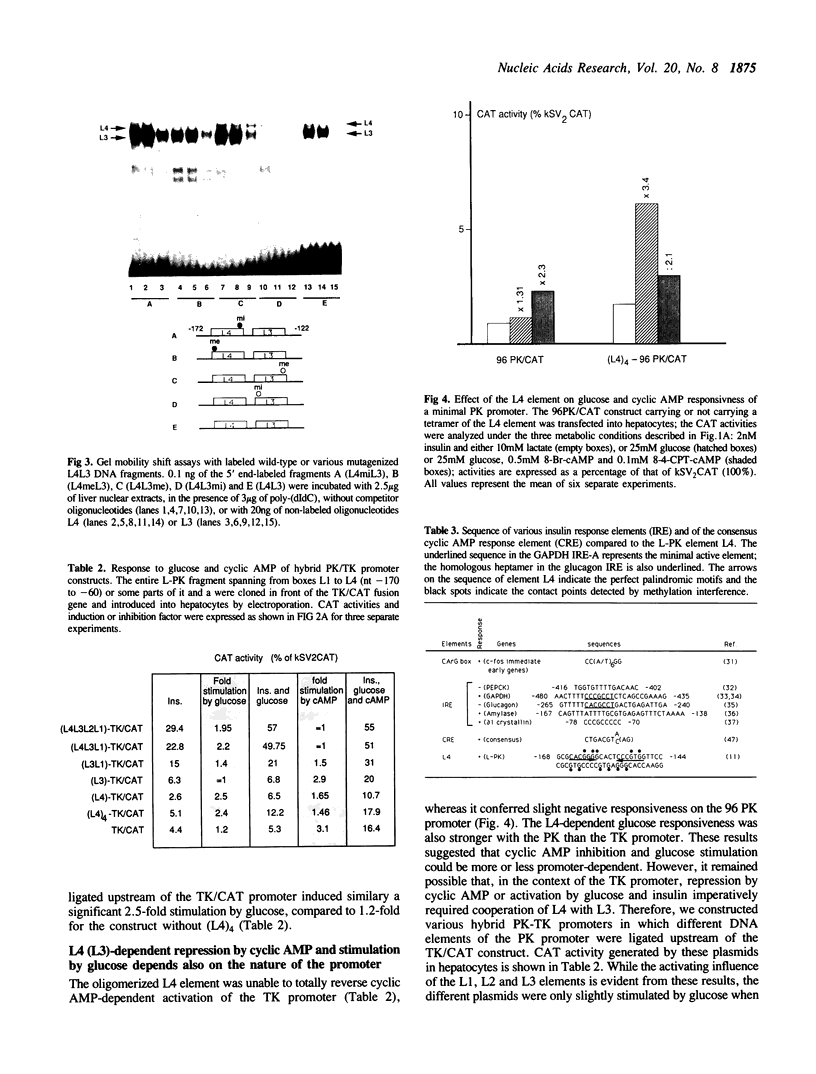
The glucose/insulin response element of the L-pyruvate kinase gene is a perfect palindrome located from nt -168 to -144 with respect to the cap site. This element (L4) is partially homologous to MLTF binding sites. Its full efficiency requires cooperation with a contiguous binding site for HNF4, termed L3 and located from nt -145 to -125. In the presence of the L4 element contiguous to L3, cyclic AMP inhibits activity of the L-PK promoter while in its absence, or when the normal L4-L3 contiguity is modified, cyclic AMP behaves as a transcriptional activator that does not seem to be sequence-specific. Therefore, we propose that the mechanism of inhibition of the L-PK gene by cyclic AMP requires precise interactions between the nucleoprotein complex built up at sites L4 and L3 and other components of the L-PK transcription initiation complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany J., Borras T., de Pablo F. Transcriptional stimulation of the delta 1-crystallin gene by insulin-like growth factor I and insulin requires DNA cis elements in chicken. Proc Natl Acad Sci U S A. 1990 May;87(9):3353–3357. doi: 10.1073/pnas.87.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. C., Lomanto M., Nasrin N., Ramaika C. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5092–5096. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Nicholas K. R., Van Wyk J. J., Topper Y. J. Insulin is essential for accumulation of casein mRNA in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5682–5684. doi: 10.1073/pnas.78.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weih F., Schmidt A., Fournier R. E., Schütz G. A cyclic AMP response element mediates repression of tyrosine aminotransferase gene transcription by the tissue-specific extinguisher locus Tse-1. Cell. 1990 Jun 1;61(5):905–916. doi: 10.1016/0092-8674(90)90201-o. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chu D. T., Davis C. M., Chrapkiewicz N. B., Granner D. K. Reciprocal regulation of gene transcription by insulin. Inhibition of the phosphoenolpyruvate carboxykinase gene and stimulation of gene 33 in a single cell type. J Biol Chem. 1988 Sep 15;263(26):13007–13011. [PubMed] [Google Scholar]
- Cognet M., Bergot M. O., Kahn A. cis-acting DNA elements regulating expression of the liver pyruvate kinase gene in hepatocytes and hepatoma cells. Evidence for tissue-specific activators and extinguisher. J Biol Chem. 1991 Apr 25;266(12):7368–7375. [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989 Jul 15;264(20):11584–11590. [PubMed] [Google Scholar]
- Decaux J. F., Marcillat O., Pichard A. L., Henry J., Kahn A. Glucose-dependent and -independent effect of insulin on gene expression. J Biol Chem. 1991 Feb 25;266(6):3432–3438. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. A., Rosenberg M. P., Johnson T. M., Howard G., Meisler M. H. Regulation of amylase gene expression in diabetic mice is mediated by a cis-acting upstream element close to the pancreas-specific enhancer. Genes Dev. 1990 Aug;4(8):1316–1321. doi: 10.1101/gad.4.8.1316. [DOI] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Dwarki V. J., Ofir R., Montminy M., Verma I. M. Negative and positive regulation by transcription factor cAMP response element-binding protein is modulated by phosphorylation. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4320–4324. doi: 10.1073/pnas.87.11.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. C., O'Brien R. M., Mitchell J. A., Davis C. M., Imai E., Forman B. M., Samuels H. H., Granner D. K. A retinoic acid response element is part of a pleiotropic domain in the phosphoenolpyruvate carboxykinase gene. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2184–2188. doi: 10.1073/pnas.88.6.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M. A., Andreone T. L., Printz R. L., Koch S., Granner D. K. Rat glucokinase gene: structure and regulation by insulin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4838–4842. doi: 10.1073/pnas.86.13.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienhofer M. C., De Medicis E., Cognet M., Kahn A. Regulation of genes for glycolytic enzymes in cultured rat hepatoma cell lines. Eur J Biochem. 1987 Dec 1;169(2):237–243. doi: 10.1111/j.1432-1033.1987.tb13603.x. [DOI] [PubMed] [Google Scholar]
- Messina J. L. Insulin's regulation of c-fos gene transcription in hepatoma cells. J Biol Chem. 1990 Jul 15;265(20):11700–11705. [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnich A., Lyonnet S., Chauvet D., Van Schaftingen E., Kahn A. Differential effects of glucose and fructose on liver L-type pyruvate kinase gene expression in vivo. J Biol Chem. 1987 Dec 15;262(35):17065–17071. [PubMed] [Google Scholar]
- Munnich A., Marie J., Reach G., Vaulont S., Simon M. P., Kahn A. In vivo hormonal control of L-type pyruvate kinase gene expression. Effects of glucagon, cyclic AMP, insulin, cortisone, and thyroid hormones on the dietary induction of mRNAs in the liver. J Biol Chem. 1984 Aug 25;259(16):10228–10231. [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Inoue H., Tanaka T. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase in diabetic rat liver by insulin and dietary fructose. J Biol Chem. 1985 Nov 15;260(26):14393–14397. [PubMed] [Google Scholar]
- O'Brien R. M., Bonovich M. T., Forest C. D., Granner D. K. Signal transduction convergence: phorbol esters and insulin inhibit phosphoenolpyruvate carboxykinase gene transcription through the same 10-base-pair sequence. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6580–6584. doi: 10.1073/pnas.88.15.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. Regulation of gene expression by insulin. Biochem J. 1991 Sep 15;278(Pt 3):609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Peavy D. E., Taylor J. M., Jefferson L. S. Correlation of albumin production rates and albumin mRNA levels in livers of normal, diabetic, and insulin-treated diabetic rats. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5879–5883. doi: 10.1073/pnas.75.12.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7224–7227. doi: 10.1073/pnas.88.16.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Straus D. S., Takemoto C. D. Insulin negatively regulates albumin mRNA at the transcriptional and post-transcriptional level in rat hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):1955–1960. [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Stewart T. N., Gilman M. Z., Blackshear P. J. Identification of c-fos sequences involved in induction by insulin and phorbol esters. J Biol Chem. 1988 Feb 5;263(4):1611–1614. [PubMed] [Google Scholar]
- Thompson K. S., Towle H. C. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J Biol Chem. 1991 May 15;266(14):8679–8682. [PubMed] [Google Scholar]
- Tremp G. L., Boquet D., Ripoche M. A., Cognet M., Lone Y. C., Jami J., Kahn A., Daegelen D. Expression of the rat L-type pyruvate kinase gene from its dual erythroid- and liver-specific promoter in transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19904–19910. [PubMed] [Google Scholar]
- Tur-Kaspa R., Teicher L., Levine B. J., Skoultchi A. I., Shafritz D. A. Use of electroporation to introduce biologically active foreign genes into primary rat hepatocytes. Mol Cell Biol. 1986 Feb;6(2):716–718. doi: 10.1128/mcb.6.2.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Munnich A., Decaux J. F., Kahn A. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase gene expression in rat liver. J Biol Chem. 1986 Jun 15;261(17):7621–7625. [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Kahn A., Raymondjean M. Analysis by cell-free transcription of the liver-specific pyruvate kinase gene promoter. Mol Cell Biol. 1989 Oct;9(10):4409–4415. doi: 10.1128/mcb.9.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Levrat F., Cognet M., Kahn A., Raymondjean M. Proteins binding to the liver-specific pyruvate kinase gene promoter. A unique combination of known factors. J Mol Biol. 1989 Sep 20;209(2):205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]
- Yamada K., Noguchi T., Matsuda T., Takenaka M., Monaci P., Nicosia A., Tanaka T. Identification and characterization of hepatocyte-specific regulatory regions of the rat pyruvate kinase L gene. The synergistic effect of multiple elements. J Biol Chem. 1990 Nov 15;265(32):19885–19891. [PubMed] [Google Scholar]
- Yamada K., Noguchi T., Miyazaki J., Matsuda T., Takenaka M., Yamamura K., Tanaka T. Tissue-specific expression of rat pyruvate kinase L/chloramphenicol acetyltransferase fusion gene in transgenic mice and its regulation by diet and insulin. Biochem Biophys Res Commun. 1990 Aug 31;171(1):243–249. doi: 10.1016/0006-291x(90)91383-4. [DOI] [PubMed] [Google Scholar]