Abstract
Wiskott-Aldrich syndrome (WAS) is an inherited immunodeficiency characterized by high incidence of autoantibody-mediated autoimmune complications. Such a feature has been associated with defective suppressor activity of WAS protein-deficient, naturally occurring CD4+CD25+Foxp3+ regulatory T cells on responder T cells. However, it remains to be established whether the altered B-cell tolerance reported in WAS patients and Was knockout (WKO) mice is secondary to abnormalities in the direct suppression of B-cell function by nTreg cells or to impaired regulation of T-helper function. Because activated nTreg cells are known to induce granzyme B–mediated B-cell killing, we decided to evaluate the regulatory capabilities of WKO nTregs on B lymphocytes. We found that preactivated WKO nTreg cells failed to effectively suppress B-cell proliferation and that such a defect was associated with reduced killing of B cells and significantly decreased degranulation of granzyme B. Altogether, these results provide additional mechanistic insights into the loss of immune tolerance in WAS.
Introduction
Wiskott-Aldrich syndrome (WAS) is a rare X-linked primary immunodeficiency caused by mutations of the WAS gene and characterized by thrombocytopenia, eczema, recurrent infections, and high incidence of malignancy and autoimmunity.1,2 Was knockout mice (WKO) share many features of the human disease, including altered immune responses and development of autoantibody-mediated autoimmunity.3–6 Current evidence implicates the WAS protein in naturally occurring regulatory T (nTreg) cell activation and function, suggesting that the autoimmune and atopic pathologic manifestation in WAS may result, at least in part, from impaired nTreg function.5,7–9
Recent studies have demonstrated that nTreg cells can suppress the function of the immune cells by FasL-independent, perforin- and granzyme-dependent killing.10–12 Such results are consistent with recent findings indicating that preactivated murine nTreg cells suppress B-cell proliferation in a granzyme B– and perforin-dependent manner13 and that nTreg cells mediate direct inhibition of B lymphocytes in autoimmune disorders associated with aberrant production of autoantibodies.14 Accumulated evidence has suggested that intrinsic B-cell abnormalities may affect both response to pathogens and peripheral immune tolerance in WAS.15,16 However, it can also be postulated that the autoantibody-mediated autoimmune complications affecting WAS patients and WKO mice5,6 may be secondary to defects in direct suppression of B-cell function by nTreg cells or to impaired intermediate regulation of T-helper function.
In this study, we have evaluated the ability of WKO nTreg cells to suppress in vitro B-cell proliferation and observed a significant reduction of regulatory function associated with defective cytotoxic activity and decreased degranulation of granzyme B. Altogether, these results point to impaired B-cell suppression as one of the possible mechanisms underlying autoimmunity in WAS.
Methods
Mice
Was−/Y and Was−/− (129S6/SvEvTac-Wastm1Sbs/J)17 and control 129S6/SvEvTac mice were used in experiments approved by the National Human Genome Research Institute Animal Care and Use Committee. Spleens from Was+/− × OT-2 (WT/OT-2) and Was−/y × OT-2 (WKO/OT-2) crossed mice were kindly provided by Dr Richard M. Siegel (National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health, Bethesda, MD).
Cell purification, culture, and characterization
Murine CD4+CD25+ (nTreg), CD4+CD25− (Tconv), and CD8+ T cells were isolated as previously described,18 activated with plate-bound anti-CD3 (1 μg/mL) and recombinant human IL-2 (100 U/mL) for 3 days and expanded with only recombinant human IL-2 (200 U/mL) for 2 days. Antigen-presenting cells (APCs) were obtained from control splenocytes by depletion of CD90.2+ cells (Miltenyi Biotec) and irradiated (30 Gy). B cells were positively selected from splenocytes using anti–mouse CD19-coated microbeads. Coculture assays were performed as previously described.18 Briefly, B cells (5 × 104) were seeded in 96-well plates with the indicated number of nTreg, Tconv, or CD8+ T cells in the presence of 3 μg/mL lipopolysaccharide (LPS) and 2 μg/mL of soluble anti-CD3 or 10μM OVA323-339 peptide, and cultured for 72 hours before assessment of proliferation by [3H]thymidine incorporation.
Intracellular staining for Foxp3, granzyme B, and perforin was performed following the manufacturer's recommendations (eBioscience). CD107a expression was determined after culture of activated nTreg or CD8+ T cells with 2 μg/mL anti-CD3 and irradiated T cell–depleted spleen cells for 8 hours in the presence of anti–mouse CD107a or isotype control, and GolgiStop (BD Biosciences PharMingen).
Apoptosis and ELISAs
Purified B cells were stimulated with LPS (5 μg/mL) for 20 hours and, when indicated, pulsed with 10μM OVA323-339 peptide for 2 hours. Viable B-cell blasts were isolated by Lympholyte-M gradient (CedarLane Labs) and cultured with LPS (0.5 μg/mL) and preactivated effector T cells in the presence or absence of anti-CD3 (2 μg/mL) for 8 hours. The used effector-to-target ratios are reported in the figures. Culture media were collected and stored at −70°C until analysis. ELISAs for granzyme B were performed according to the manufacturer's instructions (eBioscience). Cells were washed and stained with 7-amino-actinomycin D (7-AAD), annexin V, and anti–B220-phycoerythrin antibodies in annexin V–binding buffer. The percentages of annexin V+/7-AAD+/B220+ cells were determined by flow cytometry.
Results and discussion
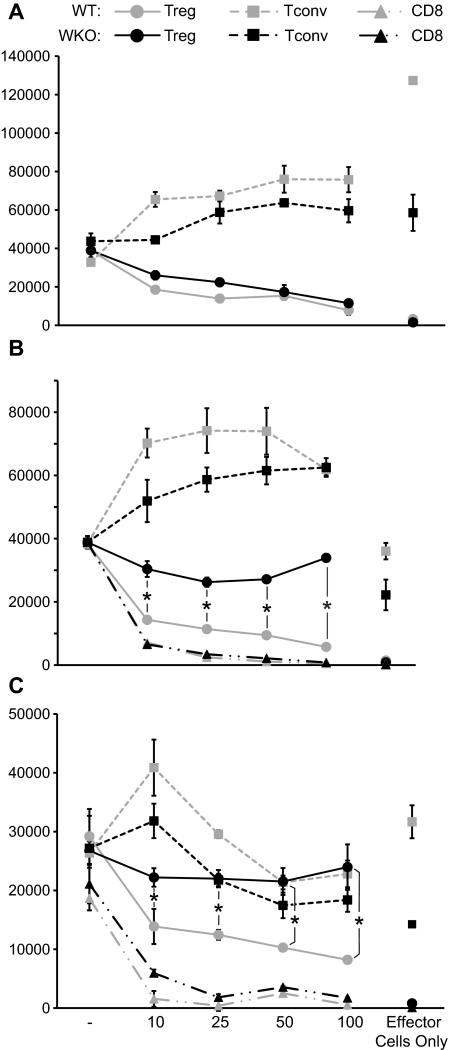
We have previously shown that preactivation with anti-CD3 and IL-2 can rescue suppression function in WKO nTreg cells.7 To confirm and expand these findings, preactivated WT and WKO nTreg cells were cocultured with highly purified WT T or B cells in the presence of LPS and anti-CD3. In these conditions, WKO nTreg cells showed suppressive activity on Tconv cells at levels comparable with WT nTreg cells (Figure 1A), whereas WKO nTreg cells failed to effectively suppress proliferation of both WT and WKO B cells (Figure 1B-C). As expected, neither WKO nor WT preactivated Tconv cells suppressed either T- or B-cell proliferation (Figure 1A-C). No difference in the ability to suppress B-cell proliferation was observed between preactivated CD8+ cells from WT and WKO mice (Figure 1B-C). Although it is reported that CD4+CD25+ T cells can respond to LPS stimulation,19 no proliferation of nTreg cells was observed at the LPS concentrations used in our experiments. Importantly, FACS analysis of WKO and WT nTreg cell populations before and after activation showed comparable expression of Foxp3 and the absence of contaminating CD8+ cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition, WKO nTreg cells showed activation characteristics comparable with WT nTreg populations as assessed by down-regulation of CD62L and up-regulation of CD44, OX-40/CD134, GITR, and CTLA4 (supplemental Figure 2).
Figure 1.
Preactivated WKO nTreg cells can suppress T-cell but not B-cell proliferation. nTreg, Tconv, and CD8+ T cells were isolated from WT and WKO mice and preactivated with anti-CD3 and IL-2. (A) Proliferation of freshly isolated Tconv (5 × 104) from WT mice cocultured with the indicated number of WT and WKO preactivated nTreg or Tconv cells in the presence of anti-CD3 (2 μg/mL) and irradiated, T-depleted APCs. (B) B cells (5 × 104) from WT mice were stimulated with LPS (3 μg/mL) and cocultured with the indicated number of preactivated nTreg, Tconv, or CD8+ cells from both WT or WKO mice in the presence of soluble anti-CD3 (2 μg/mL) and irradiated T-depleted APCs. (C) B cells isolated from WKO mice were cultured as in panels A and B. Proliferation was then assessed as incorporation of [3H]-thymidine after the cells were pulsed for the last 16 hours during a total of 72 hours of cultures. Note that the proliferation of effector T cells (nTreg, Tconv, and CD8 T cells) cultured alone in the presence of soluble anti-CD3 and LPS stimulation is also shown. Statistical significance of differences between samples was calculated using the 2-tailed Mann-Whitney test with 95% confidence intervals. Results are mean of triplicate cultures and are representative of at least 3 experiments. P < .05.
These data indicate that WKO nTregs are unable to suppress the proliferation of B cells and suggest that failure of nTreg cells to directly regulate B-cell activation and proliferation may play a role in the increase in autoantibody levels and the altered B-cell tolerance reported in WAS patients and WKO mice.5,6
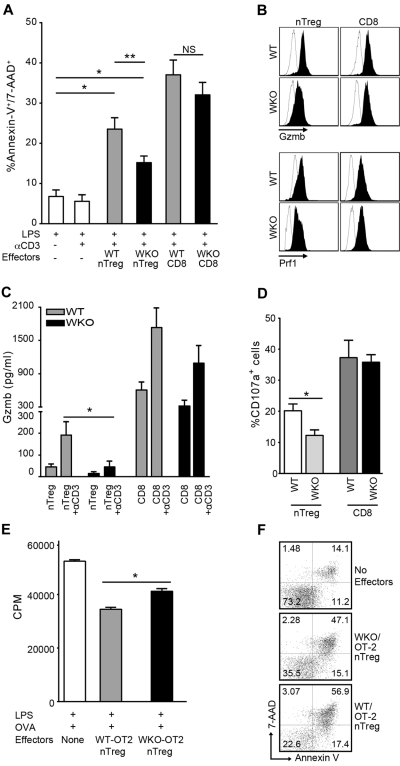
Preactivated nTreg cells suppress B-cell proliferation by inducing cell death through the perforin/granzyme pathway, in both mice and humans.13,14 To explore whether such mechanisms are affected in WKO nTreg cells, we investigated the cytotoxic activity of WKO and WT nTreg cells and observed significant reduced ability of WKO nTreg cells to induce apoptosis of B cells (Figure 2A). Interestingly, no significant differences were noted in the ability of WKO and WT CD8+ cells to lyse B-cell blasts. As expected,13 induction of B-cell death was observed only in cultures containing preactivated nTreg plus anti-CD3, whereas it was virtually absent in the absence of effector cells (regardless of anti-CD3 addition). Coculture with preactivated nTreg cells in the absence of anti-CD3 stimulation did not induce B-cell apoptosis (supplemental Figure 3). These findings point to a specific inability to induce apoptosis as the mechanism responsible for the failure of WKO nTreg cells to suppress B-cell proliferation.
Figure 2.
Defective B-cell death induction and granzyme B release by WKO nTreg cells. (A) Percentage of apoptotic B220+ cells after coculture of activated nTreg and CD8+ cells from WKO or WT mice with LPS-induced B-cell blasts at a ratio of 5:1 in the presence or absence of anti-CD3 antibody and LPS (0.5 μg/mL). Bar graph represents the results of 11 independent experiments expressed as mean ± SD. *P < .0001. **P < .05. NS indicates not significant. (B) Granzyme B (Gzmb) and perforin (Prf1) expression in WKO and WT preactivated nTreg and CD8+ cells. Open histograms represent isotype controls; and solid histograms, Gzmb- and Prf1-specific staining. Representative data from at least 3 independent experiments are shown. (C) ELISA determination of granzyme B (Gzmb) release in culture media by preactivated WT and WKO nTreg and CD8+ cells after restimulation with 2 μg/mL anti-CD3 antibody. Results are mean ± SD of 5 independent experiments. *P = .0260. Statistical significances of differences among samples were calculated using the 2-tailed Mann-Whitney test. (D) Percentage of CD107a+ after culture of WKO and WT-activated nTreg and CD8+ cells (5 × 104) with 2 μg/mL soluble anti-CD3 and irradiated T cell–depleted spleen cells (5 × 104) for 8 hours in the presence of FITC-anti–mouse CD107a or FITC-conjugated isotype control and GolgiStop (BD Biosciences PharMingen). After restimulation, the cells were stained with anti-CD107a, anti-CD4, and anti-CD8 and analyzed by flow cytometry. Results are mean ± SD of 3 independent experiments. *P = .0317. Statistical significances of differences among samples were calculated using the 2-tailed Mann-Whitney test. (E-F) Defective suppression of B-cell proliferation and LPS-induced B-cell blast killing by WKO-OT2 nTreg cells. (E) B cells (5 × 104) from WT mice were stimulated with LPS (3 μg/mL) and cocultured with equal number of preactivated nTreg cells from both WT or WKO mice in the presence of OVA323-339 peptide (10μM), and irradiated T-depleted APCs. Proliferation was then assessed as in Figure 1. (F) B-cell blasts were pulsed with OVA323-339 peptide (10μM) for 2 hours and cultured alone or with WKO or WT OT-2 nTreg cells at an effector-to-target ratio of 1:1 for 8 hours in the presence of LPS (0.5 μg/mL). Statistical significance of differences between samples was calculated using the Mann-Whitney test with 95% confidence intervals. Results are mean of triplicate cultures and are representative of at least 2 experiments.
nTreg cells are known to induce B-cell apoptosis through the release of the cytotoxic molecule granzyme B.12,13 Therefore, we tested the possibility that the defective B-cell suppression by WKO nTreg cells was the result of impaired granzyme B–mediated apoptosis. Evaluation of granzyme B and perforin intracellular expression showed comparable levels in WKO and WT cells after activation (Figure 2B). However, the amount of granzyme B secreted by WKO nTreg during the cytotoxic assays was significantly reduced compared with WT nTregs (Figure 2C). Accordingly, we detected significantly higher expression of the degranulation marker CD107a on the surface of activated WT nTreg compared with the WKO nTreg cells (Figure 2D). These results are consistent with our recent findings of defective granule secretion in WKO activated T cells6 and suggest that the observed impaired cytotoxic/suppressive activity of WKO nTreg cells on B cells may be ascribed to a vesicular granzyme B secretion defect.
To address whether the observed defective B-cell suppression by WKO nTreg also occurred in a system allowing cognate interaction between T and B cells, we tested the suppressive and cytotoxic activity of activated nTreg cells from WT/OT-2 and WKO/OT-2 mice cultured either with B cells in the presence of OVA323-339 peptide and LPS or with B-cell blasts pulsed with OVA323-339 peptide. As observed in the experiments using polyclonal, activated nTreg cells, WKO/ OT2 nTreg cells showed a significantly reduced ability to suppress B-cell proliferation (Figure 2E) and to induce B-cell blast apoptosis (Figure 2F) compared with WT-OT2 cells.
Interestingly, we observed preserved ability of WKO CD8+ cells to secrete granzyme B, up-regulate the membrane expression of CD107a, and exert cytotoxic/suppressive activity on B cells. These findings may be attributed to specific biologic characteristics of CD8+ cells, such as the high contents in granzyme B, the capacity of maintaining a stable and mature immune synapsis, and the consequent efficient release of cytotoxic granules after activation. In the context of WAS protein deficiency, the instability of the immune synapsis20 may play a role in the reduced cytotoxic activity of WKO nTreg cells, but become less of a factor for the efficient degranulation of and consequent killing by CD8+ cells.
In conclusion, we provide evidence that WKO nTreg cells show impaired ability to induce apoptosis in B cells, which is associated with a significant reduction of granzyme B secretion. These findings point to a previously unreported role of WAS protein in the nTreg granzyme B– and contact-mediated dependent suppression of target cells. Our results also suggest that the altered B-cell tolerance reported in WAS patients and WKO mice may derive from a failure of nTreg to directly regulate activated B cells and provide novel mechanistic insights into the loss of immune tolerance in WAS.
Supplementary Material
Acknowledgments
The authors thank the staff of the NHGRI Office of Laboratory Animal Medicine and the Laboratory Animal Technicians of the NIH Building 49 Central Animal Facility. They also thank Ms Julia Fekecs for the production of scientific illustrations.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A., K.A.J., M.R.K., C.S., and S.M.A. performed the experiments; M.A., T.U., and F.C. designed, analyzed, and interpreted the data; and M.A. and F.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabio Candotti, Disorders of Immunity Section, Genetics and Molecular Biology Branch/National Human Genome Research Institute, 49 Convent Dr, Bldg 49, Rm 3A04, Bethesda, MD 20892; e-mail: fabio@mail.nih.gov.
References
- 1.Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood. 2009;113(25):6288–6295. doi: 10.1182/blood-2008-12-115253. [DOI] [PubMed] [Google Scholar]
- 2.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117(4):725–738. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Shehabeldin A, da Cruz LA, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190(9):1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westerberg L, Larsson M, Hardy SJ, Fernandez C, Thrasher AJ, Severinson E. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood. 2005;105(3):1144–1152. doi: 10.1182/blood-2004-03-1003. [DOI] [PubMed] [Google Scholar]
- 5.Humblet-Baron S, Sather B, Anover S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117(2):407–418. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolov NP, Shimizu M, Cleland S, et al. Systemic autoimmunity and defective Fas ligand secretion in the absence of the Wiskott-Aldrich syndrome protein. Blood. 2010;116(5):740–747. doi: 10.1182/blood-2009-08-237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adriani M, Aoki J, Horai R, et al. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clin Immunol. 2007;124(1):41–48. doi: 10.1016/j.clim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maillard MH, Cotta-de-Almeida V, Takeshima F, et al. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204(2):381–391. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marangoni F, Trifari S, Scaramuzza S, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204(2):369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21(4):589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104(9):2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 12.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174(4):1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 13.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107(10):3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iikuni N, Lourenco EV, Hahn BH, La Cava A. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183(3):1518–1522. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westerberg LS, de la Fuente MA, Wermeling F, et al. WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood. 2008;112(10):4139–4147. doi: 10.1182/blood-2008-02-140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer-Bahlburg A, Becker-Herman S, Humblet-Baron S, et al. Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood. 2008;112(10):4158–4169. doi: 10.1182/blood-2008-02-140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9(1):81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 18.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197(4):403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims TN, Soos TJ, Xenias HS, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129(4):773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.