Abstract
In response to ultraviolet B damage, keratinocytes undergo apoptosis to eliminate damaged cells, thereby preventing tumorigenic transformation. Caffeine, the most widely consumed psychoactive substance, produces complex pharmacological actions; it has been shown to be chemopreventive in non-melamona skin cancer in mice through increasing apoptosis. Here we have investigated the molecular and cellular mechanisms in the pro-apoptotic effect of caffeine on UVB-irradiated human HaCaT keratinocytes. Pretreatment with caffeine increased UVB-induced apoptosis in HaCaT cells. Caffeine blocked UVB-induced Chk1 phosphorylation. In addition, similar to the effect of the PI3K inhibitor LY294002, caffeine also inhibited phosphorylation of AKT and up-regulation of COX-2, two critical oncogenic pathways in skin tumorigenesis. However, phosphorylation of EGFR or ERK was unaffected. Inhibiting ATR pathways by siRNA targeting ATR had little effect on UVB-induced apoptosis or AKT activation, indicating that the inhibitory effect of caffeine on apoptosis and the AKT pathway does not require the ATR pathway. Inhibiting AKT by caffeine blocked UVB-induced COX-2 up-regulation. Expression of constitutively active AKT that was not inhibited by caffeine was found to protect cells from caffeine-promoted apoptosis post-UVB irradiation, indicating that AKT is an essential inhibitory target for caffeine to promote apoptosis. Caffeine specifically sensitized cells with unrepaired DNA damage to UVB-induced apoptosis. These findings indicate that in HaCaT keratinocytes, inhibiting the AKT/COX-2 pathways through an ATR-independent pathway is a critical molecular mechanism by which caffeine promotes UVB-induced apoptosis of unrepaired keratinocytes for elimination.
Keywords: Akt PKB, Cell Death, Cyclooxygenase (COX) Pathway, DNA Damage, DNA Nucleotide Excision Repair, Skin, Caffeine, UVB
Introduction
Skin cancer is the most common type of cancer in the United States (US). Each year more than one million new cases of skin cancer are diagnosed in the US alone, accounting for 40% of all newly diagnosed cancer cases (1, 2). The incidence of skin cancers continues to rise each year. Effective prevention of skin cancer is essential to reduce this disease burden.
The major environmental factor in skin cancer is ultraviolet radiation B (UVB)2 in sunlight, which causes the formation of DNA damage products, i.e. cyclobutane pyrimidine dimers (CPD) and pyrimidine (6–4) pyrimidone photoproducts (6–4PPs) (3, 4). DNA damage subsequently triggers apoptotic responses to eliminate damaged cells (5–7). In surviving cells, failure to repair these major DNA damage products is the principal cause of skin cancer.
In cells with damaged DNA, the DNA damage response (DDR) signal-transduction pathway coordinates cell-cycle transitions, DNA replication, DNA repair, and apoptosis. The major regulators of the DNA damage response are the phosphoinositide 3-kinase (PI3K)-related protein kinases (PIKKs), including ataxia-telangiectasia mutated (ATM) and ATM and RAD3-related (ATR). ATM and ATR respond to different types of DNA damage: ATM responds to double strand breaks (DSB, typical DNA damage caused by ionizing radiation (IR)), and ATR responds to replication stress and UV-induced pyrimidine dimers (8, 9). The list of ATR substrates is rapidly expanding; however, the best studied is the Ser/Thr kinase checkpoint kinase-1 (Chk1) (10, 11). ATM activates another checkpoint protein, checkpoint kinase-2 (Chk2) (12–15). These pathways coordinate the DNA damage checkpoint function. Defects in the ATR/Chk1 and ATM/Chk2 pathways increase cancer risk (16–21).
Two further factors are known to be important in the response to UVB irradiation. First, the serine/threonine kinase AKT, also known as protein kinase B (PKB), is a downstream effector of phosphatidylinositol 3-kinase (PI3K) that has recently been a focus of intense research. It appears that AKT lies at the cross-roads of multiple cellular signaling pathways and acts as a transducer of many functions initiated by growth factors and other receptors that activate PI3K (22–24). One of the major activities of AKT is to promote cell survival (22, 25). AKT is activated in response to UVB irradiation and is frequently activated in human cancers (1, 26, 27).
Second, cyclooxygenase-2 (COX-2), the rate-limiting enzyme in arachidonic acid metabolism leading to prostaglandin synthesis, is up-regulated in murine and human NMSC (28). Overexpression of COX-2 promotes UV-induced skin tumorigenesis in mice (29). Inhibition of COX-2 by biochemical inhibitors or genetic deletion decreases chemical- or UV-induced skin tumor development (29–31). In addition, UVB radiation induces COX-2 up-regulation in human skin, mouse skin, and keratinocytes in vitro (1, 32). Extensive studies have identified multiple mechanisms mediating UVB-induced COX-2 up-regulation (1, 26).
A chemopreventive effect against UVB-induced skin malignancies is observed for caffeine, the most widely consumed psychoactive substance. Topical caffeine administration in mice reduces UVB-induced skin tumorigenesis (33, 34). Topical or oral caffeine treatment induces p53-independent apoptosis in mouse epidermis and primary human keratinocytes post-UVB irradiation (33–36). It has been proposed that the effect of caffeine is mediated by inhibiting ATR/Chk1 pathways (35, 36). However, it remains unclear whether ATR/Chk1 is the only mediating mechanism, or other apoptotic regulatory mechanisms are involved. Sun-exposed normal skin harbors numerous clones of p53-mutated premalignant keratinocytes (37–41). Here we have used human HaCaT keratinocytes that harbor UV-type p53 mutations as a model for premalignant cells to investigate the precise molecular basis for the pro-apoptotic effect of caffeine in UVB-irradiated keratinocytes and its biological specificity and consequences. We found that inhibition of AKT/COX-2 plays a major role in caffeine apoptosis-promoting effect on incompletely repaired UVB-damaged cells.
MATERIALS AND METHODS
Cell Culture
Human HaCaT keratinocytes (obtained from Professor N. Fusenig) and HeLa cells (ATCC) were maintained in a monolayer culture in 95% air/5% CO2 at 37 °C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units per ml penicillin, and 100 mg per ml streptomycin (Invitrogen, Carlsbad, CA). Normal human epidermal keratinocytes (NHEK) were obtained from Clonetics (Lonza) and cultured in KGM Gold BulletKit medium (Clonetics, Lonza) according to the manufacturer's instructions. Cells were treated with caffeine at predetermined concentrations 1 h prior to UVB irradiation and after UVB irradiation, except otherwise specified.
UVB Radiation
UVB radiation was performed as described previously (27) using UV stratalinker with UVB bulbs (peak emission at 312 nm, Stratagene). Our UVB radiation was monitored every other week to measure the exposure output and dose using a spectroradiameter (Luzchem). This UV system comprises 0% UVC, 51% UVB, and 49% UVA.
Cell Viability and Apoptosis
Cell growth and apoptosis were determined as described previously (42).
siRNA Transfection and Constitutively Active or Dominant Negative AKT Adenoviral Infection
Cells were transfected with negative control (NC) or siRNA (ON-TARGETplus SMARTpool, Dharmacon) targeting ATR or AKT1, using Amaxa Nucleofector according to the manufacturer's instructions as described previously (42). Then ATR, AKT1, or COX-2 knockdown was evaluated by immunoblotting. For overexpression of constitutively active AKT (Myr-AKT), cells were infected with an adenoviral vector expressing constitutively active AKT (A+) or dominant negative AKT (A-) as described previously (42). Empty vector was used as a negative control (EV).
Western Blotting
Protein concentrations were determined using the BCA assay (Pierce). Equal amounts of protein were subjected to electrophoresis. Western blotting was performed as described previously using film detection (27, 43). Antibodies used included phospho-AKT (serine 473, p-AKT), total AKT, phospho-ERK (p-ERK), ERK, phospho-EGFR (p-EGFR), EGFR, COX-2, Chk1, Chk2, HA (hemagglutinin, for A+), PARP, β-actin, GAPDH (Santa Cruz Biotechnology), p-Chk1 (serine 345), and p-Chk2 (threonine 68) (Cell Signaling Technology).
Determination of Two Major Forms of UVB-induced DNA Damage in Genomic DNA by Enzyme-linked Immunosorbent Assay (ELISA)
ELISA assay of CPD and 6–4PPs were performed as described previously (44). Cells were collected at different time points post-UVB and DNA was isolated using a QIAamp DNA Mini kit (Qiagen, Valencia, CA). The DNA concentration was calculated from the absorbance at 260 nm using NanoDrop 1000 (NanoDrop products, Wilmington, DE). The CPD and 6–4PP in DNA were quantified by ELISA with monoantibodies (TDM-2 for CPD and 64 m-2 for 6–4PP, COSMO BIO Co., Koto-Ku, Tokyo, Japan) as described previously (45) according to the manufacturer's instructions. The absorbance of colored products derived from o-phenylene diamine (Sigma) was measured at 492 nm with a TECAN M200 plate reader (TECAN, Research Triangle Park, NC). For examining repair kinetics, the percentage (%) of repair was calculated by comparing the absorbance at the indicated time to that of the corresponding absorbance at time 0 when there was no opportunity for repair and 100% of CPDs (or 6–4PPs) were present post-UVB.
Statistical Analyses
Statistical analyses were performed using Prism 5 (GraphPad software, San Diego, CA). Data were expressed as the mean of three independent experiments and analyzed by Student's t test and ANOVA. A p value of less than 0.05 was considered statistically significant.
RESULTS
Caffeine Increases UVB-induced Apoptosis in Human HaCaT Keratinocytes
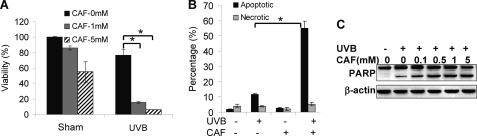
To determine the effect of caffeine on responses of HaCaT cells to UVB radiation, we pretreated cells with caffeine and analyzed cell viability. UVB (20 mJ/cm2) slightly reduced cell viability (Fig. 1A). Caffeine (1 mm) had little effect on sham-irradiated cells, whereas in UVB-irradiated cells it significantly decreased cell viability (Fig. 1A). Caffeine at 5 mm further reduced cell viability post-UVB. It is noteworthy that caffeine at 5 mm seemed to also reduce cell viability in sham-irradiated cells, although it did not reach statistical significance (p > 0.05, Student's t test).
FIGURE 1.
Caffeine promotes UVB-induced apoptosis. A, analysis of viability using the MTS assay in cells at 24 h post-UVB (20 mJ/cm2) pretreated with or without caffeine (CAF, 1 or 5 mm). B, annexin V/propidium iodide assays followed by flow cytometric analysis of apoptosis and necrosis in cells at 18 h post-UVB (20 mJ/cm2) or post-sham pretreated with or without caffeine (1 mm). C, immunoblot analysis of PARP cleavage (lower band) and β-actin in cells treated with or without UVB (20 mJ/cm2) pretreated with or without caffeine at indicated concentrations. The experiment was repeated three times independently and a representative blot was displayed. *, p < 0.05, significant difference between comparison groups using Student's t test.
To determine whether the decrease in cell viability is caused by increased apoptosis, we used the annexin V/propidium iodide assay followed by flow cytometric analysis to compare apoptosis and necrosis between vehicle and caffeine-treated cells post-UVB. UVB increased apoptosis, whereas pretreatment of cells with caffeine significantly enhanced apoptosis (Fig. 1B). No significant increase in necrosis was detected with or without UVB or caffeine treatment. Immunoblot analysis further showed that caffeine at different doses increased UVB-induced PARP cleavage, a hallmark of apoptosis (Fig. 1C). These data indicate that caffeine sensitizes human HaCaT keratinocytes to UVB-induced apoptosis.
ATR/Chk1 Is Inhibited by Caffeine but Is Dispensable for UVB-induced Apoptosis in HaCaT Cells
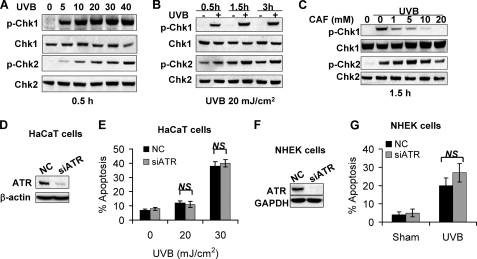
The ATR/Chk1 pathway has been shown to be inhibited by caffeine (35, 36). To determine the effect of caffeine on the ATR pathway in HaCaT cells, we examined whether caffeine inhibits ATR/Chk1 pathway and thereby increases UVB-induced apoptosis. UVB at different doses induced phosphorylation of both Chk1 and Chk2 at 0.5 h and up to 3 h (Fig. 2, A and B). Caffeine at different doses inhibited UVB-induced Chk1 phosphorylation, while it had little effect on Chk2 phosphorylation (Fig. 2C), indicating that caffeine specifically inhibits the ATR/Chk1 pathway following UVB damage.
FIGURE 2.
UVB-induced ATR/Chk1 is inhibited by caffeine but is dispensable for UVB-induced apoptosis. A, immunoblot analysis of phospho-Chk1 (p-Chk1, at serine 345), Chk1, phospho-Chk2 (p-Chk2, at threonine 68), Chk2 in cells at 0.5 h post-UVB at indicated doses (0, 5, 10, 20, 30, or 40 mJ/cm2). B, immunoblot analysis of p-Chk1, Chk1, p-Chk2, and Chk2 at different times post-UVB (20 mJ/cm2). C, immunoblot analysis of p-Chk1, Chk1, p-Chk2, and Chk2 at 1.5 h post-UVB (20 mJ/cm2) in cells treated with caffeine at different concentrations (0, 1, 5, 10, or 20 mm). D, immunoblot analysis of ATR and β-actin in HaCaT cells transfected with siRNA targeting ATR (siATR) or negative control (NC). E, annexin-V/propidium iodide assay followed by flow cytometric analysis of apoptosis in HaCaT cells transfected with NC or siATR at 18 h post-UVB (0, 20, or 30 mJ/cm2). F, immunoblot analysis of ATR and β-actin in NHEK cells transfected with siRNA targeting ATR (siATR) or negative control (NC). G, propidium iodide assay of fixed cells followed by flow cytometric analysis of apoptosis in NHEK cells transfected with NC or siATR at 18 h post-UVB (60 mJ/cm2) or -sham irradiation. NS, not statistically significant between comparison groups using Student's t test. Each experiment was repeated three times independently and a representative blot was shown.
To determine whether inhibiting the ATR/Chk1 pathway is critical for the pro-apoptotic effect of caffeine, we investigated the consequence of ATR inhibition by siRNA targeting ATR on UVB-induced apoptosis of HaCaT, NHEK, and HeLa cells (Fig. 2, D and F, and supplemental Fig. S1, B and C). ATR knockdown had little effect on UVB-induced PARP cleavage in HaCaT cells (supplemental Fig. S1A) or apoptosis in HaCaT cells (Fig. 2E), normal human epidermal keratinocytes (NHEK) (Fig. 2G), or HeLa cells (supplemental Fig. S1D). These data indicate that ATR inhibition is dispensable for UVB-induced apoptosis post-UVB, independent of the cell types tested.
Caffeine Inhibits UVB-induced AKT Activation
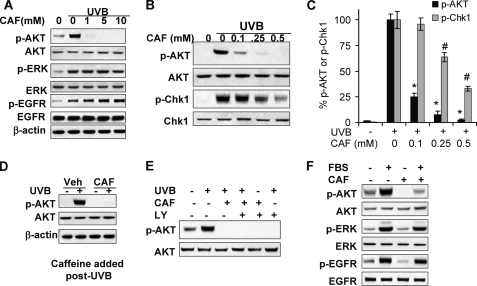
The EGFR/ERK/AKT pathway is critical for cell survival post-UVB (1, 26). To determine the molecular mechanism by which caffeine promotes UVB-induced apoptosis, we investigated the effect of caffeine on the activation of EGFR, ERK, and AKT. UVB irradiation increased phosphorylation of AKT, ERK, and EGFR (Fig. 3A). Caffeine (1, 5, or 10 mm) inhibited basal or UVB-induced AKT activation, while it had no effect on ERK or EGFR activation (Fig. 3A), indicating that caffeine specifically blocks the AKT pathway through impacting EGFR downstream pathways.
FIGURE 3.
Caffeine inhibits UVB-induced AKT activation. A, immunoblot analysis of phospho-AKT (p-AKT), AKT, phospho-ERK (p-ERK), phospho-EGFR (p-EGFR), EGFR, and β-actin in cells pretreated with caffeine at indicated concentration at 1.5 h post-UVB (20 mJ/cm2) or -sham. B, immunoblot analysis of p-AKT, AKT, p-Chk1, and Chk1 in cells pretreated with caffeine at indicated concentration at 1.5 h post-UVB (20 mJ/cm2) or -sham. C, quantification of p-AKT or p-Chk1 in B, normalized against total AKT and Chk1, respectively. D, immunoblot analysis of p-AKT, AKT, and β-actin in cells at 1.5 h post-UVB (20 mJ/cm2) with caffeine added after UVB irradiation. E, immunoblot analysis of p-AKT and AKT in cells pretreated with caffeine or LY294002 (LY, 10 μm) at 1.5 h post-UVB (20 mJ/cm2). F, immunoblot analysis of p-AKT, AKT, p-ERK, ERK, p-EGFR, and EGFR in cells cultured in low-serum (0.1% fetal bovine serum, FBS) for 24 h and then treated with 10% FBS for 30 min. Error bars show S.E. *, p < 0.05, significant difference in p-AKT in UVB-treated cells between vehicle and caffeine-treated groups using Student's t test. #, p < 0.05, significant difference in p-Chk1 in UVB-treated cells between vehicle and caffeine-treated groups using Student's t test. Each experiment was repeated three times independently, and a representative blot was shown.
To determine the precise dose effect of caffeine on the AKT and Chk1 pathways, we treated HaCaT cells with smaller doses of caffeine (0.1–0.5 mm). Caffeine in this range dramatically reduced AKT phosphorylation (Fig. 3, B and C). Although caffeine at the same concentrations also significantly reduced Chk1 phosphorylation, the inhibitory effect on Chk1 phosphorylation by caffeine at 0.25 and 0.5 mm was less extensive than that on AKT phosphorylation (Fig. 3, B and C). These data indicate that caffeine inhibited the UVB-induced AKT pathway more effectively than the Chk1 pathway. Similar to the effect of pretreatment, caffeine added after UVB irradiation effectively inhibited UVB-induced AKT activation (Fig. 3D), indicating that inhibiting AKT by caffeine is not due to its sunscreen effect (34). Similar to caffeine, the PI3K inhibitor LY294002 (LY, 10 μm) completely blocked UVB-induced AKT activation (Fig. 3E). These data suggest that caffeine specifically blocks the AKT pathway.
To further determine the effect of caffeine on AKT activation, we pretreated cells cultured in low serum and then treated them with 10% serum. Caffeine inhibited serum-induced AKT activation as well as the basal AKT activation (Fig. 3F), whereas it had no effect on phosphorylation of ERK and EGFR. These data further demonstrate that caffeine specifically blocks AKT activation without affecting EGFR or ERK activation.
Caffeine Inhibits UVB-induced Anti-apoptotic AKT Activation and COX-2 Up-regulation Independent of ATR
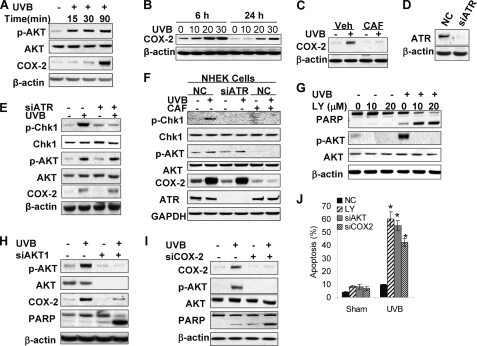
AKT activation closely interacts with COX-2 to increase cell survival in UVB-irradiated cells (1, 46). We found that UVB induces COX-2 up-regulation at 1.5 h and up to 24 h post-UVB (20 mJ/cm2) (Fig. 4, A and B). Caffeine inhibited UVB-induced COX-2 up-regulation (Fig. 4, C and F). To examine the role of ATR in caffeine effect on AKT activation and COX-2 expression, we investigated the effect of AKT knockdown using siRNA targeting ATR. In both HaCaT and NHEK cells, ATR inhibition blocked Chk1 activation, while it had no effect on AKT phosphorylation or COX-2 up-regulation (Fig. 4, E and F), indicating a dispensable role for ATR in the inhibitory effect of caffeine on AKT or COX-2.
FIGURE 4.
UVB-induced anti-apoptotic activation of AKT/COX-2 is independent of ATR. A, immunoblot analysis of p-AKT, AKT, COX-2 and β-actin in cells at different times post-UVB (20 mJ/cm2) or -sham. B, immunoblot analysis of COX-2 and β-actin in cells at 6 and 24 h post-UVB (20 mJ/cm2) or -sham. C, immunoblot analysis of COX-2 and β-actin in cells pretreated with vehicle (Veh) or caffeine (CAF, 0.25 mm) at 1.5 h post-UVB (20 mJ/cm2) or -sham. D, immunoblot analysis of ATR and β-actin in cells transfected with siRNA targeting ATR (siATR) or negative control (NC). E, immunoblot analysis of p-Chk1, Chk1, p-AKT, AKT, COX-2, and β-actin in HaCaT cells transfected with NC or siATR at 1.5 h post-UVB (20 mJ/cm2) or -sham. F, immunoblot analysis of p-Chk1, Chk1, p-AKT, AKT, COX-2, and β-actin in NHEK cells transfected with NC or siATR at 1.5 h post-UVB (20 mJ/cm2) or -sham. G, immunoblot analysis of PARP cleavage, p-AKT, AKT, and β-actin in cells treated with LY (0, 10, or 20 μm) at 1.5 h post-UVB (20 mJ/cm2) or -sham. H, immunoblot analysis of p-AKT, AKT, COX-2, PARP cleavage, and β-actin in cells transfected with NC or siAKT1 at 6 h post-UVB (20 mJ/cm2) or -sham. I, immunoblot analysis of COX-2, p-AKT, AKT, PARP cleavage, and β-actin in cells transfected with NC or siCOX-2 at 6 h post-UVB (20 mJ/cm2) or -sham. J, propidium iodide assay of fixed cells followed by flow cytometric analysis of apoptosis in cells treated with LY (10 μm) or transfected with NC, siAKT, or siCOX2 at 18 h post-UVB (20 mJ/cm2) or -sham. *, p < 0.05, significant difference in apoptosis in UVB-treated cells between NC, and LY, siAKT or siCOX2-treated groups using Student's t test. Each experiment was repeated three times independently and a representative blot was shown.
To determine the AKT-COX-2 interaction and its role in UVB-induced apoptosis, we first examined the effect of LY (10 or 20 μm) on UVB-induced apoptosis. LY dramatically increased UVB-induced PARP cleavage (Fig. 4G). AKT knockdown by siRNA targeting AKT1, the major isoform in HaCaT keratinocytes (42), increased UVB-induced PARP cleavage, and inhibited COX-2 up-regulation (Fig. 4H). COX-2 inhibition was also detected in the treatment with LY (supplemental Fig. S2A). Inhibiting COX-2 by its specific biochemical inhibitor Indomethacin (Indo) blocked AKT activation (supplemental Fig. S2B). COX-2 inhibition by siRNA targeting COX-2 abolished AKT activation and enhanced UVB-induced PARP cleavage (Fig. 4I). Cellular apoptosis analysis indicated that LY, siAKT, or siCOX2 significantly increased UVB-induced apoptosis (Fig. 4J). These data demonstrate that caffeine inhibits the UVB-induced positive feedback loop between AKT and COX-2, thus promoting UVB-induced apoptosis.
Activating AKT Antagonizes the Effect of Caffeine in Apoptosis
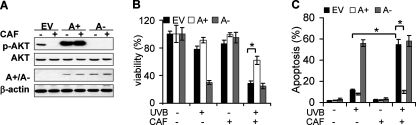
To further determine the causative role of AKT inhibition in caffeine-promoted apoptosis, we infected HaCaT cells with an adenoviral vector expressing constitutively active AKT (A+), dominant negative AKT (A-), or empty vector (EV) alone (Fig. 5A). Caffeine had no effect on AKT activation in A+ cells (Fig. 5A). Expression of A+ significantly reduced cell death in caffeine-treated cells, whereas expression of A- increased UVB-induced apoptosis and was insensitive to caffeine treatment (Fig. 5B). Apoptosis analysis showed that A+ significantly reduced caffeine-enhanced apoptosis, whereas A- resembled the effect of caffeine (Fig. 5C). These findings strongly demonstrate that caffeine promotes UVB-induced apoptosis through inhibiting the AKT pathway.
FIGURE 5.
Activating AKT antagonizes the effect of caffeine on apoptosis. A, immunoblot analysis of p-AKT, AKT, constitutively active AKT (A+) or dominant negative AKT (A-) (HA) and β-actin in cells infected with an adenoviral vector expressing A+ or A- pretreated with or without caffeine (1 mm) at 1.5 h post-UVB (20 mJ/cm2) or -sham. The experiment was repeated three times independently and a representative blot was shown. B, analysis of viability using the MTS assay in cells treated as in A at 24 h post-UVB (20 mJ/cm2). C, annexin V/propidium iodide assays followed by flow cytometric analysis of apoptosis and necrosis in cells treated as in A at 18 h post-UVB (20 mJ/cm2) or -sham. Error bars show S.E. *, p < 0.05, significant differences between comparison groups using Student's t test.
Caffeine Kills Cells with Unrepaired DNA Damage
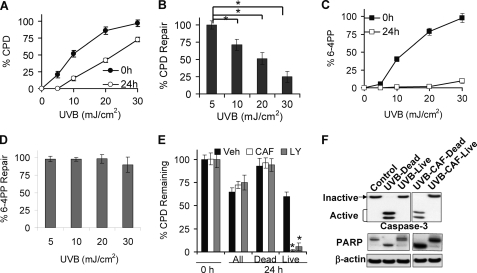
Survival of damaged cells without complete DNA repair leads to tumorigenic mutations. To determine whether caffeine-promoted apoptosis is specific for cells without complete repair, we first investigated the repair of CPDs and 6–4PPs in surviving cells following different doses of UVB radiation. Increasing doses of UVB induced increasing initial CPD and 6–4PP immediately after UVB (Fig. 6, A and B). At 24 h post-UVB, the percentage (%) of remaining CPDs or 6–4PPs decreased, indicating DNA damage repair (Fig. 6, A and B). Only partial repair of CPDs was seen at 10, 20, or 30 mJ/cm2, while 6–4PPs were completely removed at all UVB doses (Fig. 6, A and B). With increasing UVB doses, CPD repair capacity significantly decreased (p < 0.05; Student's t test between UVB doses; two-way ANOVA for different UVB doses), while 6–4PP repair capacity remained efficient (Fig. 6, C and D). These data demonstrated that surviving cells harbor CPD damage depending on the amount of initial UVB-induced DNA damage.
FIGURE 6.
Caffeine promotes UVB-induced apoptosis in cells with unrepaired DNA damage. A, ELISA analysis of the percentage (%) of CPD remaining at 0 h and 24 h post-UVB (5, 10, 20, or 30 mJ/cm2). B, ELISA analysis of the percentage (%) of 6–4PP remaining at 0 h and 24 h post-UVB (5, 10, 20 or 30 mJ/cm2). C, quantification of % CPD repair in A. D, quantification of % 6–4PP repair in B. E, ELISA analysis of % CPD remaining in pooled live and dead cells (All), dead cells, and live cells pretreated with vehicle, caffeine (1 mm), or LY294002 (10 μm) at 0 h or 24 h post-UVB (20 mJ/cm2). F, immunoblot analysis of inactive and active caspase-3, PARP cleavage and β-actin in control, UVB-treated dead cells, UVB-treated live cells, UVB/caffeine-treated dead cells and UVB/caffeine-treated live cells. Error bars show S.E. The experiment was repeated three times independently and a representative blot was shown. *, p < 0.05, significant differences between comparison groups using Student's t test.
To determine whether caffeine selectively kills cells with unrepaired damage, we separated live from dead cells and measured the percentage of the CPD levels in vehicle and caffeine-treated cells post-UVB. In pooled live and dead cells (All), caffeine had little effect on CPD repair at 24 h post-UVB (Fig. 6E). In dead cells, there was no repair of CPD. However, in live cells, CPD levels were significantly reduced in caffeine-treated cells as compared with vehicle-treated counterparts (Fig. 6E). The presence of LY294002 (LY, 10 μm) had a similar effect on the remaining CPD levels (Fig. 6E). Immunoblot analysis confirmed the absence of caspase-3 activation and PARP cleavage, two key hallmarks of apoptosis, in live cells, while in dead cells there was complete caspase-3 activation and PARP cleavage in the presence of caffeine (Fig. 6F). Similar patterns of caspase-3 activation and PARP cleavage were detected in LY-treated live or dead cells (data not shown). These findings clearly demonstrated that, similar to AKT pathway inhibition, caffeine specifically sensitizes unrepaired cells to apoptosis under UVB genotoxic stress.
DISCUSSION
In this study, we have used human HaCaT keratinocytes to determine the specific p53-independent mechanisms by which caffeine promotes UVB-induced apoptosis and specific targeting of damaged cells. We found that low doses of caffeine enhanced keratinocyte apoptosis following UVB. Indeed, inhibition of UVB-induced AKT activation mediated caffeine-promoted apoptosis. Although caffeine inhibited UVB-induced Chk1 activation, ATR inhibition did not resemble the caffeine effect on apoptosis. Our findings also demonstrated that caffeine specifically promotes apoptosis of keratinocytes with unrepaired DNA damage, and thereby offers an effective and targeted preventive approach to reducing tumorigenic transformation.
We found that ATR activation is dispensable for caffeine's proapoptotic effect following UVB in HaCaT, NHEK, and HeLa cells. Previous studies have reported that ATR/Chk1 is an important proapoptotic target for caffeine (35, 36, 47, 48). Loss of p53, downstream of Chk1, sensitizes cells to premature chromatin condensation caused by caffeine (47, 48). Lower caffeine levels induce apoptosis in unirradiated JB6 cells via a p53-dependent pathway (49). In response to UVB radiation, however, caffeine enhances UVB-induced apoptosis in mouse skin and primary human keratinocytes through a p53-independent pathway (35, 50). Caffeine-induced apoptosis post-UVB is associated with diminished Chk1 activation (35, 36). Consistent with these studies, we found that caffeine blocked Chk1 activation in HaCaT cells. However, ATR inhibition by siRNA knockdown had no effect on UVB-induced apoptosis, implying that other targets for caffeine play important roles. Although there was a trend that caffeine increases UVB-induced apoptosis in NHEK cells (Fig. 2G), but it failed to reach a statistical significance. The different findings between ours and previous studies (35) may be due to different sources of the NHEK cells and culture mediums or other unknown reasons, and require further investigations.
Through screening of critical major anti-apoptotic pathways in UVB responses, we found that the UVB-induced AKT/COX-2 axis is inhibited by caffeine, even at low doses. This inhibition of AKT by caffeine was more effective than that of Chk1, indicating that UVB-enhanced AKT activation is a major target for caffeine in human keratinocytes. Using siRNA knockdown and biochemical inhibition, we also demonstrated that AKT and COX-2 form a positive feedback loop: inhibition of one signal resulted in the loss of the other signal. AKT regulates COX-2 up-regulation at the transcription level (51), a regulatory mode that has been implicated in several other cellular models. The role of AKT has been shown in the effect of caffeine on cell proliferation and EGF-induced cell transformation in JB6 cells (52, 53). In glioma cells deficient in PTEN, a negative regulator of AKT, caffeine was found to negatively regulate AKT and to radiosensitize the cells by enhancing G1 arrest (54). In rat microglial cells, caffeine inhibits COX-2 protein synthesis (55). Our current findings suggest that caffeine inhibits AKT through a downstream of EGFR but not the ERK pathway. The role of PI3K in the caffeine effect remains controversial (53, 56). The direct molecular targets of caffeine remains to be determined in future studies.
Our findings have clearly demonstrated that inhibiting AKT mediates the pro-apoptotic function of caffeine in UVB-irradiated keratinocytes. When active AKT that caffeine does not inhibit was expressed, the pro-apoptotic effect of caffeine was blocked. Confirming the essential role of AKT (1, 22, 25, 27) and its downstream COX-2 pathway (1, 46), inhibiting AKT/COX-2 abolished the key survival machinery in UVB-damaged keratinocytes and thus sensitized these cells to UVB-induced apoptosis.
Although DNA damage induces apoptosis, UVB also induces anti-apoptotic AKT and COX-2 signaling. Surviving cells with incomplete repair can become tumorigenic through accumulating genetic mutations. Caffeine specifically promoted apoptosis of keratinocytes without efficient repair, although it did not affect the overall DNA repair capacity in human HaCaT keratinocytes. However, several previous studies have suggested that at higher concentrations caffeine affects DNA repair in different cell types and biochemical studies. In human fibroblasts irradiated with UVC, ATR inhibition by caffeine at 10 mm seems to inhibit DNA repair during S phase (57). In biochemical studies of the caffeine effect on DNA repair using purified DNA repair proteins from Escherichia coli, caffeine (1–50 mm) was found to compromise the specific binding of these DNA repair proteins with damaged DNA (58). Another example of the different observations on the role of ATR in the effect of caffeine was reported in checkpoint inhibition in cells treated with hydroxyurea and ionizing radiation, in which inhibiting the activation of either ATR or ATM is dispensable (59). It appears that the role of ATR/caffeine in DNA damage response is much more complex than it is currently known, and requires further investigations. Nevertheless, our studies have clearly shown that, in caffeine-treated cells, only cells with complete repair survived. Such effect of caffeine resembles that of AKT inhibition, further implying the importance of AKT inhibition in the caffeine effect. This specific pro-apoptotic action eliminated damaged cells and thus reduced tumorigenic transformation.
In conclusion, we have demonstrated that caffeine effectively inhibits the UVB-induced AKT/COX-2 pathway independent of ATR. Blocking the AKT/COX-2 signaling by caffeine specifically eliminates UVB-damaged keratinocytes without complete DNA repair through apoptosis. Our findings add new molecular insights into the chemopreventive action of caffeine in UVB-induced skin cancer.
Supplementary Material
Acknowledgment
We thank Dr. Ann Motten for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant ES016936 (to Y.-Y. H.). Further support was provided by UC Friends of Dermatology Research Funds.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- UVB
- ultraviolet radiation B
- 6–4PP
- pyrimidine (6–4) pyrimidone photoproducts
- A+
- constitutively active AKT
- AKT
- a serine-threonine kinase, downstream of PI3K, also called protein kinase B
- ATM
- ataxia-telangiectasia mutated
- ATR
- ATM, and RAD3-related
- Chk1
- checkpoint kinase 1
- Chk2
- checkpoint kinase 2
- COX-2
- cyclooxygenase-2
- CPD
- cyclobutane pyrimidine dimers
- EGFR
- epidermal growth factor receptor
- ERK
- extracellular signal-regulated kinase
- LY
- LY294002, a specific PI3K inhibitor
- NMSC
- non-melanoma skin cancer
- PI3K
- phosphoinositide 3-kinase.
REFERENCES
- 1. Bowden G. T. (2004) Nat. Rev. Cancer 4, 23–35 [DOI] [PubMed] [Google Scholar]
- 2. Johnson T. M., Dolan O. M., Hamilton T. A., Lu M. C., Swanson N. A., Lowe L. (1998) J. Am. Acad. Dermatol. 38, 681–686 [DOI] [PubMed] [Google Scholar]
- 3. Niggli H. J., Röthlisberger R. (1988) J. Invest. Dermatol. 91, 579–584 [DOI] [PubMed] [Google Scholar]
- 4. Vink A. A., Berg R. J., de Gruijl F. R., Roza L., Baan R. A. (1991) Carcinogenesis 12, 861–864 [DOI] [PubMed] [Google Scholar]
- 5. Cleaver J. E. (2005) Nat. Rev. Cancer 5, 564–573 [DOI] [PubMed] [Google Scholar]
- 6. Cleaver J. E., Lam E. T., Revet I. (2009) Nat. Rev. Genet 10, 756–768 [DOI] [PubMed] [Google Scholar]
- 7. Hoeijmakers J. H. (2001) Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 8. Cimprich K. A., Cortez D. (2008) Nat. Rev. Mol. Cell Biol. 9, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou B. B., Elledge S. J. (2000) Nature 408, 433–439 [DOI] [PubMed] [Google Scholar]
- 10. Brown E. J., Baltimore D. (2003) Genes Dev. 17, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Q., Guntuku S., Cui X. S., Matsuoka S., Cortez D., Tamai K., Luo G., Carattini-Rivera S., DeMayo F., Bradley A., Donehower L. A., Elledge S. J. (2000) Genes Dev. 14, 1448–1459 [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuoka S., Huang M., Elledge S. J. (1998) Science 282, 1893–1897 [DOI] [PubMed] [Google Scholar]
- 13. Chaturvedi P., Eng W. K., Zhu Y., Mattern M. R., Mishra R., Hurle M. R., Zhang X., Annan R. S., Lu Q., Faucette L. F., Scott G. F., Li X., Carr S. A., Johnson R. K., Winkler J. D., Zhou B. B. (1999) Oncogene 18, 4047–4054 [DOI] [PubMed] [Google Scholar]
- 14. Matsuoka S., Rotman G., Ogawa A., Shiloh Y., Tamai K., Elledge S. J. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 10389–10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn J. Y., Schwarz J. K., Piwnica-Worms H., Canman C. E. (2000) Cancer Res. 60, 5934–5936 [PubMed] [Google Scholar]
- 16. Brown E. J., Baltimore D. (2000) Genes Dev. 14, 397–402 [PMC free article] [PubMed] [Google Scholar]
- 17. Menoyo A., Alazzouzi H., Espín E., Armengol M., Yamamoto H., Schwartz S., Jr. (2001) Cancer Res. 61, 7727–7730 [PubMed] [Google Scholar]
- 18. Swift M., Reitnauer P. J., Morrell D., Chase C. L. (1987) New Engl. J. Med. 316, 1289–1294 [DOI] [PubMed] [Google Scholar]
- 19. Renwick A., Thompson D., Seal S., Kelly P., Chagtai T., Ahmed M., North B., Jayatilake H., Barfoot R., Spanova K., McGuffog L., Evans D. G., Eccles D., Easton D. F., Stratton M. R., Rahman N. (2006) Nat. Genet. 38, 873–875 [DOI] [PubMed] [Google Scholar]
- 20. Hannan M. A., Hellani A., Al-Khodairy F. M., Kunhi M., Siddiqui Y., Al-Yussef N., Pangue-Cruz N., Siewertsen M., Al-Ahdal M. N., Aboussekhra A. (2002) Carcinogenesis 23, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 21. Hirao A., Cheung A., Duncan G., Girard P. M., Elia A. J., Wakeham A., Okada H., Sarkissian T., Wong J. A., Sakai T., De Stanchina E., Bristow R. G., Suda T., Lowe S. W., Jeggo P. A., Elledge S. J., Mak T. W. (2002) Mol. Cell. Biol. 22, 6521–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kandel E. S., Hay N. (1999) Exp. Cell Res. 253, 210–229 [DOI] [PubMed] [Google Scholar]
- 23. Brazil D. P., Hemmings B. A. (2001) Trends Biochem. Sci. 26, 657–664 [DOI] [PubMed] [Google Scholar]
- 24. Lawlor M. A., Alessi D. R. (2001) J. Cell Sci. 114, 2903–2910 [DOI] [PubMed] [Google Scholar]
- 25. Datta S. R., Brunet A., Greenberg M. E. (1999) Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 26. Bode A. M., Dong Z. (2003) Sci. STKE 2003, RE2. [DOI] [PubMed] [Google Scholar]
- 27. Ming M., Han W., Maddox J., Soltani K., Shea C. R., Freeman D. M., He Y. Y. (2010) Oncogene 29, 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. An K. P., Athar M., Tang X., Katiyar S. K., Russo J., Beech J., Aszterbaum M., Kopelovich L., Epstein E. H., Jr., Mukhtar H., Bickers D. R. (2002) Photochem. Photobiol. 76, 73–80 [DOI] [PubMed] [Google Scholar]
- 29. Fischer S. M., Pavone A., Mikulec C., Langenbach R., Rundhaug J. E. (2007) Mol. Carcinog. 46, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pentland A. P., Schoggins J. W., Scott G. A., Khan K. N., Han R. (1999) Carcinogenesis 20, 1939–1944 [DOI] [PubMed] [Google Scholar]
- 31. Tiano H. F., Loftin C. D., Akunda J., Lee C. A., Spalding J., Sessoms A., Dunson D. B., Rogan E. G., Morham S. G., Smart R. C., Langenbach R. (2002) Cancer Res. 62, 3395–3401 [PubMed] [Google Scholar]
- 32. Buckman S. Y., Gresham A., Hale P., Hruza G., Anast J., Masferrer J., Pentland A. P. (1998) Carcinogenesis 19, 723–729 [DOI] [PubMed] [Google Scholar]
- 33. Lu Y. P., Lou Y. R., Xie J. G., Peng Q. Y., Liao J., Yang C. S., Huang M. T., Conney A. H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12455–12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu Y. P., Lou Y. R., Xie J. G., Peng Q. Y., Zhou S., Lin Y., Shih W. J., Conney A. H. (2007) Carcinogenesis 28, 199–206 [DOI] [PubMed] [Google Scholar]
- 35. Heffernan T. P., Kawasumi M., Blasina A., Anderes K., Conney A. H., Nghiem P. (2009) J. Invest Dermatol. 129, 1805–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Y. P., Lou Y. R., Peng Q. Y., Xie J. G., Nghiem P., Conney A. H. (2008) Cancer Res. 68, 2523–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jonason A. S., Kunala S., Price G. J., Restifo R. J., Spinelli H. M., Persing J. A., Leffell D. J., Tarone R. E., Brash D. E. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 14025–14029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Gruijl F. R. (2002) Exp. Dermatol. 11, Suppl. 1, 37–39 [DOI] [PubMed] [Google Scholar]
- 39. de Gruijl F. R., Rebel H. (2008) Photochem. Photobiol. 84, 382–387 [DOI] [PubMed] [Google Scholar]
- 40. Ling G., Persson A., Berne B., Uhlén M., Lundeberg J., Ponten F. (2001) Am. J. Pathol. 159, 1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakazawa H., English D., Randell P. L., Nakazawa K., Martel N., Armstrong B. K., Yamasaki H. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han W., Ming M., He T. C., He Y. Y. (2010) J. Biol. Chem. 285, 11369–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He Y. Y., Pi J., Huang J. L., Diwan B. A., Waalkes M. P., Chignell C. F. (2006) Oncogene 25, 3680–3688 [DOI] [PubMed] [Google Scholar]
- 44. Ming M., Shea C. R., Guo X., Li X., Soltani K., Han W., He Y. Y. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 22623–22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakagawa A., Kobayashi N., Muramatsu T., Yamashina Y., Shirai T., Hashimoto M. W., Ikenaga M., Mori T. (1998) J. Invest. Dermatol. 110, 143–148 [DOI] [PubMed] [Google Scholar]
- 46. Chun K. S., Akunda J. K., Langenbach R. (2007) Cancer Res. 67, 2015–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nghiem P., Park P. K., Kim Y., Vaziri C., Schreiber S. L. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9092–9097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nghiem P., Park P. K., Kim Ys Y. S., Desai B. N., Schreiber S. L. (2002) J. Biol. Chem. 277, 4428–4434 [DOI] [PubMed] [Google Scholar]
- 49. He Z., Ma W. Y., Hashimoto T., Bode A. M., Yang C. S., Dong Z. (2003) Cancer Res. 63, 4396–4401 [PubMed] [Google Scholar]
- 50. Lu Y. P., Lou Y. R., Peng Q. Y., Xie J. G., Conney A. H. (2004) Cancer Res. 64, 5020–5027 [DOI] [PubMed] [Google Scholar]
- 51. Tang Q., Gonzales M., Inoue H., Bowden G. T. (2001) Cancer Res. 61, 4329–4332 [PubMed] [Google Scholar]
- 52. Hashimoto T., He Z., Ma W. Y., Schmid P. C., Bode A. M., Yang C. S., Dong Z. (2004) Cancer Res. 64, 3344–3349 [DOI] [PubMed] [Google Scholar]
- 53. Nomura M., Ichimatsu D., Moritani S., Koyama I., Dong Z., Yokogawa K., Miyamoto K. (2005) Mol. Carcinog. 44, 67–76 [DOI] [PubMed] [Google Scholar]
- 54. Sinn B., Tallen G., Schroeder G., Grassl B., Schulze J., Budach V., Tinhofer I. (2010) Mol. Cancer Ther. 9, 480–488 [DOI] [PubMed] [Google Scholar]
- 55. Fiebich B. L., Lieb K., Hüll M., Aicher B., van Ryn J., Pairet M., Engelhardt G. (2000) Neuropharmacology 39, 2205–2213 [DOI] [PubMed] [Google Scholar]
- 56. Foukas L. C., Daniele N., Ktori C., Anderson K. E., Jensen J., Shepherd P. R. (2002) J. Biol. Chem. 277, 37124–37130 [DOI] [PubMed] [Google Scholar]
- 57. Auclair Y., Rouget R., Affar el B., Drobetsky E. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17896–17901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Selby C. P., Sancar A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3522–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cortez D. (2003) J. Biol. Chem. 278, 37139–37145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.