Abstract
Regulation of inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) by IP3 and Ca2+ allows them to initiate and regeneratively propagate intracellular Ca2+ signals. The distribution and mobility of IP3R determines the spatial organization of these Ca2+ signals. Until now, there has been no systematic comparison of the distribution and mobility of the three mammalian IP3R subtypes in a uniform background. We used confocal microscopy and fluorescence recovery after photobleaching to define these properties for each IP3R subtype expressed heterologously in COS-7 cells. IP3R1 and IP3R3 were uniformly distributed within the membranes of the endoplasmic reticulum (ER), but the distribution of IP3R2 was punctate. The mobile fractions (Mf = 84 ± 2 and 80 ± 2%) and diffusion coefficients (D = 0.018 ± 0.001 and 0.016 ± 0.002 μm2/s) of IP3R1 and IP3R3 were similar. Other ER membrane proteins (ryanodine receptor type 1 and sarco/endoplasmic reticulum Ca2+-ATPase type 1) and a luminal protein (enhanced GFP with a KDEL retrieval sequence) had similar mobile fractions, suggesting that IP3R1 and IP3R3 move freely within an ER that is largely, although not entirely, continuous. IP3R2 was less mobile, but IP3R2 mobility differed between perinuclear (Mf = 47 ± 4% and D = 0.004 ± 0.001 μm2/s) and near-plasma membrane (Mf = 64 ± 6% and D = 0.013 ± 0.004 μm2/s) regions, whereas IP3R3 behaved similarly in both regions. We conclude that IP3R1 and IP3R3 diffuse freely within a largely continuous ER, but IP3R2 is more heterogeneously distributed and less mobile, and its mobility differs between regions of the cell.
Keywords: Calcium Channels, Calcium Intracellular Release, Fluorescence, Membrane Proteins, Protein Targeting, Receptors, FRAP, IP3 Receptors, Protein Mobility
Introduction
Inositol 1,4,5-trisphosphate (IP3)2 receptors (IP3R) are tetrameric intracellular Ca2+ channels that are expressed in the membranes of the endoplasmic reticulum (ER) of almost all animal cells (1, 2). The intrinsic channel opens when the IP3R binds both Ca2+ and IP3, with IP3 effectively tuning the sensitivity of the IP3R to Ca2+ (3, 4). The interplay between IP3 and the Ca2+ released by IP3R allows active IP3R to recruit regeneratively the activity of nearby IP3R. This Ca2+-induced Ca2+ release allows Ca2+ signals to grow from tiny local events, arising from openings of single IP3R, into larger events (“Ca2+ puffs”), reflecting the coordinated opening of several clustered IP3R, and ultimately into regenerative Ca2+ waves that may invade the entire cell (5–7). This hierarchical recruitment of IP3R is an essential feature of the spatially organized Ca2+ signals that substantially underpin the versatility of Ca2+ as an intracellular messenger (8).
Regulation of IP3R by Ca2+ is one feature that allows regenerative recruitment of IP3R activity, but the other is the spatial relationship between IP3R. Only closely spaced IP3R are likely to be influenced by each others' activities because cytoplasmic Ca2+ buffers effectively insulate more widely separated IP3R from each other. Persuasive evidence from analyses of both Ca2+ signals (6, 7, 9) and IP3R distribution (10–13) in intact cells and from patch-clamp recording of IP3R in the nuclear envelope (14–17) has established that IP3R can assemble into clusters. The relationships between these observations, whether IP3R assemble into clusters only when stimulated (16–18), and whether the three IP3R subtypes differ in these behaviors are unresolved. An issue that has become contentious is whether functional IP3R are mobile. Our analyses of IP3R in the nuclear envelope suggest that IP3R are randomly distributed and rapidly assemble into clusters only when stimulated with IP3 (16, 17). This suggestion is consistent with analyses of IP3R motility using fluorescence recovery after photobleaching (FRAP), which suggest that most IP3R are mobile (11, 19–23), but high-resolution analyses of the local Ca2+ signals evoked by IP3 suggest that these sparse events are repeatedly initiated at the same point (7, 18). This suggests either that the small fraction of the cellular complement of IP3R that generates these events is immobile or that mobile IP3R initiate events only when they visit specific regions of a cell. Further understanding of IP3R mobility within cells is required to resolve these issues. Here, we extend previous studies of IP3R mobility by using confocal microscopy and FRAP to examine, for the first time, the distribution and mobility of each IP3R subtype in a single cell type, COS-7 cells.
EXPERIMENTAL PROCEDURES
Constructs
Rat IP3R1 (GenBankTM accession number GQ233032) (24) lacking the S1 splice region (25), mouse IP3R2 (accession number GU980658) (12), and rat IP3R3 (accession number GQ233031) (26) were used. The EYFP-IP3R1 plasmid was prepared as described (27, 28). The plasmid encoding EGFP-IP3R1 was made from a previously described plasmid for ECFP-IP3R1 (29) by exchanging ECFP for EGFP. In brief, pENTR-ECFP-IP3R1 was digested with MluI and NheI to excise ECFP, which was then replaced with EGFP (similarly excised from pENTR-EGFP). The vector was recombined into the pcDNA3.2/DEST expression vector using Gateway LR Clonase enzyme mixture (Invitrogen). The EGFP-IP3R2 plasmid was prepared from a previously described construct, pENTR-IP3R2 (30). PCR was used to amplify an N-terminal fragment of IP3R2 (bases 1–3297) using the following primers (5′–3′): forward (P1), AGCATGCGCGCATGTCTGACAAAATGTCCAGCTTCCTCTACATT; and reverse (P2), TATCGCTCGAGTAACTGCACCTGTTTAAAGGCCTG. This fragment was digested with BssHII and XhoI and cloned into pENTR-EGFP-IP3R1 digested with MluI and XhoI. This replaced the entire IP3R1 sequence with the first 3297 bases of the IP3R2 sequence, resulting in pENTR-EGFP-NtermIP3R2. This construct and pENTR-IP3R2 were each digested with HaeII, and the HaeII fragment from pENTR-IP3R2 was replaced with the HaeII fragment from pENTR-EGFP-NtermIP3R2 to give pENTR-EGFP-IP3R2. This vector was recombined into the pcDNA3.2/DEST expression vector. A similar approach was used to construct the FLAG-IP3R2 plasmid. PCR was used to amplify an N-terminal fragment of IP3R2 from pENTR-IP3R2 using the following primers (5′–3′): forward (P3), GCGTCGACGCCACCATGGACTACAAAGACGATGACGATAAGATGTCTGACAAAATGT; and reverse primer P2 (described above). The fragment was digested with SalI and XhoI and cloned in pENTR-1A. The N-terminal fragment and pENTR-IP3R2 were digested with HaeII. The HaeII fragment from pENTR-IP3R2 was then replaced with the HaeII fragment of the N-terminal to generate pENTR-FLAG-IP3R2. This vector was recombined into the pcDNA3.2/DEST expression vector. For ECFP-IP3R3, the existing clone of pENTR-ECFP-IP3R1 (29) was used. PCR was used to amplify an N-terminal fragment of IP3R3 (bases 1–4600) using the following primers (5′–3′): forward (P4), TGCATACGCGTATGAATGAAATGTCCAGCTTTCTTCACATC; and reverse (P5), CAAGGACACTCGAGCAGCCGGGTGGTGGAC. The PCR product was digested with MluI and XhoI, and it was then used to replace the MluI-XhoI-excised fragment of pENTR-ECFP-IP3R1 to give pENTR-ECFP-NtermIP3R3. pENTR-IP3R3 was digested with MfeI and EcoRI, and the excised fragment was used to replace the MfeI-EcoRI fragment of the pENTR-ECFP-NtermIP3R3 vector to give pENTR-ECFP-IP3R3. This vector was recombined into the pcDNA3.2/DEST expression vector. The EGFP-IP3R3 construct was made from the ECFP-IP3R3 plasmid. In brief, pENTR-ECFP-IP3R3 was digested with MluI and NheI to excise ECFP, which was then replaced with EGFP. The coding sequences of all IP3R were confirmed by sequencing. The plasmid encoding rabbit SERCA1 (sarco/endoplasmic reticulum Ca2+-ATPase type 1) C-terminally tagged with EGFP was provided and sequenced by Dr. Malcolm East (University of Southampton, Hampshire, United Kingdom) (31). The plasmid encoding the EGFP sequence flanked by an N-terminal preprolactin leader sequence and a C-terminal KDEL motif (EGFP-KDEL) was provided and sequenced by Dr. Peter Haggie (University of California, San Francisco) (32). The plasmid encoding EGFP-ryanodine receptor type 1 (RyR1) was prepared as described (33).
Cell Culture and Transfection
COS-7 cells were grown in minimal essential medium supplemented with 10% fetal bovine serum and 2 mm l-glutamine at 37 °C in 5% CO2. Cells (80–90% confluent) were transfected using Lipofectamine 2000 (1 μl/1 μg of DNA) (28). For immunofluorescence, cells grown on 13-mm diameter coverslips were transfected with 1 μg of DNA. For subcellular fractionation and FRAP assays, cells grown in 6-well plates and 35-mm dishes with glass bases (Scientific Laboratory Supplies, Yorkshire, United Kingdom), respectively, were transfected with 2–4 μg of DNA. For cotransfection with plasmids for two IP3R subtypes, 0.5 μg of DNA of each plasmid was used. Cells were used 24 h after transfection.
Confocal Microscopy
Transfected cells on coverslips were fixed with 3.5% paraformaldehyde in PBS (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, and 2 mm KH2PO4, pH 7.4) at pH 7.4 for 5–20 min at 20 °C, washed with PBS, permeabilized with Triton X-100 (0.1% in PBS) for 5–10 min at 20 °C, and blocked by incubation with BSA (5% in PBS) for 10 min at 20 °C (28). Similar results were obtained after fixation with methanol/acetone (1:1, v/v) for 10 min at −20 °C (supplemental Fig. S1). All immunostaining was performed in PBS with 5% BSA, with three washes between steps. Calreticulin was visualized by incubating first with a rabbit anti-calreticulin antiserum (1:100; Calbiochem) for 2.5 h at 20 °C and then with an Alexa Fluor 633-labeled goat anti-rabbit secondary antibody (1:500; Molecular Probes) for 1 h at 20 °C. To identify FLAG-IP3R2, cells were incubated with a mouse anti-FLAG antibody (1:1000; Sigma) for 2 h at 37 °C and then with an Alexa Fluor 488-labeled goat anti-mouse secondary antibody. Endogenous and/or heterologously expressed IP3R2 or IP3R3 was identified using affinity-purified rabbit antiserum to IP3R2 (1:100; kindly provided by Dr. David Yule, University of Rochester) (34) or a mouse anti-IP3R3 monoclonal antibody (1:100; Transduction Laboratories). Incubation with the primary antibodies for 2 h at 37 °C was followed by incubation with an Alexa Fluor 555-labeled goat anti-rabbit (or anti-mouse) secondary antibody (1:500; Molecular Probes). Coverslips were washed three times with PBS and once with water and then mounted in ProLong mounting medium (Molecular Probes).
Imaging was performed with a ×63 1.4-numerical aperture oil-immersion objective using a Leica TCS SP2 AOBS confocal microscope. ECFP, EGFP, EYFP, Alexa Fluor 488, Alexa Fluor 555, and Alexa Flour 633 were excited with the 458-, 488-, 514-, 488-, 541-, and 633-nm lines, respectively. Signals were collected using emission filters with detection bands of 468–550 (for ECFP alone; 468–500 when imaged simultaneously with EYFP), 500–565 (for EGFP alone; 500–525 when imaged with Alexa Fluor 555), 520–600, 500–580, 550–650, and 640–750 nm, respectively. No bleed-through was observed in the paired labeling. Images were exported as tiff files and processed using Adobe Photoshop.
Subcellular Fractionation and Western Blotting
Cells were washed with PBS, scraped into ice-cold PBS with protease inhibitors (one tablet/10 ml; Roche Applied Science), and disrupted by 30 passages through a 25-gauge needle. The lysate was centrifuged at 30,000 × g for 30 min, and the supernatant (S1, cytosolic proteins) was collected. The pellet was resuspended in 0.1 m Na2CO3 at pH 11.5, incubated on ice for 45 min to dissociate peripheral membrane proteins, and centrifuged at 30,000 × g for 30 min, and the second supernatant (S2, peripheral membrane proteins) was collected. The pellet (P, integral membrane proteins) was resuspended in PBS containing protease inhibitors and 1% Triton X-100 (28).
Samples (equivalent numbers of cells for each fraction) were loaded onto precast NuPAGE 3–8% Tris acetate gels (Invitrogen). SDS-PAGE, transfer (Invitrogen), and blotting were performed as described previously (28). Membranes were blocked by incubation in PBS containing 0.1% Tween (PBS-T) and 1% BSA for ∼12 h and then incubated with primary antibody for 1 h in PBS-T with 1% BSA. Rabbit anti-GFP (1:1000; Abcam), mouse anti-FLAG (1:1000; Sigma) and mouse anti-IP3R3 (1:1000) antibodies were used as primary antibodies. The membranes were then washed with PBS-T and incubated with a donkey anti-rabbit (1:2000) or goat anti-mouse (1:1000) secondary antibody (Santa Cruz Biotechnology) coupled to horseradish peroxidase for 1 h in PBS-T containing 1% BSA. The anti-FLAG antibody was itself preconjugated to peroxidase. Bands were detected using SuperSignal West Pico chemiluminescent substrate (Pierce).
FRAP
FRAP experiments were performed at 20 °C in culture medium supplemented with 20 mm Hepes at pH 7.3 using a Leica TCS SP5 AOBS microscope fitted with a ×63 1.4-numerical aperture oil-immersion objective and a pinhole diameter set for 1 Airy unit. Cells were illuminated with the 488-nm argon laser line (Lasos CW, 100 milliwatts), and signals were collected at 500–565 nm. Photobleaching experiments were performed using the Leica FRAP application wizard. Typically, fluorescence was recorded at 800-ms intervals (90-ms intervals for EGFP-KDEL) from 10 frames at 2% laser power before bleaching a 2.5-μm diameter region of interest (ROI) for 0.8 s at 100% laser power. Post-bleach images were then collected at 800-ms intervals using 2% laser power for up to 1000 s (typically 300 s). ROI were chosen to entirely exclude the nucleus, immediate perinuclear area (1–2 μm from the nucleus), and the plasma membrane. Only ROI where at least 70% of the initial fluorescence was bleached were used for analysis. Across all the experiments reported here, bleaching caused fluorescence within the ROI to decrease as follows: IP3R1, 86 ± 2%; IP3R2, 89 ± 1%; IP3R3, 92 ± 1%; RyR1, 80 ± 4%; SERCA, 86 ± 2%; and EGFP-KDEL, 81 ± 1%.
Analysis
For every experiment, three reference regions were selected: the bleached ROI, a background region from outside the cell, and the whole cell (see Fig. 3A). All images were first corrected by subtracting background fluorescence. The average pixel intensity within the background area was subtracted from every pixel within the cell (ROI and whole cell); the corrected values were used for all subsequent analyses. Loss of fluorescence due to both image acquisition and bleaching of the ROI causes the whole cell fluorescence to decline with time. We corrected for this loss of fluorescence, which varied between both experiments and constructs (IP3R1, 6 ± 1%; IP3R2, 22 ± 4%; IP3R3, 7 ± 1%; RyR1, 35 ± 3%; SERCA, 4 ± 1%; and EGFP-KDEL, 6 ± 1%), by expressing the fluorescence recorded from the ROI at each time as a fraction of that recorded from the whole cell at the same time. For convenience, all FRAP records were also normalized by expressing this corrected fluorescence from the ROI as a fraction of its pre-bleach value (35). The half-time for recovery (t½ = ln 2/k) was calculated by nonlinear curve fitting of the fluorescence recovery to a single exponential association equation (GraphPad Prism Version 5): Ft = F0 + (Fmax − F0)(1 − e−kt), where Ft is the corrected fluorescence recorded from the ROI at time t; F0 and Fmax are the fluorescence values obtained by extrapolation to immediately after the bleach and at infinite time after recovery, respectively; and k is the first-order rate constant for recovery. The mobile fraction (Mf) was calculated from (Fmax − F0)/(1 − F0). Diffusion coefficients (D) were calculated from a two-dimensional diffusion equation: D = (r2γ)/(4t½), where r is the radius of the ROI (1.25 μm), and γ is a correction factor for bleaching (∼1 for a circular beam) (36). Statistics were computed using Student's t test or one-way analysis of variance (Bonferroni comparison test, 95% confidence intervals) as appropriate. All results are expressed as means ± S.E.
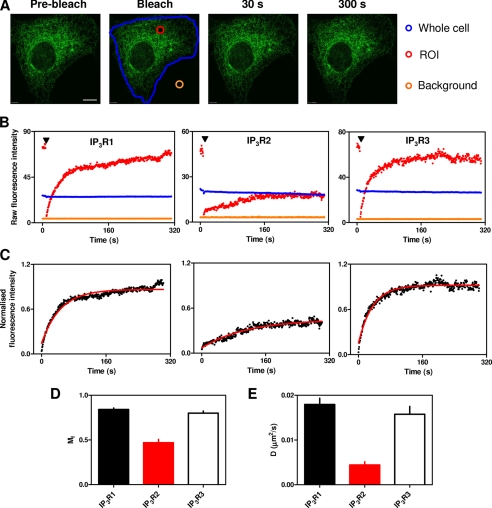
FIGURE 3.
Mobilities of IP3R subtypes. A, a typical FRAP experiment is shown for EGFP-IP3R1. Fluorescence was recorded from the entire cell (blue outline), the bleached ROI (red circle), and a background area outside the cell (orange circle). Typical images are shown before and after bleaching. Scale bar = 10 μm. B, raw fluorescence intensities are shown for the three regions color-coded as described for A. The arrowheads denote bleaching. C, normalized fluorescence intensities are shown after correction for background and loss of fluorescence with time. Red lines show the fitted monoexponential recovery curves (see “Experimental Procedures”). Mf (D) and D (E) are shown for each IP3R subtype. The results in D and E are means ± S.E. from 23–30 cells. Supplemental Fig. S5 provides a comparison of the same data analyzed using another commonly used equation to describe the recovery phase.
RESULTS AND DISCUSSION
Differential Subcellular Distributions of IP3R Subtypes
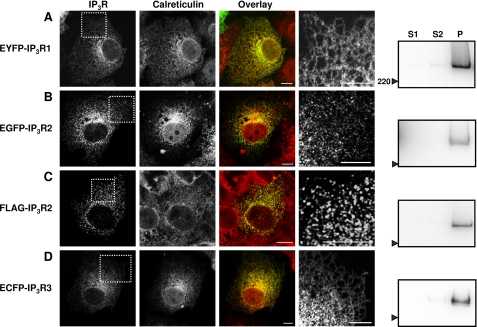
After transient expression in COS-7 cells, EYFP-IP3R1 (or EGFP-IP3R1; data not shown) and ECFP-IP3R3 (or EGFP-IP3R3; data not shown) were uniformly localized to the ER. Each was distributed with a characteristic reticular pattern, and each co-localized with calreticulin, a luminal ER protein (Fig. 1, A and D). After crude fractionation, most IP3R were detected in the pellet (see “Experimental Procedures”). Western blot analysis with antiserum to GFP or IP3R3 revealed single bands with the sizes expected for single subunits of IP3R1 (∼340 kDa) and IP3R3 (∼330 kDa) (Fig. 1, A and D, fifth column). These results confirm previous analyses of IP3R1 (27, 28, 37–39) and IP3R3 (19, 26, 39) distribution in COS cells using both immunocytochemistry of endogenous IP3R (26, 37) and expression of tagged IP3R (19, 27, 28, 38, 39).
FIGURE 1.
Subcellular distribution of IP3R subtypes expressed in COS-7 cells. A–D, typical confocal images of COS-7 cells expressing the indicated constructs. The first and second columns show the distribution of the expressed IP3R and of calreticulin, respectively. The third column shows overlays of the distribution of the two proteins (IP3R in green and calreticulin in red). The fourth column shows an enlargement of the boxed areas shown in the first column. Images are representative of three independent transfections. Scale bars = 10 μm. The fifth column shows Western blots probed with antibodies to GFP (A and B), FLAG (C), or IP3R3 (D) after fractionation of the cells into S1 (cytosolic proteins), S2 (peripheral proteins), and P (pellet; integral membrane proteins) fractions. Each lane (lanes S1, S2, and P) was loaded with material derived from the same number of cells. The position of the 220-kDa molecular mass marker is shown. Each blot is representative of three independent transfections and fractionations.
The distribution of EGFP-IP3R2 (Fig. 1B) was different from that of IP3R1 and IP3R3. Rather than uniformly populating the reticular ER, the distribution of EGFP-IP3R2 was punctate, with the puncta apparently overlying the ER. Similar results were obtained with FLAG-IP3R2 visualized using an anti-FLAG antibody (Fig. 1C), with EGFP-IP3R2 and a different fixative (supplemental Fig. S1A), with endogenous IP3R2 (see Fig. 4E), and with live cell imaging of EGFP-IP3R2 (supplemental Fig. S1B). Others have also reported punctate distributions of native IP3R2 (40) and heterologously expressed IP3R2 (12), although in one study of COS-1 cells, there was no discernible difference between the distributions of IP3R1 and IP3R2 (38). IP3R2 tagged with either EGFP or FLAG were recovered in the pellet fraction after subcellular fractionation (Fig. 1, B and C, fifth column), and each had the size expected (∼335 and ∼310 kDa, respectively). Enlarged images of the subcellular distribution of the three IP3R subtypes highlight the different patterns of IP3R2 expression (Fig. 1, A–D, fourth column). Representative fields of cells are shown in supplemental Fig. S2. The punctate distribution of IP3R2 in unstimulated COS-7 cells is consistent with evidence that although all three IP3R subtypes can cluster when cells are stimulated (10, 11, 13), clustering of IP3R2 appears to occur at resting levels of IP3 (12).
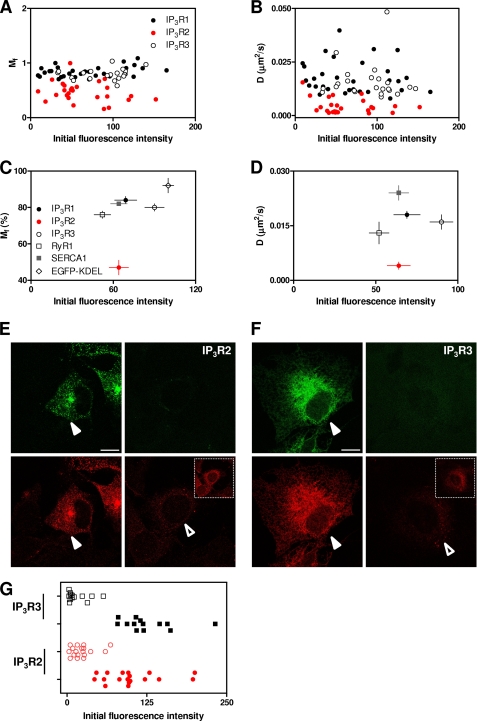
FIGURE 4.
Expression of EGFP-tagged IP3R subtypes does not perturb measurements of the mobile fraction or diffusion coefficient. Mf (A) and D (B) are shown for each cell expressing each IP3R subtype as a function of the background-corrected initial pre-bleach fluorescence intensity. Because each IP3R subtype has the same tag, the latter provides a measure of the relative expression level of each subtype. The results demonstrate considerable, but comparable, variation in the levels of expression of each EGFP-IP3R subtype and no relationship between the expression level and measured Mf or D. The relationship between the average levels of expression for each IP3R subtype, RyR1, SERCA1, and EGFP-KDEL are shown for Mf (C) and D (D). The results (x and y coordinates) are means ± S.E. for 13–30 cells. E, typical confocal images of COS-7 cells show fields that include cells transfected with EGFP-IP3R2 and used for FRAP analyses (left panels, closed arrowheads) or non-transfected cells (right panels, open arrowhead). The upper panels show EGFP fluorescence (green), and the lower panels show immunostaining for IP3R2 (red). The inset shows the same field imaged at higher laser intensity to show more clearly the immunostaining of endogenous IP3R2. F, cells transfected with EGFP-IP3R3 and immunostained for IP3R3 are shown in the same format as described for E. Scale bars (E and F) = 10 μm. G, from fields similar to those shown in E and F, cells expressing EGFP-IP3R at levels suitable for FRAP analyses were identified and compared with neighboring cells lacking EGFP-IP3R. The levels of immunostaining in perinuclear ROI were then quantified for the two cell populations for each IP3R subtype. The results (means ± S.E. for 13–18 cells) show immunostaining for each non-transfected (open symbols) and transfected (closed symbols) cell.
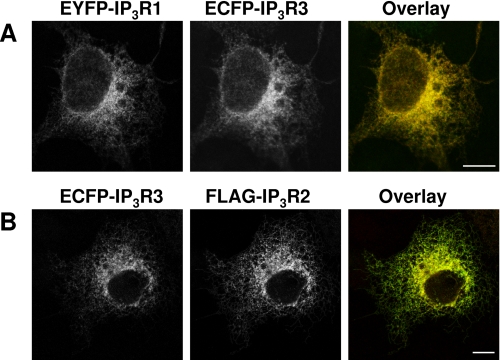
Most cells express more than one IP3R subtype (1, 41), and the different subtypes assemble into both homo- and heterotetramers (39). COS-1 cells express IP3R3 (∼62%) and IP3R2 (∼37%) (42), and heterotetrameric assemblies of IP3R1 and IP3R3 have been detected when they are heterologously expressed in COS-7 cells (39). Co-assembly of transfected IP3R with native subunits is unlikely to distort our results because there is minimal association of endogenous and transfected IP3R (39). Because many native IP3R are likely to be hetero-oligomeric, we therefore examined the distribution of IP3R in cells cotransfected with equal amounts of plasmids encoding two IP3R subtypes. When EYFP-IP3R1 was cotransfected with ECFP-IP3R3, both were, as expected, uniformly distributed within the ER (Fig. 2A). Coexpression of EYFP-IP3R1 with FLAG-IP3R2 proved difficult. Most cells expressed only one IP3R subtype, and in the very rare cases where both were expressed, the distributions were ambiguous (data not shown). Coexpression of FLAG-IP3R2 with ECFP-IP3R3 resulted in a distribution that was indistinguishable from that of ECFP-IP3R3, with no evidence of puncta (Fig. 2B). Others have shown that a splice variant of IP3R2 (IP3R2m2−) that lacks a functional IP3-binding site fails to cluster, and its coexpression with either IP3R1 or IP3R3 prevents ATP from evoking their clustering (12). Collectively, these results demonstrate the complexity of the interactions within heterotetrameric IP3R, but the most important point for the present work is that expressed IP3R2 can assemble with IP3R3 without perturbing the subcellular distribution of the latter. This provides persuasive evidence that the punctate distribution of IP3R2 is unlikely to result from its misfolding. These results provide justification for using COS-7 cells to measure, for the first time, the relative mobilities of the three IP3R subtypes in exactly the same cellular environment.
FIGURE 2.
Coexpression of pairs of IP3R subtypes results in a uniform reticular distribution. A and B, COS-7 cells were transiently transfected with equal amounts of the indicated constructs. The first and second columns show the distribution of each IP3R subtype, and the third column shows the overlay (with the first construct in green and the second in red). Scale bars = 10 μm. Images are representative of three independent transfections.
Differential Mobilities of IP3R Subtypes
FRAP was used to measure the Mf (mobile fraction) and t½ (half-time for recovery of fluorescence), from which D (lateral diffusion coefficient) was calculated (see “Experimental Procedures”). Control experiments with fixed cells confirmed that there was no recovery of fluorescence within 5–6 min in the photobleached area in cells expressing EGFP-tagged IP3R1 (supplemental Fig. S3). We note, however, not least because EYFP-tagged proteins are often used for both FRAP and FRET analyses, that there was substantial reversible bleaching of EYFP constructs (supplemental Fig. S4 and Table S1). In subsequent FRAP analyses, we avoided EYFP-tagged proteins.
Fig. 3A shows typical images during a FRAP experiment with EGFP-IP3R1, highlighting the three regions selected for detailed analysis: the ROI (the 2.5-μm diameter bleached area), the entire cell, and a background area lying outside the cell. In all experiments, the ROI was selected to lie in the perinuclear region, at a distance >2 μm from the nucleus or the plasma membrane. Fig. 3B shows time courses of the raw fluorescence intensities from each of these three regions for typical cells expressing EGFP-tagged IP3R1, IP3R2, or IP3R3. The time courses of the recovery of fluorescence within the bleached area (ROI), corrected for both background fluorescence and the decrease in overall fluorescence of the cell due to bleaching of the ROI and imaging (see “Experimental Procedures”), were each fitted to single exponential equations (Fig. 3C), from which Mf and D were calculated (Fig. 3, D and E). An alternative analysis (36, 43), derived via a theoretical examination of FRAP kinetics, provided results consistent with the conclusions derived from our simpler monoexponential curve fitting, although with slightly increased values for both Mf and D for all proteins (supplemental Fig. S5).
The results demonstrate that most IP3R1 and IP3R3 are mobile (Mf = 84 ± 2 and 80 ± 2%, respectively) and with indistinguishable diffusion coefficients (D = 0.018 ± 0.001 and 0.016 ± 0.002 μm2/s, respectively) (Table 1). Comparable measurements of IP3R2 revealed a smaller mobile fraction (47 ± 4%) and a lesser diffusion coefficient (0.004 ± 0.001 μm2/s) (Table 1).
TABLE 1.
D and Mf for IP3R, RyR, SERCA, and EGFP-KDEL
The results (means ± S.E. from n cells) show D and Mf derived from experiments similar to those shown in Figs. 3 and 4 and supplemental Fig. S7. ND, not determined.
| D | Mf | n | |
|---|---|---|---|
| μm2/s | % | ||
| IP3R1 | 0.018 ± 0.001 | 84 ± 2 | 30 |
| IP3R2 | 0.004 ± 0.001 | 47 ± 4 | 26 |
| IP3R3 | 0.016 ± 0.002 | 80 ± 2 | 23 |
| RyR1 | 0.013 ± 0.003 | 76 ± 2 | 13 |
| SERCA1 | 0.024 ± 0.002 | 82 ± 1 | 22 |
| EGFP-KDEL | ND | 92 ± 4 | 17 |
FRAP Measurements without Perturbing IP3R Mobility
An inevitable concern with FRAP analyses is that heterologous expression of a fluorescent protein might overwhelm the cellular machinery that normally determines the distribution and mobility of the native protein. To minimize such problems, we selected cells with the lowest acceptable levels of expression of EGFP-IP3R compatible with the FRAP analyses. By performing FRAP experiments in parallel under identical conditions for each IP3R subtype, we could compare the levels of expression of each EGFP-tagged IP3R subtype in each cell with the measured Mf and D. The results demonstrate, first, that the expression levels of the three IP3R subtypes were comparable and, second, that for each there was no correlation across an almost 15-fold range of expression level between either Mf or D and the amount of EGFP-IP3R expressed (Fig. 4, A–D).
To allow direct comparison of the endogenous levels of expression of IP3R with those prevailing after expression of EGFP-IP3R, cells prepared for FRAP experiments were immunostained with antibodies that recognize IP3R2 or IP3R3, the predominant endogenous IP3R subtypes in COS cells (42). The antibodies selectively recognize both endogenous and tagged IP3R subtypes. Cells in which the expression of EGFP-IP3R2 (or EGFP-IP3R3) matched our criteria for analysis by FRAP were compared with those in which there was no detectable expression of EGFP, and for each category, we compared the cell immunostaining for IP3R2 (or IP3R3) in a perinuclear ROI. The results show that in the cells used for FRAP, background-corrected immunofluorescence (in arbitrary units of fluorescence) was 101 ± 10 (n = 18) for transfected IP3R2 and 125 ± 11 (n = 13) for transfected IP3R3, compared with 23 ± 4 and 16 ± 5 in non-transfected cells, respectively (Fig. 4, E–G). These results establish that in the FRAP analyses, the average levels of IP3R2 and IP3R3 expression were increased by ∼4- and 8-fold, respectively. We are unaware of any previous attempt to compare quantitatively the levels of overexpression required to perform FRAP analyses. Although the average levels of IP3R expression were increased in the cells used for FRAP analyses, there was considerable variability between individual cells (Fig. 4G). It is noteworthy that IP3R expression levels in native cells with the highest levels of expression and in transfected cells with the lowest levels were similar (and considerably overlapped for IP3R2) (Fig. 4G). These results, together with our demonstration that there is no correlation across an ∼15-fold difference in expression level between IP3R expression and Mf or D (Fig. 4, A and B), provide evidence that under the conditions of our experiments, FRAP is likely to report faithfully the motility of the endogenous IP3R population.
Comparison of the Mobility of IP3R with That of Other ER Proteins
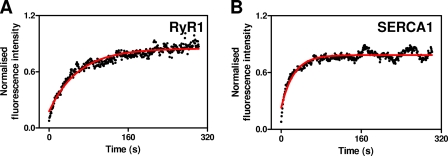
In parallel FRAP analysis of EGFP-RyR1, which is likely to have transmembrane structures similar to IP3R (44) and which is also expressed in the ER, both D (0.013 ± 0.003 μm2/s) and Mf (76 ± 2%) (Fig. 5A and Table 1) were similar to those determined for IP3R1 and IP3R3. SERCA1-EGFP, an ER membrane protein with fewer transmembrane domains than a tetrameric IP3R (10 versus 24), had the same Mf (82 ± 1%) as IP3R1 and IP3R3 but a slightly increased D (0.024 ± 0.002 μm2/s) (Fig. 5B and Table 1). The difference in D between IP3R and SERCA1, which is similar to that reported for SERCA2a and IP3R1 (23), is rather larger than expected for two freely diffusing proteins for which mobility is determined solely by the number of transmembrane domains (supplemental Fig. S6). There are several possible explanations for the disparity. SERCA may move by both diffusion and active transport and thereby provide an inappropriate benchmark for comparison with proteins moving only by diffusion. The mobility of IP3R may be hampered by association with cytoskeletal structures (19, 20, 23). Protein 4.1, for example, selectively associates with IP3R1 (20, 45), and disrupting actin filaments increases the diffusion of IP3R1 in Purkinje cells (23) and hippocampal neurons (20). Disruption of microtubules has also been reported to increase the mobility of IP3R1 (20) and IP3R3 (19). Alternatively, assembly of IP3R1 or IP3R3 into small clusters of approximately seven IP3R would be sufficient to account for the lesser D relative to SERCA (see legend to supplemental Fig. S6 for further details). Our measurements of D (Table 1), whether they reflect movement of single or clustered IP3R, indicate that IP3R1 or IP3R3 can move ∼1 μm within the two dimensions of a lipid membrane in ∼10 s (mean displacement, |r| = √4Dt) (46).
FIGURE 5.
Mobilities of EGFP-RyR1 and SERCA1-EGFP. A and B, normalized fluorescence intensities from the ROI after photobleaching as a function of time for EGFP-RyR1 and SERCA1-EGFP, respectively. Red lines show the monoexponential curve fits to the recovery phase. The results show typical examples of the results summarized in Table 1.
An elegant functional analysis of SH-SY5Y neuroblastoma cells, which express predominantly IP3R1 (42), assumed that movement of the sites from which the smallest Ca2+ release events originate reflected movement of single IP3R and thereby estimated D for functional IP3R1 to be between 0.003 μm2/s (47) and 0.012 μm2/s (18). It is unclear, however, whether this estimate, which suggests that IP3R1 diffuses considerably more slowly than suggested by our FRAP analysis (Table 1), reflects the immobility of a site to which freely moving IP3R transiently attach or the mobility of the IP3R themselves. In addition, it is not clear whether the Ca2+ signals arise from an isolated IP3R or a single active IP3R within a larger cluster. We note, too, that there is substantial variation in D determined for IP3R1 in different cells (0.004–0.26 μm2/s) (supplemental Table S2).
EGFP expressed within the lumen of the ER (EGFP-KDEL) diffused too quickly for D to be measured, but it was possible to measure Mf (supplemental Fig. S7). Mf determined for this luminal protein (92 ± 4%) was similar to that determined for most of the membrane proteins (IP3R1, IP3R3, and SERCA1). Only RyR1 (76 ± 2%) and IP3R2 (47 ± 4%) had significantly smaller Mf (Table 1). The consistent Mf between several ER membrane proteins and a luminal protein suggests that the immobile fraction probably arises from discontinuities within the ER rather than immobile proteins within a continuous organelle. We conclude that the majority (and perhaps all) of IP3R1 and IP3R3 move freely within the membranes of the ER and with similar diffusion coefficients. However, IP3R2 is different: it has a significantly smaller D and Mf (Table 1).
Subcellular Heterogeneity in the Motility of IP3R2
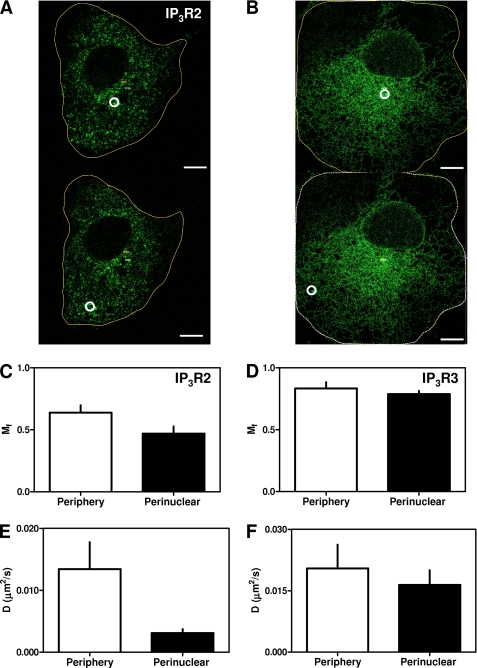
D for IP3R2, which has not to our knowledge been reported previously, is significantly lower than for IP3R1 or IP3R3 (Table 1). If this lesser D were due only to clustering, it would require the clusters to include >900 IP3R2 (supplemental Fig. S6). This is implausible, given the number of puncta and likely density of IP3R (probably <105 IP3R/cell) (48). Therefore, the slower diffusion of IP3R2 relative to IP3R1 and IP3R3 is not a direct consequence of the propensity of IP3R2 to cluster. This suggests that clustering and reduced mobility are independent properties of IP3R2. Further studies are required to establish whether these distinctive behaviors of IP3R2 are expressed in all cell types or whether they require additional endogenous proteins that may be selectively expressed in only some cells, such as COS-7 cells.
We used FRAP to examine the mobility of IP3R2 and IP3R3 in different subcellular regions (Fig. 6, A and B). IP3R2 receptors adjacent (∼2 μm) to the plasma membrane were considerably more mobile (Mf = 64 ± 6% and D = 0.013 ± 0.004 μm2/s, n = 15) than those in the perinuclear region (Mf = 47 ± 6% and D = 0.003 ± 0.001 μm2/s, n = 15) (Fig. 6, C and E). By contrast, IP3R3 (the other major endogenous IP3R subtype in COS cells) had similar properties in the perinuclear (Mf = 79 ± 2% and D = 0.016 ± 0.004 μm2/s, n = 10) (a subset of the data presented in Table 1) and peripheral (Mf = 83 ± 5% and D = 0.020 ± 0.006 μm2/s, n = 10) regions (Fig. 6, D and F). These results establish that both the diffusion and mobile fraction of IP3R2 differ between subcellular regions.
FIGURE 6.
Differential mobility of IP3R2 in different subcellular regions. Typical FRAP experiments are shown for EGFP-IP3R2 (A) and EGFP-IP3R3 (B), with ROI (white circles) chosen to capture IP3R within the perinuclear (upper panels) or peripheral (lower panels) regions of the cell. Images are shown after recovery periods of at least 300 s. Scale bars = 10 μm. The results are typical of recordings from 10–15 cells from three independent transfections. Summary results (means ± S.E., n = 10–15) are shown for Mf (C and D) and D (E and F) determined for each IP3R subtype in the cell periphery (open bars) or perinuclear region (closed bars).
Conclusions
Using FRAP methods that seem unlikely to perturb the mobility of IP3R (Fig. 4), we have compared the mobility of all three mammalian IP3R subtypes in the same cellular background. We conclude that IP3R1 and IP3R3 move freely and with similar diffusion coefficients within ER membranes that seem largely, although not entirely, continuous throughout the cell (Figs. 3 and 5 and Table 1). IP3R2 is different in that it forms puncta (Fig. 1), and its mobility differs between subcellular regions (Fig. 6). In perinuclear regions, 53% of IP3R2 diffuse slowly, and the remainder are immobile, whereas close to the plasma membrane, 36% are immobile, and the rest diffuse at rates similar to those of the other IP3R subtypes. We conclude that IP3R1 and IP3R3 diffuse freely within a largely continuous ER, but IP3R2 is more heterogeneously distributed and less mobile, and its mobility differs between regions of the cell. The latter may be important for initiation of IP3-evoked Ca2+ release because IP3R2 is the most sensitive of the IP3R subtypes to IP3 (12, 30) and perhaps thereby the most likely to initiate Ca2+ signals.
Supplementary Material
Acknowledgments
We thank Drs. S. Dedos, A. Parker, G. Meur, F. Wolfram, and Z. Ding (all originally in the Department of Pharmacology, University of Cambridge) for providing IP3R and RyR plasmids or helping with their preparation. The plasmid for SERCA1-EGFP was provided by Dr. Malcolm East. EGFP-KDEL was provided by Dr. Peter Haggie. The rabbit anti-IP3R2 antibody was provided by Dr. David Yule. We thank Drs. J. Skepper and G. Hammond (University of Cambridge) for help with confocal microscopy and FRAP, respectively.
This work was supported by Medical Research Council UK Grant G0700843 and Wellcome Trust Grant 085295.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7, Tables S1 and S2, and additional references.
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- IP3 receptor(s)
- ER
- endoplasmic reticulum
- FRAP
- fluorescence recovery after photobleaching
- EYFP
- enhanced yellow fluorescent protein
- EGFP
- enhanced green fluorescent protein
- ECFP
- enhanced cyan fluorescent protein
- RyR1
- ryanodine receptor type 1
- ROI
- region(s) of interest.
REFERENCES
- 1. Taylor C. W., Genazzani A. A., Morris S. A. (1999) Cell Calcium 26, 237–251 [DOI] [PubMed] [Google Scholar]
- 2. Foskett J. K., White C., Cheung K. H., Mak D. O. (2007) Physiol. Rev. 87, 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marchant J. S., Taylor C. W. (1997) Curr. Biol. 7, 510–518 [DOI] [PubMed] [Google Scholar]
- 4. Mak D. O., McBride S., Foskett J. K. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15821–15825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bootman M. D., Berridge M. J. (1995) Cell 83, 675–678 [DOI] [PubMed] [Google Scholar]
- 6. Marchant J., Callamaras N., Parker I. (1999) EMBO J. 18, 5285–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith I. F., Parker I. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6404–6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berridge M. J., Lipp P., Bootman M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 9. Bootman M. D., Berridge M. J. (1996) Curr. Biol. 6, 855–865 [DOI] [PubMed] [Google Scholar]
- 10. Wilson B. S., Pfeiffer J. R., Smith A. J., Oliver J. M., Oberdorf J. A., Wojcikiewicz R. J. H. (1998) Mol. Biol. Cell 9, 1465–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chalmers M., Schell M. J., Thorn P. (2006) Biochem. J. 394, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. (2005) J. Biol. Chem. 280, 10305–10317 [DOI] [PubMed] [Google Scholar]
- 13. Tateishi Y., Hattori M., Nakayama T., Iwai M., Bannai H., Nakamura T., Michikawa T., Inoue T., Mikoshiba K. (2005) J. Biol. Chem. 280, 6816–6822 [DOI] [PubMed] [Google Scholar]
- 14. Mak D. O., Foskett J. K. (1997) J. Gen. Physiol. 109, 571–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mak D. O., McBride S., Raghuram V., Yue Y., Joseph S. K., Foskett J. K. (2000) J. Gen. Physiol. 115, 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman T., Taylor C. W. (2009) Channels 3, 226–232 [DOI] [PubMed] [Google Scholar]
- 17. Taufiq-Ur-Rahman, Skupin A., Falcke M., Taylor C. W. (2009) Nature 458, 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith I. F., Wiltgen S. M., Shuai J., Parker I. (2009) Sci. Signal. 2, ra77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferreri-Jacobia M., Mak D. O., Foskett J. K. (2005) J. Biol. Chem. 280, 3824–3831 [DOI] [PubMed] [Google Scholar]
- 20. Fukatsu K., Bannai H., Zhang S., Nakamura H., Inoue T., Mikoshiba K. (2004) J. Biol. Chem. 279, 48976–48982 [DOI] [PubMed] [Google Scholar]
- 21. Gibson C. J., Ehrlich B. E. (2008) Cell Calcium 43, 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cruttwell C., Bernard J., Hilly M., Nicolas V., Tunwell R. E., Mauger J. P. (2005) Biol. Cell 97, 699–707 [DOI] [PubMed] [Google Scholar]
- 23. Fukatsu K., Bannai H., Inoue T., Mikoshiba K. (2010) J. Neurochem. 114, 1720–1733 [DOI] [PubMed] [Google Scholar]
- 24. Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. (1990) J. Biol. Chem. 265, 12679–12685 [PubMed] [Google Scholar]
- 25. Cardy T. J., Taylor C. W. (1998) Biochem. J. 334, 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. (1993) J. Biol. Chem. 268, 11356–11363 [PubMed] [Google Scholar]
- 27. Parker A. K., Gergely F. V., Taylor C. W. (2004) J. Biol. Chem. 279, 23797–23805 [DOI] [PubMed] [Google Scholar]
- 28. Pantazaka E., Taylor C. W. (2010) Biochem. J. 425, 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolfram F., Morris E., Taylor C. W. (2010) Biochem. J. 428, 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tovey S. C., Dedos S. G., Rahman T., Taylor E. J., Pantazaka E., Taylor C. W. (2010) J. Biol. Chem. 285, 12979–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Newton T., Black J. P., Butler J., Lee A. G., Chad J., East J. M. (2003) Biochem. J. 371, 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dayel M. J., Hom E. F., Verkman A. S. (1999) Biophys. J. 76, 2843–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meur G., Parker A. K., Gergely F. V., Taylor C. W. (2007) J. Biol. Chem. 282, 23096–23103 [DOI] [PubMed] [Google Scholar]
- 34. Betzenhauser M. J., Fike J. L., Wagner L. E., 2nd, Yule D. I. (2009) J. Biol. Chem. 284, 25116–25125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rabut G., Ellenberg J. (2004) in Live Cell Imaging: A Laboratory Manual (Goldman R. D., Spector D. L. eds) pp. 101–126, Cold Spring Harbor Laboratory Press, Woodbury, NY [Google Scholar]
- 36. Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. (1976) Biophys. J. 16, 1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laflamme K., Domingue O., Guillemette B. I., Guillemette G. (2002) J. Cell. Biochem. 85, 219–228 [PubMed] [Google Scholar]
- 38. Ramos-Franco J., Bare D., Caenepeel S., Nani A., Fill M., Mignery G. (2000) Biophys. J. 79, 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joseph S. K., Bokkala S., Boehning D., Zeigler S. (2000) J. Biol. Chem. 275, 16084–16090 [DOI] [PubMed] [Google Scholar]
- 40. Sheppard C. A., Simpson P. B., Sharp A. H., Nucifora F. C., Ross C. A., Lange G. D., Russell J. T. (1997) J. Neurochem. 68, 2317–2327 [DOI] [PubMed] [Google Scholar]
- 41. Vermassen E., Parys J. B., Mauger J. P. (2004) Biol. Cell 96, 3–17 [DOI] [PubMed] [Google Scholar]
- 42. Wojcikiewicz R. J. H. (1995) J. Biol. Chem. 270, 11678–11683 [DOI] [PubMed] [Google Scholar]
- 43. Kwon G., Axelrod D., Neubig R. R. (1994) Cell. Signal. 6, 663–679 [DOI] [PubMed] [Google Scholar]
- 44. Samso M., Wagenknecht T., Allen P. D. (2005) Nat. Struct. Mol. Biol. 6, 539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fukatsu K., Bannai H., Inoue T., Mikoshiba K. (2006) Biochem. Biophys. Res. Commun. 342, 573–576 [DOI] [PubMed] [Google Scholar]
- 46. Berg H. C. (1983) Random Walks in Biology, Princeton University Press, Princeton, NJ [Google Scholar]
- 47. Wiltgen S. M., Smith I. F., Parker I. (2010) Biophys. J. 99, 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tovey S. C., Dedos S. G., Taylor E. J., Church J. E., Taylor C. W. (2008) J. Cell Biol. 183, 297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.