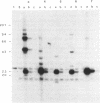
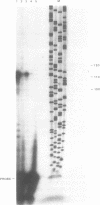
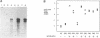Abstract
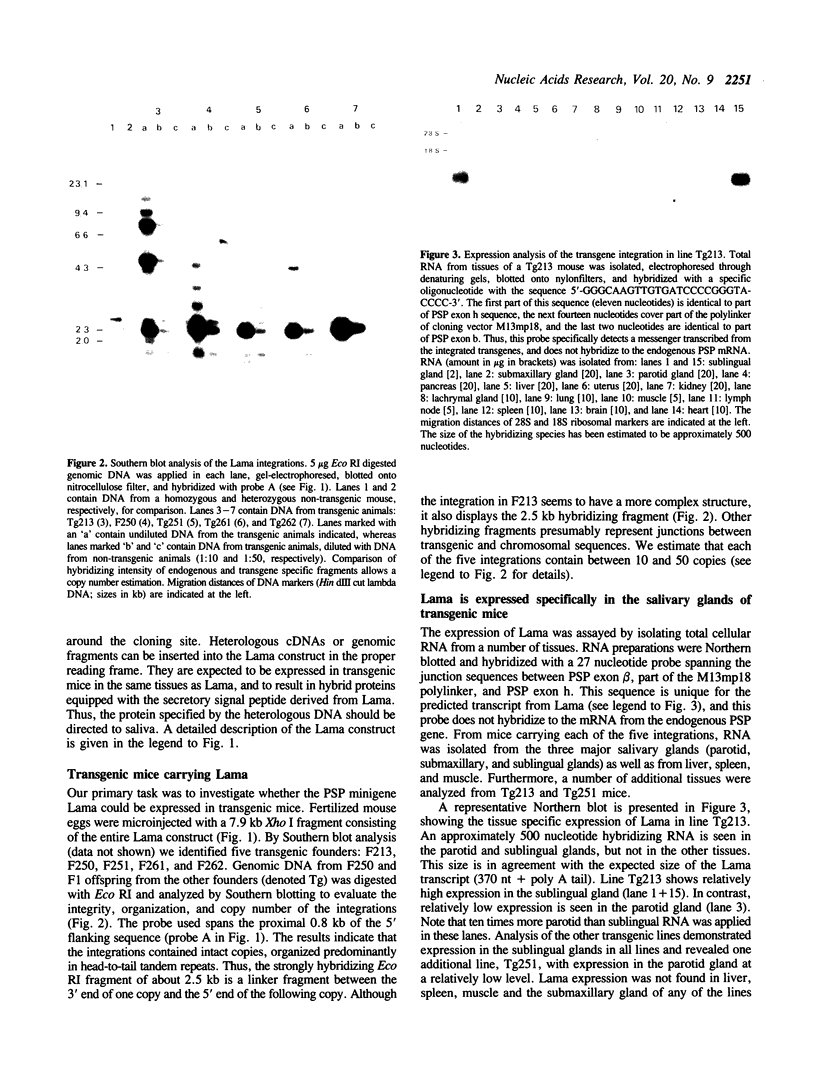
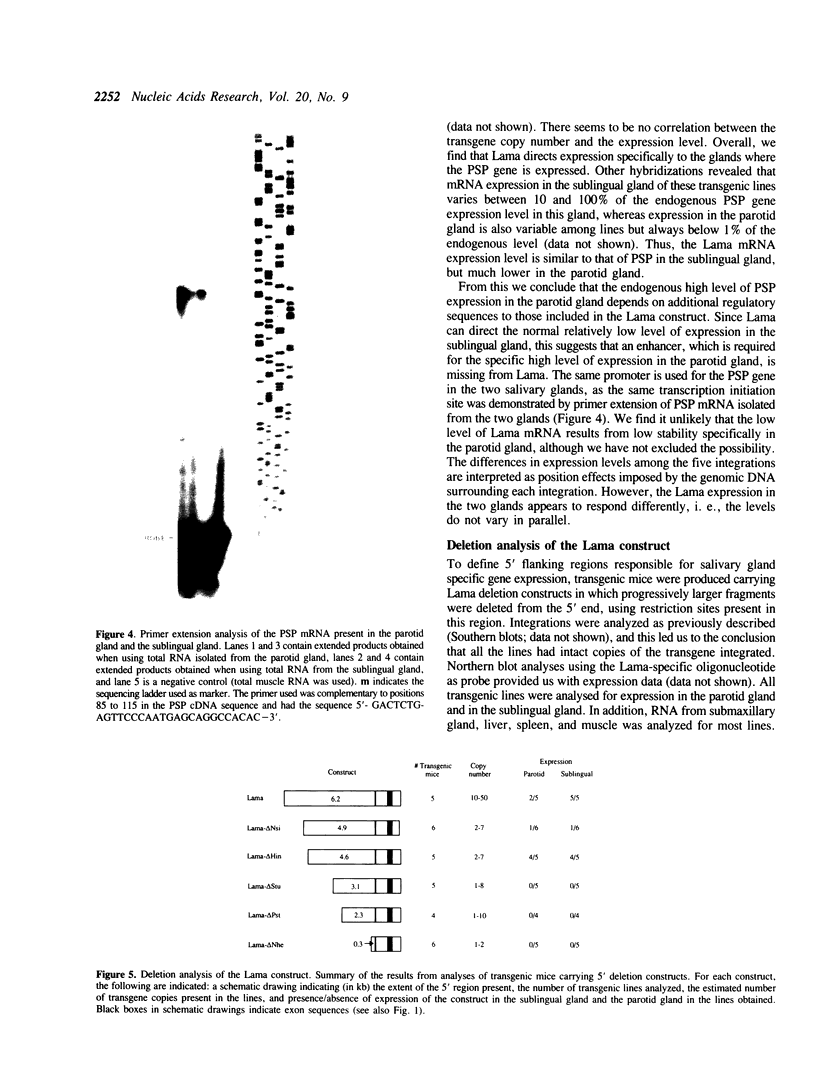
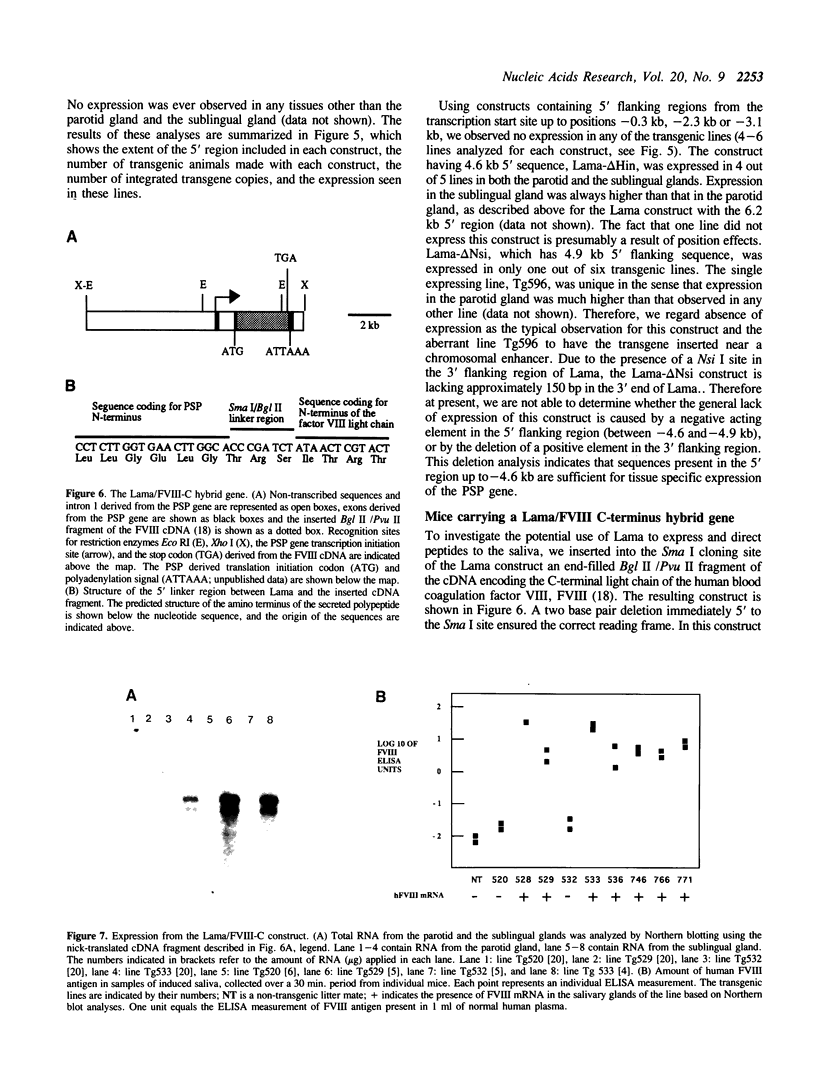
Using a DNA construct, named Lama, derived from the murine parotid secretory protein (PSP) gene, we have obtained salivary gland specific gene expression in transgenic mice. Lama is a PSP minigene and allows analysis of the PSP gene 5' regulatory region by transgenesis. We show here that the regulatory region included in Lama with 4.6 kb of 5' flanking sequence is sufficient to direct expression specifically to the salivary glands. The expression level in the parotid gland is only about one percent of the PSP mRNA level, while that of the sublingual gland is near the PSP mRNA level. This suggests significant differences in the PSP gene regulation in the two glands. In addition, Lama is a secretory expression vector in which cDNAs or genomic fragments can be inserted. We demonstrate that the Lama construct can direct the expression of a heterologous cDNA encoding the C-terminal peptide of human factor VIII to salivary glands and that the corresponding peptide is secreted into saliva.
Full text
PDF
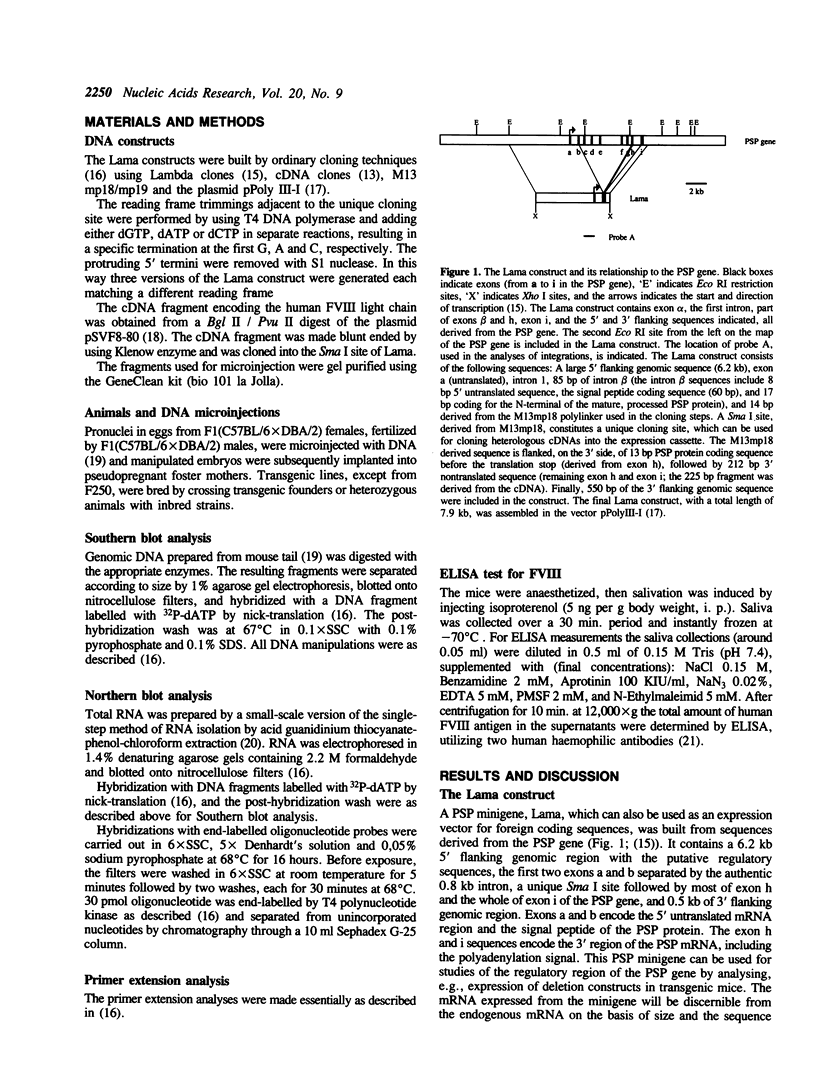


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann D. K., Smith M. K., Carlson D. M. Molecular evolution of the mouse proline-rich protein multigene family. Insertion of a long interspersed repeated DNA element. J Biol Chem. 1988 Aug 5;263(22):10887–10893. [PubMed] [Google Scholar]
- Archibald A. L., McClenaghan M., Hornsey V., Simons J. P., Clark A. J. High-level expression of biologically active human alpha 1-antitrypsin in the milk of transgenic mice. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5178–5182. doi: 10.1073/pnas.87.13.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. L., Pachl C., Quiroga M., Rosenberg S., Haigwood N., Nordfang O., Ezban M. The functional domains of coagulation factor VIII:C. J Biol Chem. 1986 Sep 25;261(27):12574–12578. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fay P. J., Chavin S. I., Schroeder D., Young F. E., Marder V. J. Purification and characterization of a highly purified human factor VIII consisting of a single type of polypeptide chain. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7200–7204. doi: 10.1073/pnas.79.23.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth J. P. Genetic variation in mouse salivary amylase rate of synthesis. Biochem Genet. 1979 Aug;17(7-8):665–682. doi: 10.1007/BF00502125. [DOI] [PubMed] [Google Scholar]
- Ingerslev J., Stenbjerg S. Enzyme linked immunosorbent assay (ELISA) for the measurement of factor VIII coagulant antigen (CAg) using haemophilic antibodies. Br J Haematol. 1986 Feb;62(2):325–332. doi: 10.1111/j.1365-2141.1986.tb02936.x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Keller S. A., Samuelson L. C., Osborn L., Rosenberg M. P., Meisler M. H. A salivary amylase transgene is efficiently expressed in liver but not in parotid gland of transgenic mice. Nucleic Acids Res. 1989 Aug 25;17(16):6613–6623. doi: 10.1093/nar/17.16.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Madsen H. O., Hjorth J. P. Molecular cloning of mouse PSP mRNA. Nucleic Acids Res. 1985 Jan 11;13(1):1–13. doi: 10.1093/nar/13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K., Jakobsen B. K., Mikkelsen B. M., Harmark K., Nielsen J. T., Hjorth J. P. Coordination of murine parotid secretory protein and salivary amylase expression. EMBO J. 1986 Aug;5(8):1891–1896. doi: 10.1002/j.1460-2075.1986.tb04441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Schibler U. Structure and expression of the parotid secretory protein gene of mouse. J Mol Biol. 1986 Dec 5;192(3):567–576. doi: 10.1016/0022-2836(86)90277-9. [DOI] [PubMed] [Google Scholar]
- Simons F. E., Simons K. J., Chung M., Yeh J. The comparative pharmacokinetics of H1-receptor antagonists. Ann Allergy. 1987 Dec;59(6 Pt 2):20–24. [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]