Abstract
Objective
Study the effect of participation in a mindfulness training program (Mindfulness Based Stress Reduction) on degree of bother from hot flashes and night sweats.
Methods
Randomized trial of 110 late perimenopausal and early post-menopausal women experiencing average of ≥5 moderate or severe hot flashes (including night sweats)/day. A wait list control was used, with three-month post-intervention follow-up. Main outcome was degree of bother from hot flashes and night sweats in previous 24 hours. Secondary measures: hot flash intensity, quality of life, insomnia, anxiety, perceived stress.
Results
Baseline average hot flash frequency was 7.87 (SD 3.44) and 2.81 night sweats (SD 1.76)/day. Mean bothersomeness score was 3.18 (SD 0.55) (‘moderately bothered/extremely bothered’). All analyses were intent to treat, and controlled for baseline values. Within-woman changes in bother from hot flashes differed significantly by treatment arm (week × treatment arm interaction P=0.042). At completion of intervention, bother in the MBSR arm decreased on average by 14.77% versus 6.79% for WLC. At 20 weeks total reduction in bother for MBSR was 21.62% and 10.50% for WLC. Baseline-adjusted changes in hot flash intensity did not differ between treatment arms (week × treatment arm interaction P=0.692). The MBSR arm made clinically significant improvements in quality of life (P=0.022), subjective sleep quality (p=0.009), anxiety (P=0.005), and perceived stress (P=0.001). Improvements were maintained 3 months post-intervention.
Conclusions
Our data suggest that MBSR may be a clinically significant resource in reducing the degree of bother and distress women experience from hot flashes and night sweats.
Keywords: Mindfulness, menopause, hot flashes, sleep, quality of life, perceived stress
Introduction
Approximately 40% of postmenopausal women report that their hot flashes and night sweats negatively affect their quality of life by interfering with their work, leisure, mood, concentration, sleep and sexual activity.1 Hormonal therapy (HT) provided relief for most women, but since the publication of its possible health risks,2–5 the search for other treatments of similar efficacy has not yet been successful.6 While non-hormonal oral therapies offer some relief, their adverse effects and cost may prohibit their use for many women.7–11 Women’s need for relief from their hot flashes is indicated by the substantial number who resume oral HT despite the possible risks,12–14 and by the number turning to complementary and alternative treatments15,16 despite lack of evidence for their efficacy.17–20
A substantial proportion of women report negative emotions in association with their hot flashes, including psychological distress, social embarrassment, and anxiety.21–25 This distress is also associated with greater reported hot flash frequency,24,26 and may lead women to evaluate their hot flashes as more bothersome, over and above the frequency at which they occur.21,23,26–40 Thus, women’s distress may reflect not only the severity of their physical symptoms, but also their psychological reactions to these unpredictable and socially problematic bodily states.21,23,26–35,41 Programs designed to affect these reactions however, have focused mainly on reducing hot flash frequency and have had mixed and limited results,20,35,42–47 In recognition of the opportunity this approach represents however, two NIH-convened panels48; Santoro, 2007 #7519} have recommend the investigation of new behavioral treatments to increase women’s resilience to hot flashes.
Mindfulness training involves patients learning to recognize and discriminate more accurately between the components of experience such as thoughts, feelings, and sensations, and developing a non-reactive awareness of these.49 The psychological distance created through mindfulness reduces the urgency of thoughts and affective states, enabling the person to observe, appraise and be less reactive to events in their internal and external environment.50 Following an initial feasibility study,35 our group tested the effect of participation in a widely-available mindfulness training program (Mindfulness-Based Stress Reduction (MBSR)) on the degree of bother that women experienced from their hot flashes. MBSR has been shown to be effective in supporting patients in coping with a wide range of symptom-related challenges51 including reduced sleep disturbance,52,53 perceived stress,54 anxiety55,56 and panic.57,58 We also examined changes in hot flash intensity (hot flash frequency × severity) and in four psychosocial variables affected by hot flashes.
Methods
Study population and eligibility criteria
Participants were women in late menopausal transition and early post-menopause59 experiencing an average of ≥5 moderate or severe hot flashes (including night sweats)/day during the past week. The frequency and severity criteria were chosen to maximize eligibility in relation to hot flash prevalence,59–62 and are consistent with other studies,63–66 and reviews.59,67–70
Additional criteria included willingness to keep a daily diary of the time and intensity and bother from hot flashes, and endeavoring to maintain present exercise, dietary pattern, and dosage of any soy supplements or menopausal remedies (including isoflavone intake). Exclusion criteria included medical conditions or medications that may affect hot flashes (such as a history of thyroid disease, or selective estrogen receptor modulators). Patients with breast cancer were eligible six months after completion of their treatment. Women were asked to report the initiation of any medications during the study, and those taking selective serotonin reuptake inhibitors (SSRI’s) and anxiolytics were asked to maintain their present dosage or to report immediately any change. The study was approved by the University of Massachusetts Medical School Institutional Review Board.
The target sample size, after accounting for attrition, was 50 women/treatment arm, as recommended by Sloan et al.71 Using these recommendations, this sample size yields an estimated effect size for the between-arm difference of 0.58 standard deviations, equivalent to approximately 3 units/day for hot flash intensity; smaller between-group differences are unlikely to be clinically meaningful.
Recruitment
Multiple strategies were employed for recruitment, such as newspaper, radio and cable advertising; University of Massachusetts Medical School (UMMS) intranet messaging and poster displays; a “Tell a Friend” campaign that encouraged women to pass the word to a friend or colleague; presentations at worksites and at a menopause symposium; health fairs and community events; Registry of Motor Vehicle Electronic Billboard; provider referral; and a direct mail campaign. Advertisements offered eligible women the opportunity to participate at no cost in the MBSR program as a possible means of managing their hot flashes. Enrollment took place between November 2005 and September 2007, and comprised eight cycles of women, enrolled at 3-month intervals to coincide with the beginning of the four MBSR class cycles that run through the year at UMMS Center for Mindfulness, Worcester MA.
Following a phone interview to establish eligibility, women completed the daily hot flash diary over the following seven days to confirm eligibility, and provide baseline hot flash data. At the following clinic interview, the study manager scanned the completed diary to confirm hot flash eligibility, explained the behavioral demands of the intervention and assessment procedures, and completed the informed consent process. Women who consented to participate completed the pre-program assessment questionnaires, and were then randomly assigned to the intervention or to a wait-list control (WLC) group. Women completed the study assessments again at end of intervention and at 20 weeks after enrollment, and returned them by mail. Randomization was performed using the Stata’s ralloc command which generates a sequence of group assignments randomly permuted in blocks of several sizes. The size and order of the blocks are also random: block sizes of 4 and 6 were used in this study. The programmer generated the random allocation sequence and uploaded the table containing the random sequence of group assignments to an Access database. Based on this table, the participant was automatically assigned to a group by the study manager clicking the “Randomize” button. All data entry personnel were blinded to group allocation.
Intervention
Women randomized to the intervention arm were able to select one of three concurrent MBSR classes (evening and daytime). Classes were conducted by Center for Mindfulness instructors and who were blinded to the study outcomes. Classes comprised approximately 25 participants and included others who had been referred and self-referred to the MBSR program with a variety of diagnoses and stress-related conditions. As such, study participants were a minority in each class and did not have to reveal to others their reason for being in the class unless they chose to do so.
The MBSR protocol has been described in detail elsewhere.72 Briefly, participants attended 8 weekly 2½ hour classes, plus an all-day class on a weekend day during the 6th week. The curriculum included training in mindfulness through: a) a body scan, a gradual moving of attention through the body from feet to head while lying in a supine position, bringing awareness particularly to bodily sensations; b) sitting meditation, in which attention is brought to the flow of bodily sensations (particularly the sensations of breathing), thoughts, and emotions; and c) mindful stretching exercises, intended to develop awareness during movement. In-class didactic material emphasized the application of mindfulness in everyday life, and contained suggestions for its application in responding to distressing symptoms and stressful situations. A variety of informal mindfulness practices were assigned between sessions, and class participants received two CDs of guided instruction to be practiced at home for 45 minutes, six days/week.
Control
participants were assigned to a wait-list comparison group, and offered participation in the MBSR program at no cost following their final study assessments.
Outcome Measures
The primary outcome measure was overall degree of bother from hot flashes and night sweats in the previous 24 hours.73 Bother was recorded in a hot flash diary in which women also recorded the number of mild, moderate and severe hot flashes and night sweats. Hot flash diaries have been used to measure severity of hot flashes and degree of bother in a number of clinical trials.71,74 Women completed the diary each day during the intervention period (9 weeks), and for 1 week during the 12, 16, and 20 follow-up weeks and returned them by mail. Bother was reported at the end of each day on a four-point scale with a range of ‘not at all’ (1); ‘slightly bothered’ (2); ‘moderately bothered’ (3); ‘extremely bothered’ (4). Weekly scores (averaging daily scores over 7 days) were computed to smooth out day-to-day variability. All participants received weekly contact/reminder calls from study staff to check on possible problems related to the study intervention and/or to be reminded of completing their hot flash diaries.
Secondary outcomes included hot flash intensity, and psychosocial measures of stress/distress (quality of life, subjective sleep quality, anxiety, and perceived stress), and treatment adherence. The hot flash intensity score was calculated as the sum of hot flashes, weighted by the severity ratings (mild=1 through severe=3). Scales for the psychosocial variables included: Menopause-Related Quality of Life (MENQOL),75 a validated self-administered instrument listing 29 menopause-related symptoms in four domains: vasomotor, physical, psychosocial, and sexual. Women report the degree of bother that they have experienced from each symptom over the past month. A lower score represents better quality of life; Women’s Health Initiative Insomnia Rating Scale (WHIIRS)76,77 is a validated 5-item scale of subjective sleep quality that assesses sleep initiation and maintenance. Higher scores are indicative of poorer sleep quality; Hospital Anxiety and Depression Scale-Anxiety (HADS-A)78 is a 7-item self-administered measure assessing anxiety. Higher scores indicate greater psychological morbidity. HADS-A has been found valid and reliable for non-psychiatric medical populations,79 is widely used as a screening instrument in clinical settings,80 and is not confounded by somatic symptoms of anxiety that relate also to physical disorders. A cut-off ≥8 is usually recommended for clinically significant anxiety.79,81 The HADS-A was added to the assessments a few months after enrollment started, as findings emerged21,22,24 on the possible relationship of anxiety to hot flashes. Baseline data were available for 66% of participants.Perceived Stress Scale (PSS) (14-item version)82 posits that people appraise potentially threatening or challenging events in relation to their available coping resources, and measures the degree to which situations in life are appraised as unpredictable, uncontrollable and overwhelming. Higher scores indicate a greater degree of perceived stress.
Treatment Adherence was assessed by percent intervention attendance (MBSR classes attended/total classes × 100), and amount of reported home practice. Information about the latter was gathered through a log in which women recorded the number of minutes of formal mindfulness practiced each day during the intervention, and during each of the 12, 16 and 20-week hot flash diary follow-ups. During the intervention the completed logs were returned each week by placing them in a locked box in the MBSR classroom which was collected by the study manager. Logs were not seen by the MBSR instructors.
In addition, information was collected on demographics, socioeconomic status, medical history, smoking, previous meditation experience, yoga or Tai-Chi experience, and on a number of variables known to affect hot flashes, such as BMI, alcohol intake, and physical activity.
Statistical Methods
The two treatment arms were characterized at baseline using frequencies for categorical variables and means and standard deviations for continuous variables. To assess the effect of the intervention on hot flash bother and intensity, percent change from baseline was modeled using linear mixed modeling83 as a function of week (weeks 1 through 9, 12, 16, and 20, treated as a categorical variable to allow for non-linear trajectories), treatment arm, and their interaction, with adjustment for baseline value of the outcome being modeled to account for possible regression to the mean.84 Similar analyses were conducted for the psychosocial variables, modeling change from baseline at weeks 9 and 20; absolute change rather than percent change was modeled because these scales have established clinical norms. All analyses were intention to treat. Primary analyses included all participants with a baseline measure and at least one follow-up measure. To assess the impact of missing data, analyses were repeated carrying the last observation forward; in all cases, results were similar (data not shown). Adjustment for baseline characteristics85 that differed by treatment arm had little impact on treatment-related differences; thus, results adjusted only for the baseline outcome value are presented.
Results
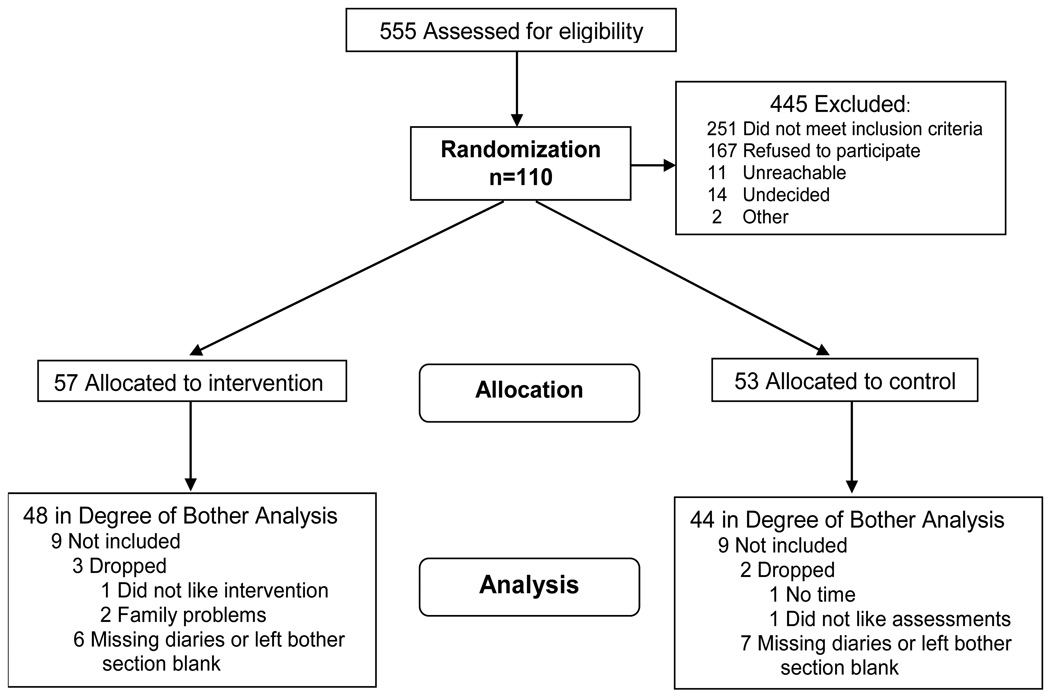
One hundred and ten women aged between 47 to 69 years (mean 53, SD 4.9) years consented to participate, with 57 randomized to the MBSR intervention and 53 to the wait list control. The flow of participants through the study is reported in the CONSORT diagram (Figure 1). Primary analysis included 92 women for bother. 99 women were included in the analysis for hot flash intensity. Retention was 90.9% overall; 87.7% for the MBSR arm, and 94.3% for the control arm. Retention data and reasons for dropping are specified in Figure 1. For the diary outcomes, 63.6% of the women provided degree of overall bother on at least 80% of diaries, and 74.5% provided hot flash number and severity on at least 80% of diaries. There were no differences at baseline between women who provided relevant data on at least 80% of the diaries and those who did not.
Figure 1.
CONSORT Diagram: Flow of participants through the study.
The baseline characteristics of the study sample are shown inTable 1. Study participants were predominantly white (99%), married (71%), with at least post-high school education (92%) and working full-time (65%). At baseline women reported an average of 8.12 hot flashes (SD 3.49) and 2.8 night sweats (SD 1.75)/day. As would be expected, daytime hot flash frequency and hot flash intensity were highly correlated (Spearman correlation = 0.91), consistent with Sloan et al.’s71 correlation of 0.95. The mean bother score was 3.19 (SD 0.56). Expressed in the original scale, this is between “moderately bothered” and “extremely bothered.” The mean insomnia score at baseline (11.85) was approximately at the 85th percentile for that scale,77 and above the cutoff (≥10) indicating problematic sleep disturbance.76. The sample PSS mean (23.9) at baseline was 0.5 SD above the norm for U.S. women.86 With the exception of anxiety and bother, other baseline characteristics were similar between groups. The mean MBSR arm HADS-Anxiety was significantly higher than that of the WLC (9.6 compared to 7.4; P=0.019), and was above the cut-off (≥8) for possible/probable clinical anxiety.87
Table 1.
Baseline characteristics of study groups (a)
| Characteristic | Total sample | Wait list | MBSR | P(b) | |
|---|---|---|---|---|---|
| n=110 | n= 48 | n=57 | |||
| Age (years) | 53.1 (4.9) | 53.8 (4.4) | 52.5 (5.4) | 0.169 | |
| White/Caucasian | 99 (1) | 100 (48) | 98.2 (54) | 0.534 | |
| Hispanic/Latina | 1 (1) | 0 (0) | 1.8 (1) | ||
| Marital status | Married /living with partner | 71.3 (77) | 67.9 (36) | 74.5 (41) | 0.447 |
| Single/widowed | 28.7 (31) | 32.1 (17) | 25.5 (14) | ||
| Education | High School | 8.4 (9) | 7.8 (4) | 8.9 (5) | 0.641 |
| College or some college | 56.1 (60) | 60.8 (31) | 51.8 (29) | ||
| Post graduate | 35.5 (38) | 31.4 (16) | 39.3 (22) | ||
| Employment | Working full time | 65.4 (70) | 67.9 (36) | 63.0 (34) | 0.737 |
| Working part time | 18.7 (20) | 15.1 (8) | 22.2 (12) | ||
| Retired | 4.7 (5) | 3.8 (2) | 5.6 (3) | ||
| How hard to pay for the very basics (food, heating, etc) | Not very hard at all | 75.5 (71) | 70.5 (31) | 80.0 (40) | 0.283 |
| Somewhat hard, or very hard | 24.5 (23) | 29.5 (13) | 20.0 (10) | ||
| Smoking | Current | 7.8 (8) | 6.3 (3) | 9.3 (5) | 0.910 |
| Ex-smoker | 43.1(44) | 43.8 921) | 42.6 (23) | ||
| Never | 49.0 (50) | 50 (24) | 48.1 (26) | ||
| Physical activity | None/light | 16.7 (18) | 13.7 (7) | 19.3 (11) | 0.181 |
| Moderate | 45.4 (49) | 39.2 (20) | 50.9 (29) | ||
| Intense | 38.0 (41) | 47.1 (24) | 29.8 (17) | ||
| Alcohol intake (# drinks during past month) | 3.7 (7) | 5.2 (9.5) | 2.33 (2.9) | 0.642 | |
| Soy/Isoflavone supplements (percent taking) | 6.4 (6) | 3.8 (2) | 8.9 (5) | 0.679 | |
| Had previous meditation experience | 38.3 (41) | 34.6 (18) | 41.8 (23) | 0.444 | |
| Practices yoga or tai-chi | 11.8 (11) | 11.4 (5) | 12.2 (6) | 0.895 | |
| BMI | 28.2 (6.4) | 26.53 (4.7) | 29.8 (7.3) | 0.247 | |
| Hot flashes frequency (average/day) | 8.12 (3.49) | 7.59 (3.16) | 8.16 (3.72) | 0.127 | |
| Night Sweats (average/night) | 2.8 (1.75) | 2.89 (1.92) | 2.73 (1.6) | 0.637 | |
| Hot flashes intensity score (frequency × severity) | 18.96 (9.88) | 17.37 (9.29) | 20.44 (10.25) | 0.120 | |
| Hot flashes overall bothersome score | 3.19 (0.56) | 3.07 (0.54) | 3.29 (0.55) | 0.035 | |
| Hospital Anxiety & Depression Scale-Anxiety | 8.6 (4.0) | 7.4 (3.3) | 9.6 (4.3) | 0.019 | |
| Perceived Stress | 23.9 (8.1) | 23.2 (8.6) | 24.5 (7.7) | 0.392 | |
| Insomnia Score (subjective sleep quality) | 11.9 (4.2) | 11.4 (4.0) | 12.3 (4.4) | 0.269 | |
| Overall QOL | 4.28 (1.02) | 4.22 (1.0) | 4.33 (1.1) | 0.588 | |
Values are means (SD) or % (n)
Fisher’s exact, T test or Wilcoxon Missing observations were omitted
Degree of Bother from Hot Flashes
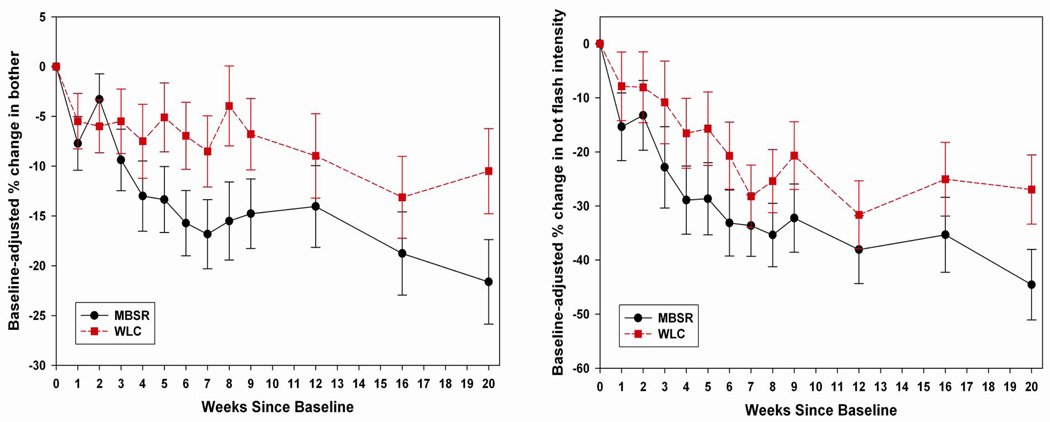
Percent change in bother, controlling for baseline bother, is illustrated in Figure 2. At the end of the intervention (week 9), the MBSR arm had decreased on average by 14.77% (95% CI 7.94% to 21.61%, P<0.0001) versus 6.79% for WLC (95% CI −0.24% to 13.81%, P=0.062) (MBSR-WLC difference P=0.116). Adjusting for baseline bother, mean bother in the MBSR participants was 2.71 (95% CI 2.49 to 2.93) and in the WLC participants was 2.82 (95% CI 2.60 to 3.04). Between weeks 9 and 20, MBSR arm bother decreased an additional 6.85% (95% CI −0.33 to 14.02, P=0.065) versus 3.71% in WLC (95% CI −3.39% to 10.81%, P=0.308) (MBSR-WLC difference P=0.544). At 20 weeks the total baseline-adjusted reduction in bother for MBSR was 21.62% (95% CI 13.29 to 29.95, P<0.0001) and 10.50% (95% CI 2.11 to 18.88, P=0.016) for WLC (MBSR-WLC difference P=0.070). Corresponding baseline-adjusted means on the original scale were 2.50 (95% CI 2.25 to 2.75) for MBSR and 2.72 (95% CI 2.47 to 2.97) for WLC, in the “slightly bothered” to “moderately bothered” range. Overall trajectories for within-woman change differed significantly by treatment arm (week × treatment arm interaction P=0.042).
Figure 2.
Mean percent change since baseline (± standard error) in hot flash bother, and hot flash intensity, adjusted for baseline values, by treatment arm, weekly during the intervention (weeks 1–9) and at 12-, 16-, and 20-week follow-up.
Daytime Hot Flash Intensity
Percent change in daytime hot flash intensity is presented in Figure 2. Overall, trajectories did not differ between treatment arms (week × treatment arm interaction P=0.692). At the end of the intervention, hot flash intensity in the MBSR arm had decreased on average by 32.25% (95% CI 19.87% to 44.63%, P<0.0001) versus 20.69% in WLC (95% CI 8.41% to 32.97%, P=0.0013) (MBSR-WLC difference P=0.198). Between weeks 9 and 20, average additional decrease for MBSR was 12.31% (95% 0.14 to 24.48, P=0.050) versus 6.28% in WLC (95% CI −5.42% to 17.97%, P=0.295) (MBSR-WLC difference P=0.485). At 20 weeks, the total baseline-adjusted reduction in hot flash intensity was 44.56% (95% CI 31.81 to 57.31, P<0.0001) for MBSR and 26.97% (95% CI 14.44, 39.50, P<0.0001) for WLC (MBSR-WLC difference P=0.057). Corresponding results for daytime hot flash frequency were very similar, as would be expected given the very high correlation between the two outcomes, and thus are not presented here.
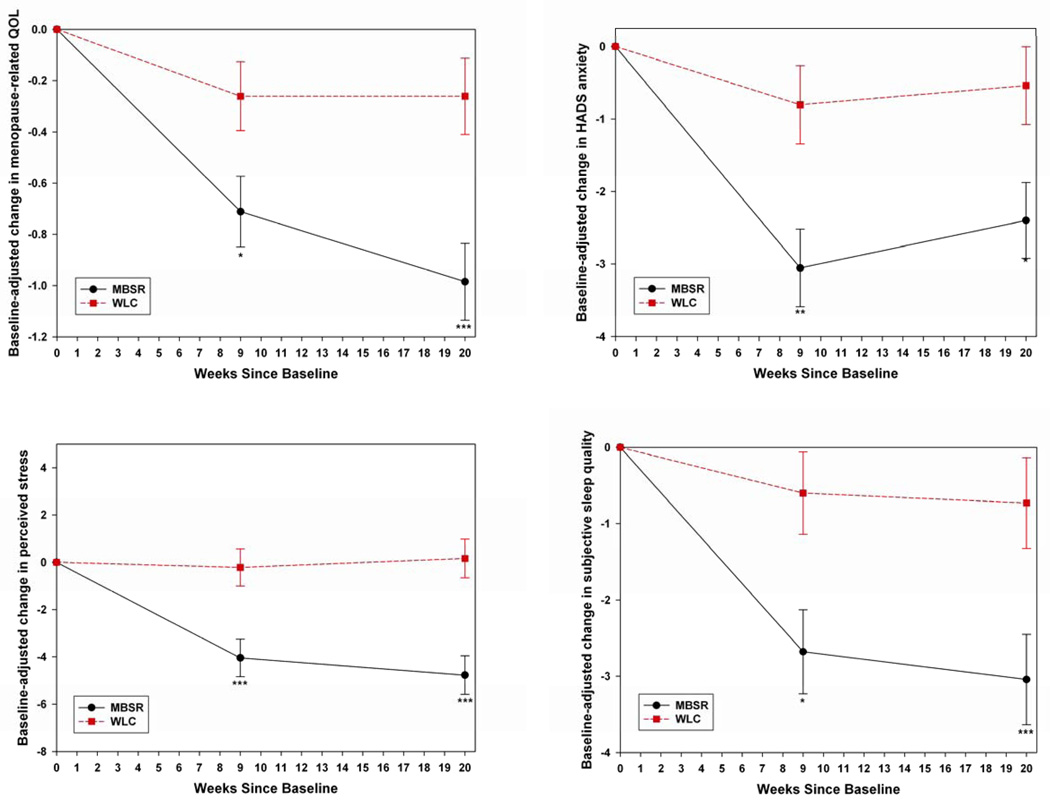
Changes in psychosocial variables, controlling for baseline values, are contained in Table 2 and illustrated in Figure 3.
Table 2.
Changes in Psychosocial Outcomes at Study Weeks 9 and 20 for MBSR and Wait-list Control Participants
| Baseline to 9 Week Change | 9 to 20 Week Change | |||||
|---|---|---|---|---|---|---|
| MBSR Arm Mean (SE) |
Control Arm Mean (SE) |
P | MBSR Arm Mean (SE) |
Control Arm Mean (SE) |
P | |
| Overall QOL | −0.71 (0.14) | −0.26 (0.13) | 0.022 | −0.27 (0.12) | 0.00 (0.12) | 0.114 |
| Sleep Quality | −2.68 (0.55) | −0.60 (0.54) | 0.009 | −0.36 (0.57) | −0.13 (0.55) | 0.766 |
| HADS Anxiety | −3.05 (0.53) | −0.80 (0.54) | 0.005 | 0.65 (0.51) | 0.26 (0.50) | 0.586 |
| Perceived Stress | −4.04 (0.80) | −0.22 (0.78) | 0.001 | −0.73 (0.70) | 0.38 (0.55) | 0.262 |
Figure 3.
Mean absolute change since baseline (± standard error) in psychosocial outcomes, adjusted for baseline values, by treatment arm at weeks 9 and 20. For between-arm differences, * indicates P<0.05, ** P<0.01, and *** P<0.001.
Quality of Life
The mean pre-post intervention (week 9) difference in overall QOL scores was significantly larger in the MBSR group. Subsequent within-woman change between 9 and 20 weeks did not differ by treatment arm. The change in the MBSR quality of life means between baseline and 20 weeks (4.3 to 3.3) represents a clinically significant improvement in overall quality of life.88
Sleep Quality
Average pre-post intervention change in sleep quality was significantly greater in MBSR than in the WLC. While subsequent within-woman change did not differ significantly by treatment arm, the difference between the group’s means at 20 weeks (11.1 WLC versus 8.9 MBSR) remained larger than the difference of 0.5 standard deviations considered clinically meaningful for this scale. The MBSR arm mean had improved to below the cutoff indicative of problematic sleep disturbance (≥10).76
HADS Anxiety
Between baseline and end of intervention, there was a significant reduction in anxiety scores in the MBSR arm compared to the WLC. The between-arm difference in mean within-woman change between weeks 9 and 20 was not significant. At the end of the intervention the MBSR mean (6.5) had decreased into the sub-clinical range (<8), and remained so at 20 weeks (7.2).
Perceived Stress
Between baseline and end of intervention, the MBSR arm had a significantly larger reduction in perceived stress than the WLC. There was no difference in the arms between weeks 9 and 20. The mean score for the MBSR arm at end of intervention (20.1) and at 20 weeks (20.4) had decreased to the normative value for US women on this scale.86
Intervention Adherence
The average MBSR class attendance was 81%. Average reported out-of class mindfulness practice was 35.0 (SD 19.6) minutes/day during the intervention, and 24.8 (SD 19.3) minutes/day during the follow-up period.
Discussion
The reported health risks associated with the use of HT2–4 have left women seeking other treatments for hot flashes. In view of the role of psychosocial factors in hot flash distress and bother,21,23–41 we tested the effect of participation in MBSR on hot flash bother, intensity, and psychological distress. The MBSR arm reported a significantly greater reduction over time in degree of overall bother from their hot flashes and night sweats compared to a wait list control group - after 9 weeks, hot flash bother had declined by an average of 14.77% in the MBSR arm, versus 6.79% in the WLC arm. This between-arm difference was maintained after the intervention (weeks 9 through 20), and at 20 weeks hot flash bother in the MBSR arm was reduced by 21.62% versus 10.50% in controls. Expressed in terms of the conceptual labels for categories of degree of bother, the sample mean at baseline was moderately/extremely bothered. At 20 weeks, baseline-adjusted means were in the “slightly bothered” to “moderately bothered” range, and trajectories for within-woman change differed significantly by treatment arm. While the clinical significance of change in bother has yet to be established, our result stands in contrast to trials of cognitive behavioral interventions to reduce hot flash bother which found no reduction.25,45 It also compares favorably with a trial of venlafaxine (a serotonin-norepinephrine reuptake inhibitor) versus placebo on hot flash frequency, which found an 18% reduction in bother (there was a 6% increase in the placebo arm) associated with the reductions in hot flash frequency.74 Significantly however, even the relatively mild side effects of the drug caused women to discontinue its use in the long term. Additionally, the 44.56% reduction in hot flash intensity at 20 weeks in the present trial compares favorably with the 49–55% (depending on dose) reduction in hot flashes intensity achieved with citalopram (an SSRI), which was recommended as an effective treatment for hot flashes.11
The clinically meaningful improvements in quality of life88 and sleep quality76 are also notable. Reductions in sleep quality occur in about 40% of women during perimenopause,89 and women experiencing distress from hot flashes report disturbed sleep37,90 that leads to fatigue and reduced quality of life.91 The results of the present study are consistent with these prior findings. The mean sleep disturbance score at baseline (11.85) was more than 1 SD above the WHIIRS norm for white women (50 – 59 years = 6.45 (SD 4.43)77) and above the scale cutoff for problematic sleep disturbance (≥10).76 At 20 weeks, the MBSR arm mean (8.8) had improved to below that cutoff. Further, the improvement represented by the difference in the MBSR WHIIRS means at baseline and 20 weeks (12.3 - 8.8 = 3.5) compares favorably with the mean reduction (5.0) in this scale over 12-weeks in women embarking on HT.92 In contrast, the sleep scores in the WLC arm did not change (11.4 - 11.1) over that period, and remained in the range for problematic sleep disturbance.
The clinical importance of stress, and the role of mental distress in hot flash frequency, severity and bother21,23,24,26,36–40 highlight our positive findings in these dimensions. Higher perceived stress scores are associated with immune dysregulation in a dose-response manner93 in older adults,94,95 and with work absenteeism.96 In this respect, the study sample perceived stress mean at baseline (23.9) was 0.5 SD above the norm for that scale for U.S. women (20.2, SD=7.8).86 At end of intervention, and at 20-weeks the MBSR arm means (20.2 and 20.4 respectively) had declined to the population norm, while the WLC arm means were unchanged from baseline (23.2, 23.3, and 23.4). The magnitude of the reduction in PSS means is comparable to other studies of MBSR with stressed populations.54 The degree of mental distress among women in the sample is suggested by the baseline HADS-Anxiety mean score (8.6) being at the clinical cut-off (≥8) for that scale.87 The MBSR arm mean score decreased from 9.6 at baseline to 6.5 at the end of intervention, and within the non-clinical range. The consistency of these findings contrast with other behavioral programs intended to affect psychological distress in perimenopausal women which have had mixed and limited results.20,35,42–47
Several other features of the results warrant comment. First, the improvement in bother from hot flashes was not accompanied by a significant reduction in hot flash intensity, suggesting that participation in MBSR may be useful to women in coping better with their existing hot flashes. Secondly, the improvements observed in the MBSR arm during the intervention were maintained at 3 months of follow-up even without post-program ‘booster’ sessions. Finally, it is noteworthy that the MBSR procedure was not altered for the purposes of the present study. Nor were the participants instructed to apply their mindfulness training in any specific way to their menopausal symptoms. Rather, participants attended the same ‘real world’ MBSR program (rather than one adapted to this population) that is widely available in the United States and Europe, and the drop out rate (12.3%) was similar to that reported in studies of MBSR with non-clinical samples (15%51,54).
This study has a number of limitations. Most notably, it did not include an active control program and so it was not possible to estimate the degree to which similar improvements in hot flash bother might result from a comparison program that incorporated such factors as the additional attention that MBSR women received through interaction with classmates and instructors. However, while studies of pharmacological treatments to reduce the frequency and/or intensity of hot flashes report a placebo effect of up to 30%,71 behavioral programs to support coping with (rather than reducing) hot flashes have not indicated that a placebo effect can be expected in a subjective variable such as bother.18 A menopause education group that was well received by perimenopausal women raised their knowledge of menopause, but had no significant impact on the bother they experienced from hot flashes.97 Additionally, the SSRI trial (referred to above)11 that found a reduction in hot flash intensity similar to that found in the present study, reported an intensity reduction in the placebo arm that was similar to that observed in our WLC arm (23% and 28% reduction, respectively). The study is further limited by the fact that only 63% of women provided 80% or more of bothersome ratings, although no differences at baseline were found between the women who provided ratings, and those who did not. Lastly, the sample was predominantly white and educated, and thus it provides no indication of the feasibility or effectiveness of MBSR for minority women.
Conclusions
Taken together, our data suggest that MBSR may be a clinically significant resource in reducing the degree of bother women experience from hot flashes and night sweats,
Acknowledgments
Research was supported by: NIH NCCAM Grant #R21 AT002910.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov identifier: NCT00317304.
Conflicts of interest/ financial disclosures: None reported.
This trial was approved by the University of Massachusetts Medical School Institutional Review Board Committee for the Protection of Human Subjects in Research – Protocol Docket #11737.
Contributor Information
James Carmody, Email: james.carmody@umassmed.edu, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
Sybil Crawford, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
Elena Salmoirago-Blotcher, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
Katherine Leung, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
Linda Churchill, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
Nicholas Olendzki, Division of Preventive and Behavioral Medicine University of Massachusetts Medical School.
References
- 1.Williams RE, et al. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62(2):153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. [see comment] JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 3.Herrington DM, Klein KP. Randomized clinical trials of hormone replacement therapy for treatment or prevention of cardiovascular disease: a review of the findings. Atherosclerosis. 2003;166(2):203–212. doi: 10.1016/s0021-9150(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, et al. Relationship between menopausal hormone therapy and risk of ductal, lobular, and ductal-lobular breast carcinomas. Cancer Epidemiology Biomarkers & Prevention. 2008;17(1):43. doi: 10.1158/1055-9965.EPI-07-0558. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, et al. Women's Health Initiative Investigators. Non-small cell lung cancer and estrogen plus progestin use in postmenopausal women in the Women's Health Initiative randomized clinical trial. Reactions. 2009;1257:4. [Google Scholar]
- 6.Ness J, Aronow WS, Beck G. Menopausal symptoms after cessation of hormone replacement therapy. Maturitas. 2006;53(3):356–361. doi: 10.1016/j.maturitas.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD, et al. Nonhormonal therapies for menopausal hot flashes systematic review and meta-analysis. JAMA. 2006;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 8.Albertazzi P. A review of non-hormonal options for the relief of menopausal symptoms. Treatments in endocrinology. 2006;5(2):101. doi: 10.2165/00024677-200605020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Krebs EE, et al. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstetrics & Gynecology. 2004;104(4):824. doi: 10.1097/01.AOG.0000140688.71638.d3. [DOI] [PubMed] [Google Scholar]
- 10.Hickey M, Saunders CM, Stuckey BGA. Non-hormonal treatments for menopausal symptoms. Maturitas. 2007;57(1):85–89. doi: 10.1016/j.maturitas.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Barton DL, et al. Phase III, Placebo-Controlled Trial of Three Doses of Citalopram for the Treatment of Hot Flashes: NCCTG Trial N05C9. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthrie JR, et al. Health care-seeking for menopausal problems. Climacteric. 2003;6(2):112–117. [PubMed] [Google Scholar]
- 13.Buist DSM, et al. Hormone therapy prescribing patterns in the United States. Obstetrics & Gynecology. 2004;104(5, Part 1):1042. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 14.Newton KM, et al. Hormone therapy initiation after the Women's Health Initiative. Menopause. 2008;15(3):487. doi: 10.1097/gme.0b013e318154b9a5. [DOI] [PubMed] [Google Scholar]
- 15.Bair YA, et al. Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women's Health Across the Nation. Menopause. 2008;15(1):32. doi: 10.1097/gme.0b013e31813429d6. [DOI] [PubMed] [Google Scholar]
- 16.Gold EB, et al. Cross-sectional analysis of specific complementary and alternative medicine (CAM) use by racial/ethnic group and menopausal status: the Study of Women's Health Across the Nation (SWAN) Menopause. 2007;14(4):612. doi: 10.1097/gme.0b013e31802d975f. [DOI] [PubMed] [Google Scholar]
- 17.Nedrow A, et al. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Archives of Internal Medicine. 2006;166(14):1453. doi: 10.1001/archinte.166.14.1453. [DOI] [PubMed] [Google Scholar]
- 18.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. [see comment] Annals of Internal Medicine. 2002;137(10):805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JS, Neal JG. Other complementary and alternative medicine modalities: acupuncture, magnets, reflexology, and homeopathy. The American Journal of Medicine. 2005;118(12S2):109–117. doi: 10.1016/j.amjmed.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: a systematic review. Menopause. 2008;15(1):193. doi: 10.1097/gme.0b013e31805c08dc. [DOI] [PubMed] [Google Scholar]
- 21.Hanisch LJ, et al. Hot flashes and panic attacks: a comparison of symptomatology, neurobiology, treatment, and a role for cognition. Psychological bulletin. 2008;134(2):247. doi: 10.1037/0033-2909.134.2.247. [DOI] [PubMed] [Google Scholar]
- 22.Thurston RC, et al. Association between hot flashes, sleep complaints, and psychological functioning among healthy menopausal women. International Journal of Behavioral Medicine. 2006;13(2):163–172. doi: 10.1207/s15327558ijbm1302_8. [DOI] [PubMed] [Google Scholar]
- 23.Freeman EW, et al. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12(3):258. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 24.Thurston RC, et al. Emotional Antecedents of Hot Flashes During Daily Life. Psychosomatic Medicine. 2005;67(1):137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 25.Keefer L, Blanchard EB. A behavioral group treatment program for menopausal hot flashes: Results of a pilot study. Applied psychophysiology and biofeedback. 2005;30(1):21–30. doi: 10.1007/s10484-005-2171-1. [DOI] [PubMed] [Google Scholar]
- 26.Gold EB, et al. Longitudinal Analysis of the Association Between Vasomotor Symptoms and Race/Ethnicity Across the Menopausal Transition: Study of Women's Health Across the Nation. American Journal of Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delmonte MM. Some cognitive aspects of meditation practice. Perceptual & Motor Skills. 1983;57(3, Pt 2):1160–1162. doi: 10.2466/pms.1983.57.3f.1160. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson DW, Delprato DJ. Multiple component self-control program for menopausal hot flashes. Journal of Behavior Therapy and Experimental Psychiatry. 1983;14(2):137–140. doi: 10.1016/0005-7916(83)90031-9. [DOI] [PubMed] [Google Scholar]
- 29.Ballinger S. Psychosocial stress and symptoms of menopause: a comparative study of menopause clinic patients and non-patients. Maturitas. 1985;7:315–327. doi: 10.1016/0378-5122(85)90055-6. [DOI] [PubMed] [Google Scholar]
- 30.Gannon L, Hansel S, Goodwin J. Correlates of menopausal hot flashes. J Beh Med. 1987;10(3):277–285. doi: 10.1007/BF00846541. [DOI] [PubMed] [Google Scholar]
- 31.Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychology. 1990;9(5):529–545. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- 32.Hunter M, Liao K. A psychological analysis of menopausal hot flushes. British Journal of Clinical Psychology. 1995;34:589–599. doi: 10.1111/j.2044-8260.1995.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 33.Nedstrand E, et al. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas. 1998;31(1):29–34. doi: 10.1016/s0378-5122(98)00058-9. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds F. Relationships between catastrophic thoughts, perceived control and distress during menopausal hot flushes: exploring the correlates of a questionnaire measure. Maturitas. 2000;36(2):113–122. doi: 10.1016/s0378-5122(00)00142-0. [DOI] [PubMed] [Google Scholar]
- 35.Carmody J, Crawford S, Churchill L. A pilot study of mindfulness-based stress reduction for hot flashes. Menopause. 2006;13(5):760–769. doi: 10.1097/01.gme.0000227402.98933.d0. [DOI] [PubMed] [Google Scholar]
- 36.Avis NE, et al. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women's Health Study. Annals of Epidemiology. 1994;4(3):214–220. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 37.Bromberger JT, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) Journal of affective disorders. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosworth HB, et al. Depressive symptoms, menopausal status, and climacteric symptoms in women at midlife. Psychosomatic Medicine. 2001;63(4):603–608. doi: 10.1097/00006842-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Gallicchio L, et al. Correlates of depressive symptoms among women undergoing the menopausal transition. Journal of Psychosomatic Research. 2007;63(3):263–268. doi: 10.1016/j.jpsychores.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Dennerstein L, et al. A population-based study of depressed mood in middle-aged, Australian-born women. Menopause. 2004;11(5):563. doi: 10.1097/01.gme.0000113844.74462.f6. [DOI] [PubMed] [Google Scholar]
- 41.Hunter M, Rendall M. Bio-psycho-socio-cultural perspectives on menopause. Best Practice & Research Clinical Obstetrics & Gynaecology. 2007;21(2):261–274. doi: 10.1016/j.bpobgyn.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Keefer L, Blanchard EB. Hot flash, hot topic: conceptualizing menopausal symptoms from a cognitive-behavioral perspective. Applied psychophysiology and biofeedback. 2005;30(1):75–82. doi: 10.1007/s10484-005-2176-9. [DOI] [PubMed] [Google Scholar]
- 43.Nedstrand E, et al. Applied relaxation and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Maturitas. 2005;51(2):154–162. doi: 10.1016/j.maturitas.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 44.Booth-LaForce C, Thurston RC, Taylor MR. A pilot study of a Hatha yoga treatment for menopausal symptoms. Maturitas. 2007;57(3):286–295. doi: 10.1016/j.maturitas.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter JS, et al. Cognitive-behavioral intervention for hot flashes. Oncology Nursing Forum. 2007;34(1):1–8. doi: 10.1188/07.ONF.E1-E8. [DOI] [PubMed] [Google Scholar]
- 46.Avis NE, et al. A randomized, controlled pilot study of acupuncture treatment for menopausal hot flashes. Menopause. 2008;15(6):1070. doi: 10.1097/gme.0b013e31816d5b03. [DOI] [PubMed] [Google Scholar]
- 47.Alder J, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychotherapy and psychosomatics. 2006;75(5):298. doi: 10.1159/000093951. [DOI] [PubMed] [Google Scholar]
- 48.National Institutes of Health. National Institutes of Health State-of-the-Science Conference Statement: Management of Menopause-Related Symptoms. Ann Intern Med. 2005;142(12, Part 1):1003–1013. [PubMed]
- 49.Carmody J. Evolving Conceptions of Mindfulness in Clinical Settings. Journal of Cognitive Psychotherapy. 2009;23(3):270–280. [Google Scholar]
- 50.Teasdale J. Metacognition, mindfulness and the modification of mood disorders. Clinical Psychology and Psychotherapy. 1999;6:146–155. [Google Scholar]
- 51.Grossman P, et al. Mindfulness-based stress reduction and health benefits: A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 52.Carlson LE, et al. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29(4):448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 53.Carlson LE, Garland SN. Impact of Mindfulness-Based Stress Reduction (MBSR) on Sleep, Mood, Stress and Fatigue Symptoms in Cancer Outpatients. International Journal of Behavioral Medicine. 2005;12(4):278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 54.Carmody J, Baer RA. Relationships between mindfulness practice and Levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- 55.Tacon AM, et al. Mindfulness meditation, anxiety reduction, and heart disease: a pilot study. Family & Community Health. 2003;26(1):25–33. doi: 10.1097/00003727-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Hofmann SG, et al. The Effect of Mindfulness-Based Therapy on Anxiety and Depression: A Meta-Analytic Review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabat-Zinn J. Psychosocial Factors: Their Importance and Management. In: Ockene IS, Ockene JK, editors. Prevention of Coronary Heart Disease. Boston: Little Brown; 1992. [Google Scholar]
- 58.Miller J, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 59.Soules MR, et al. Executive summary: stages of reproductive aging workshop (STRAW) Fertility and Sterility. 200l;76(5):874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 60.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 61.Gold EB, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. American Journal of Epidemiology. 2000;152(5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 62.Freeman EW, et al. Psychometric properties of a menopausal symptom list. Menopause. 2003;10(3):258–265. doi: 10.1097/00042192-200310030-00014. [DOI] [PubMed] [Google Scholar]
- 63.Faure ED, Chantre P, Mares P. Effects of a standardized soy extract on hot flushes: a multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2002;9(5):329–334. doi: 10.1097/00042192-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Tice JA, et al. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. [see comment] JAMA. 2003;290(2):207–214. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 65.Guttuso TJ, et al. Gabapentin's Effects on Hot Flashes in Postmenopausal Women: A Randomized Controlled Trial. [Article] Obstetrics & Gynecology. 2003;101(2):337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 66.van de Weijer PH, Barentsen R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas. 2002;42(3):187–193. doi: 10.1016/s0378-5122(02)00080-4. [DOI] [PubMed] [Google Scholar]
- 67.Sowers MF, et al. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 68.Dudley EC, et al. Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric. 1998;1(1):18–25. doi: 10.3109/13697139809080677. [DOI] [PubMed] [Google Scholar]
- 69.Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatric Clinics of North America. 2003;26(3):563–580. doi: 10.1016/s0193-953x(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 70.WHO, World Health Organization Scientific GroupTech, and S.R.S.N. WHO. Geneva: 1966. Research on the menopause in the 1990s. [Google Scholar]
- 71.Sloan JA, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19(23):4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 72.Kabat-Zinn J. Full catastrophe living: using the wisdom of your body and mind to face stress, pain and illness. New York: Delacorte; 1990. [Google Scholar]
- 73.Carpenter JS, et al. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncology Nursing Forum Online. 2002;29(3):E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 74.Carpenter JS, et al. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. The Oncologist. 2007;12(1):124. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 75.Hilditch JR, et al. A comparison of the effects of oral conjugated equine estrogen and transdermal estradiol-17 beta combined with an oral progestin on quality of life in postmenopausal women. Maturitas. 1996;24(3):177–184. doi: 10.1016/s0378-5122(96)82007-x. [DOI] [PubMed] [Google Scholar]
- 76.Levine DW, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale (WHIIRS) Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 77.Levine DW, et al. Factor structure and measurement invariance of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 78.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 79.Bjelland I, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 80.Snaith RP. The hospital anxiety and depression scale. Health and quality of life outcomes. 2003;1(1):29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olssøn I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC psychiatry. 2005;5(1):46. doi: 10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohen S, Kamark T, Mermelstern R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 83.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley and Sons; 2004. [Google Scholar]
- 84.Chuang-Stein C, Tong DM. The impact and implication of regression to the mean on the design and analysis of medical investigations. Statistical Methods in Medical Research. 1997;6(2):115. doi: 10.1177/096228029700600203. [DOI] [PubMed] [Google Scholar]
- 85.Pocock SJ, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21(19):2917–2930. doi: 10.1002/sim.1296. [DOI] [PubMed] [Google Scholar]
- 86.Cohen S, Williamson C. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Sage: 1988. [Google Scholar]
- 87.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. Journal of psychosomatic research. 1997;42(1):17. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 88.Lewis JE, Hilditch JR, Wong CJ. Further psychometric property development of the Menopause-Specific Quality of Life questionnaire and development of a modified version, MENQOL-Intervention questionnaire. Maturitas. 2005;50(3):209–221. doi: 10.1016/j.maturitas.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30(1):41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 90.Dennerstein L, et al. A prospective population-based study of menopausal symptoms. Obstetrics & Gynecology. 2000;96(3):351–358. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 91.Kravitz H, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 92.Levine DW, et al. Validation of the Women's Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosomatic medicine. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 93.Cohen S, Janicki-Deverts D, Miller GE. Psychological Stress and Disease. JAMA. 2007;298(14):1685. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 94.Goldman N, et al. Perceived stress and physiological dysregulation in older adults. Stress. 2005;8(2):95–105. doi: 10.1080/10253890500141905. [DOI] [PubMed] [Google Scholar]
- 95.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 96.Jacobson BH, et al. The relationship between perceived stress and self-reported illness-related absenteeism. American journal of health promotion: AJHP. 1996;11(1):54–61. doi: 10.4278/0890-1171-11.1.54. [DOI] [PubMed] [Google Scholar]
- 97.Hunter M, O'Dea I. An evaluation of a health education intervention for mid-aged women: five year follow-up of effects upon knowledge, impact of menopause and health. Patient Education and Counseling. 1999;38(3):249. doi: 10.1016/s0738-3991(98)00143-8. [DOI] [PubMed] [Google Scholar]