Abstract
Sirtuin-1 (SirT1) is a nutrient-sensing deacetylase whose levels and activity increase with caloric restriction to preserve euglycemia and promote efficient energy utilization. Focusing on data obtained in vivo, we review how SirT1 orchestrates the adaptive response to fasting by stimulating hepatic gluconeogenesis and fatty acid oxidation, increasing circulating adiponectin levels, and limiting immune activation. Finally, we consider its viability as a therapeutic target for the treatment of type 2 diabetes.
Keywords: SirT1, Sirtuin-1, Glucose Metabolism, Lipid Metabolism, Insulin Resistance, Type 2 Diabetes
Background
Sirtuin 1 (SirT1), the mammalian homolog of yeast silent information regulator 2 (Sir2), is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase that influences a diverse assortment of cellular processes through interactions with targets such as p53, NFκB, PGC-1α, FOXOs, and the histones H3 and H4 [1]. In the process of removing acetyl groups from acetyl-lysine protein residues, the SirT1/Sir2-catalyzed reaction hydrolyzes NAD+ to yield 2′-O-acetyl-ADPR and nicotinamide [2]. This unusual requirement for NAD+ and the knowledge that Sir family members influence longevity in yeast [3] led early investigators to hypothesize that the expression and activity of these enzymes might be regulated by changes in cellular energy/redox status (e.g. an increase in the NAD+/NADH ratio) and account for the increases in lifespan observed during caloric restriction [4]. Subsequent work, with some exceptions [5], substantiated this notion, showing that Sir2 levels increase with nutrient deprivation, and that caloric restriction does not extend lifespan in Sir2/SirT1 deficient Drosophila, yeast, or mice [6–11]. Furthermore, overexpression of Sir2 in Drosophila increases lifespan, and this increase is not further augmented by caloric restriction [10].
As a result of these findings in model organisms, there has been a surge in interest in understanding the role of SirT1 in mammals, with the hope of developing new therapies to combat diseases of aging [12]. Although this work is incomplete, existing data support the contention that mammalian SirT1 expression and activity are regulated by nutrient availability, and orchestrate the adaptive response to caloric restriction. Given that many of the metabolic changes wrought by reduced energy intake are beneficial--increases in circulating adiponectin levels, improved lipid profiles, and decreased inflammation--SirT1 based therapies may hold promise for the treatment of insulin resistance and type 2 diabetes [13].
SirT1, gluconeogenesis and fatty acid oxidation
During states of negative energy balance such as fasting or prolonged caloric restriction, a key function of the liver is to produce glucose through glycogenolysis and gluconeogenesis in order to maintain euglycemia. In the latter case, the coordinated actions of transcription factors, such as cyclic AMP response element binding protein (CREB), CREB regulated transcription coactivator 2 (CRTC2), forkhead box O1 (FOXO1), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), induce expression of essential gluconeogenic enzymes, including glucose-6-phosphatase (G6Pase), fructose-1,6-bisphosphatase (FBPase) and phosphoenolpyruvate carboxylase kinase (PEPCK) [14–16] to increase gluconeogenic capacity. SirT1 intercedes in this process by deacetylating FOXO1 and PGC-1α, thereby increasing their ability to promote transcription of their gluconeogenic targets and inhibit the expression of glycolytic genes such as glucokinase [17]. Additionally, SirT1 antagonizes STAT3-mediated repression of gluconeogenesis by deacetylating STAT3 and in turn decreasing its phosphorylation [18]. Accordingly, reduction of hepatic SirT1 gene expression in mice using viral techniques in vivo results in lower expression of gluconeogenic genes and mild hypoglycemia, whereas overexpression of SirT1 results in mild hyperglycemia [19]. This finding was also observed in studies where, in a rat model of type 2 diabetes, in vivo knockdown of SirT1 using antisense oligonucleotides (ASOs) reduced hepatic mRNA expression of gluconeogenic enzymes (PEPCK, FBPase, and G6Pase) as a result of increased STAT3, FOXO1, and PGC-1α acetylation. These changes were associated with reduced plasma glucose concentrations and improved whole body insulin sensitivity that was entirely attributable to an increased hepatic responsiveness to insulin [20]. Interestingly, during prolonged starvation, SirT1 downregulates the expression of key gluconeogenic genes due to deacetylation and subsequent ubiquitination of CRTC2 [21]. Further research is necessary to delineate the opposing roles of SirT1 in acute fasting/caloric restriction and starvation.
In addition to its role in controlling carbohydrate metabolism, SirT1 is also a key regulator of hepatic lipid metabolism. It was reported that hepatic deletion of SirT1 protected mice from high-fat diet induced glucose intolerance by reducing lipid synthesis and subsequently reducing fasting plasma glucose and fed insulin concentrations [22]. However, these findings were not seen in a separate liver-specific SirT1 KO study [23], a discrepancy that was attributed to differences in dietary fat and cholesterol content [4]. In this second model [23], the notable metabolic phenotype was a profound decrease in hepatic expression of peroxisome proliferator-activated receptor alpha (PPARα) target genes that support increased fatty acid oxidation, resulting in elevated levels of triglyceride, fatty acids, and cholesterol in liver. The authors went on to show that SirT1/PPARα interaction is required for the appropriate stimulation of PPARα regulated genes [23].
SirT1 has also been shown to regulate hepatic lipid metabolism via the activation of AMPK and PGC-1α. Adenovirus-mediated overexpression of SirT1 led to an approximately two-fold increase in phospho-AMPK levels in both HepG2 cells and livers of C57BL/6 mice and prevented hepatic lipid accumulation in HepG2 cells [24]. Similarly, viral knockdown of SirT1 increased hepatic fatty acid and cholesterol levels, and was associated with enhanced PGC-1α acetylation [19]. Activation of AMPK, PGC-1α, and PPARα are essential events in the liver during times of fasting and caloric restriction, facilitating expression of genes that allow for efficient lipid handling and oxidation [25]. These data suggest that SirT1 is a critical mediator of this adaptive response and therefore central to the maintenance of hepatic lipid balance.
In further support of this hypothesis, three distinct in vivo mouse models of SirT1 overexpression are protected from high-fat diet induced steatosis in a manner that is consistent with increased AMPK, PGC-1α, and PPARα activity [26–28]. In two models where glucose tolerance was assessed after high-fat feeding, improved insulin sensitivity was observed in SirT1 overexpressing animals [26, 28]. Although this appears at odds with data showing that SirT1 is a positive regulator of gluconeogenesis, we believe these findings to be compatible for two reasons: First, hepatic lipid overload is a known insulin resistance trigger [29–32], and in the setting of chronic high fat feeding, the insulin sensitizing effects of lower liver lipids predominates over direct effects on gluconeogenic gene transcription. Second, whole-body SirT1 overexpression increases the production of insulin-sensitizing hormones, such as adiponectin, which positively impact hepatic insulin sensitivity via activation of AMPK and prevention of ectopic lipid deposition [33]. When hepatocytes are isolated from SirT1 overexpressing mice and stimulated with cAMP, higher gluconeogenic gene expression is observed, corroborating the effects of acute SirT1 overexpression or knockdown in liver [26, 28].
Recent reports also suggest that SirT1 may reduce hepatic lipogenesis by inhibiting the activity of SREBP-1c. Deacetylated SREBP-1 has reduced affinity for the promoters of its lipogenic target genes, and increased susceptibility to ubiquitination and degradation. The prevention of lipogenesis via SREBP-1 antagonism may be a complementary means by which increases in SirT1 expression and activity prevent steatosis during high fat feeding as well as a physiologic function of the enzyme to block lipogenesis during the fasted state [34, 35].
Taken together, these results indicate that SirT1 expression and/or activity increase with fasting to increase expression of fatty acid oxidative and gluconeogenic genes while decreasing expression of lipogenic genes; and that in the context of high fat feeding, the beneficial effects of SirT1 on lipid homeostasis predominate, leading to an overall improvement in hepatic insulin sensitivity. Thus, SirT1 is a key regulator of the hepatic response to fasting and caloric restriction.
SirT1, adiponectin, and metabolic thrift
During times of fasting and caloric restriction, mammals dramatically increase their metabolic efficiency, reducing metabolic rate, decreasing locomotor activity, and conserving fat mass [36]. The adipocyte-derived hormone adiponectin, whose abundance in circulation is elevated by caloric restriction and suppressed by overnutrition, is an important molecular mediator of these adaptations. Modest two-fold overexpression of adiponectin on the ob/ob background results in dramatic increases in body weight (~60 g in controls versus ~100 g in overexpressers) and adiposity in association with decreases in locomotor activity and metabolic rate. Remarkably, despite their massive obesity, the adiponectin overexpressers have levels of blood glucose, insulin, and triglycerides indistinguishable from those of lean animals, highlighting the therapeutic potential of strategies that increase adiponectin levels to treat insulin resistance and type 2 diabetes [37].
Keeping with its putative role as an important sensor and coordinator of the mammalian response to caloric restriction, SirT1 stimulates adiponectin production. In differentiated 3T3-L1 adipocytes, SirT1-mediated FOXO1 deacetylation enhances its association with C/EBP, augmenting adiponectin transcription [38]. Recently, this observation was corroborated in vivo using BAC-mediated whole-body overexpression of SirT1 (roughly a two-fold increase). In three models--regular chow fed, high fat chow fed, and regular chow fed crossed to the db/db background--SirT1 overexpression led to ~40% higher circulating levels of adiponectin [26], whereas in vivo knockdown of SirT1 in adipose tissue causes a large decrease in plasma adiponectin concentrations [20].
This line of SirT1 overexpressing mouse also exhibits reduced levels of voluntary locomotor activity and oxygen consumption. And, as expected, it is protected from high-fat diet induced or leptin receptor-mutation induced glucose intolerance. Moreover, on the db/db background, the SirT1 overexpressing mouse has a significantly higher body weight at 15 weeks of age that also may be attributable to elevated adiponectin levels [26].
A diametrically opposite phenotype is seen in whole-body SirT1−/− mice, however. Although metabolic phenotyping data from this strain call for cautious interpretation due to the extensive developmental defects observed in homozygous knockouts (craniofacial malformations, skin inflammation, and small size), they are complementary to observations made in SirT1 overexpressing animals. Instead of being more energy efficient, SirT1−/− animals consume similar amounts of calories as wild-type controls, despite being 30–50% smaller, with this relative hyperphagia driven by large increases in whole-body oxygen consumption [6].
Collectively, these observations show that SirT1 is an important participant in the switch from the ad libitum fed to the calorie-restricted condition in adipose tissue as well as the liver, spurring the production of efficiency promoting endocrine factors and transcriptional changes that improve organismal adaptation to energy shortages.
SirT1 and inflammation
For reasons that remain inadequately understood, obesity, insulin resistance, and type 2 diabetes are accompanied by a state of low-grade inflammation. Activation of NFκB and MAPK, central effectors in the inflammatory cascade, is observed in liver and adipose tissue in these conditions [39], and is accompanied by recruitment of cells of the immune system, such as monocytes and macrophages [40]. Although the extent and mechanism of its contribution to the pathogenesis of type 2 diabetes is debated, this inflammatory cascade is unquestionably capable of influencing insulin sensitivity, likely through local cytokine effects on insulin sensitive tissues, but perhaps more importantly through cytokine-induced lipolysis, which leads to ectopic lipid accumulation and resultant insulin resistance [41].
Interestingly, the genomic region flanking SirT1 in both mice [42] and humans [43] contains several NFκB binding elements, suggesting that it is regulated by NFκB activation. Moreover, SirT1 has been shown to deacetylate NFκB p65 [44] (reducing its ability to induce expression of its targets) and histones to silence the expression of inflammatory genes [45, 46]. Given the apparent reciprocal relationship between SirT1 and NFκB and the marked suppression of SirT1 expression during insulin resistance and obesity [47, 48], SirT1 may be an important contributor to the etiology of the accompanying low-grade inflammatory state.
In agreement with this idea, a conserved observation between different research groups studying SirT1 is that animals with altered levels of SirT1 expression or activity exhibit inflammatory phenotypes. For instance, the SirT1−/−mouse develops an autoimmune condition, featuring the accumulation of immunoglobulin aggregates in liver and kidney [49], which may be explained in part by the recently identified role for SirT1 in regulating T-cell tolerance [50]. Dysregulation of inflammatory processes is also observed in tissue-specific knockouts of SirT1. Selective deletion of SirT1 in liver and subsequent microarray experiments identified elevated expression of NFκB pathway components and qPCR experiments confirmed increased levels of cytokines [23]. This result is consistent with four recent observations: (1) increased inflammation in liver and fat of fat-fed SirT1+/− mice [51]; (2) increased numbers of activated macrophages in liver and fat from fat-fed myeloid-specific SirT1−/− mice [52]; (3) decreased cytokine expression in livers of fat-fed SirT1/Dnajc12 overexpressing mice and an altered response to lipopolysaccharide (LPS) treatment [28]; and (4) decreased Kupffer cell cytokine production capacity and inflammation in livers from fat-fed DBC1−/− mice, which have constitutively high levels of SirT1 activity [27]. Thus, it is probable that the reduction in SirT1 activity accompanying obesity, insulin resistance and type 2 diabetes may contribute to not only low-grade inflammation, but also overt inflammatory pathologies such as nonalcoholic steatohepatitis (NASH).
The vexing question that emerges from these observations is: why would natural selection favor a system where inflammation is regulated by nutrient availability? In the case of caloric restriction, a reasonable explanation for this is that immune activation is energetically expensive. It has been shown in bumblebees that artificial activation of the immune system significantly increases mortality in nutrient-limited settings [53]. Thus, the robust inhibitory effect of SirT1 on NFκB activity is consistent with its role in regulating the fate of available nutrients during times of energy crisis, diverting calories away from the immune system to spare them for essential survival processes. However, why overnutrition causes inflammation remains a mystery. One possibility is that cells of the immune system and the cytokines they generate are bona fide metabolic regulators (e.g. effectors of adipose tissue remodeling, etc.) and that obesity-associated inflammation is intended. Another is that SirT1 is a dual-purpose protein that is nutrient regulated but also serves as an epigenetic inflammatory checkpoint. In this scenario, negative regulation of SirT1 by overnutrition would incidentally decrease the activation threshold required for inflammatory gene transcription. At present, however, the purpose of SirT1’s interaction with inflammatory networks is unknown, as are the circumstances in which this interaction might occur outside of obesity and caloric restriction.
SirT1 in the brain and beyond
A role was also recently proposed for SirT1 in the neural control of food intake. Genetic deletion of SirT1 in orexigenic Agrp neurons, and administration of EX527, a SirT1 inhibitor, were found to reduce food intake and body weight in rodents via the melanocortin pathway [54, 55]. This putative orexigenic function of SirT1 is congruent with its increased expression and activity in the hypothalamus [56] (including in nuclei critical to the regulation of energy balance [57]) during fasting. However, knockout of SirT1 in anorexigenic POMC neurons leads to obesity, arguing against it functioning exclusively as an orexigenic molecule in the hypothalamus [58]. One possibility is that regulation of its expression and activity is population specific. Another is that SirT1 is required for neuronal homeostasis (e.g. NFκB repression) and deleting it in an anorexigenic cell type causes obesity while deleting it in an orexigenic cell type causes leanness by disrupting the normal biology of these circuits. Future research will be required to differentiate between these possibilities.
Finally, β-cell specific SirT1 overexpression increases glucose-stimulated insulin secretion by repressing UCP2, while SirT1 loss of function has the opposite effect [59, 60]. Because SirT1 decreases in the pancreas with fasting while UCP2 increases, this mechanism may explain the attenuation of insulin secretion caused by caloric restriction. However, the SirT1−/− mouse has normal postprandial glucose levels [6], and wild-type (or lower) levels of insulin were noted in two strains of whole body SirT1 overexpressing mice fed either regular chow or high fat diet [26, 28]. Thus, more work is required to quantify the extent of pancreatic SirT1 ‘s contribution to the maintenance of glucose homeostasis.
SirT1 as a therapeutic target for metabolic disease
The prevalence of metabolic disease has been increasing steadily worldwide in recent years, making the need for new therapies pressing. Despite the complex regulation of metabolic homeostasis by SirT1, data from in vivo overexpression models and DBC1−/− mice strongly suggest that increasing SirT1 expression and/or activity improves glucose tolerance under conditions of metabolic stress produced by dietary or genetic overnutrition. Because SirT1 expression is suppressed in obesity and type 2 diabetes and activation or increased expression of SirT1 stimulates hepatic fatty acid oxidation, augments insulin secretion, increases circulating adiponectin levels, and reduces inflammation, discovery of SirT1 activators would allow clinicians to treat multiple features of diabetes simultaneously. As a result, the engineering of sirtuin activating compounds (STACs) has become a subject of intense interest.
Indeed, soon after the life-extending properties of Sir2 were described in model organisms, resveratrol, a polyphenol found in red wine, was reported to activate the enzymatic activity of sirtuins [61]. Subsequent studies in which rodents fed a high-fat, high-calorie diet supplemented with resveratrol indicated that this molecule could diminish metabolic pathology caused by such feeding regimens [62, 63]. These findings led to development of the SRT series of compounds for treatment of type 2 diabetes, and publications showing their benefits in ameliorating metabolic disease in rodents [64, 65]. However, the apparent ability of resveratrol [66, 67] and the SRT family of compounds to stimulate SirT1 enzymatic activity requires the presence of a fluorophore in the substrate peptide employed in the in vitro assay, indicating that the original observations that these compounds were SirT1 activators was likely an assay artifact [68]. Although some SRT compounds stimulate SirT1 activity against acetyl-4/5mers comprised of only unmodified amino acids via an allosteric mechanism, the significance of this finding is uncertain given that the sequence of these short polypeptides no longer matches the SirT1 deacetylation site surrounding Lys382 of p53 [69]. In summary, despite the bioactivity and promise of resveratrol and SRT1720 in vivo as future therapeutics, their effects on metabolism do not appear to be caused by specific activation of SirT1.
Thus, the key challenge in harnessing the clinical promise of SirT1 is to develop activators that specifically stimulate its activity against native substrates. However, even if this project is ultimately successful, it is also likely that SirT1 regulation of metabolism may be far more complex than is currently appreciated, and increasing SirT1 activity could induce global histone deacetylation in many tissues where this may not be beneficial. For example, histone deacetylase inhibitors (HDACs) are currently used to treat psychiatric disorders and some forms of cancer. Thus, while exciting, SirT1-activating therapies should be pursued with caution and in a tissue targeted manner.
Concluding remarks
SirT1 is a nutrient-responsive protein that facilitates the whole-body response to reduced food availability by inducing hepatic gluconeogenesis, fatty acid oxidation and adiponectin production while repressing lipogenesis and inflammation. The induction of SirT1 by caloric restriction and salubrious effects of increased SirT1 on aging are conserved from yeast to humans, indicating that it provides a useful means of coping with energy stress for a diverse array of species. Nevertheless, despite a decade of intense work by many groups, and the emergence of the broad picture of SirT1 functions in metabolic control, much awaits discovery.
One important endeavor will be to identify genes regulated by SirT1 at the level of histone acetylation in metabolic tissues, which in turn should refine our understanding of SirT1’s coordination of nutrient homeostasis. While much effort has been focused on SirT1’s role in deacetylating important metabolic transcription factors, epigenetic effects of SirT1 are largely unexplored. Learning more about SirT1’s epigenetic targets will help illuminate other tissue-specific roles of this enzyme in vivo by exposing large classes of genes it regulates (e.g. NFκB). To support these efforts, it is necessary to study the effects of selectively and inducibly perturbing SirT1 expression in tissues important for the preservation of glucose and lipid homeostasis, such as liver, skeletal muscle, adipose tissue and macrophages in vivo, as in vitro studies have indicated an important role for SirT1 in these tissue types [48, 70, 71]. This approach will clarify the tissues and junctures responsible for the complex phenotypes observed in existing SirT1 knockout and transgenic animals. Finally, more work is needed to identify other mechanisms that regulate SirT1 activity and expression, encompassing transcriptional, post-transcriptional and post-translational factors. AROS and DBC1 have been identified as posttranslational regulators of SirT1 activity [72–74], and PPARδ as a transcriptional regulator of SirT1 expression [75], but there are likely others that control how its expression, intracellular targeting and activity are altered by energy status, high fat feeding or specific nutrients. Hopefully these and other future studies will provide additional insights into the metabolic functions of this enigmatic enzyme that lead to novel therapies for metabolic diseases.
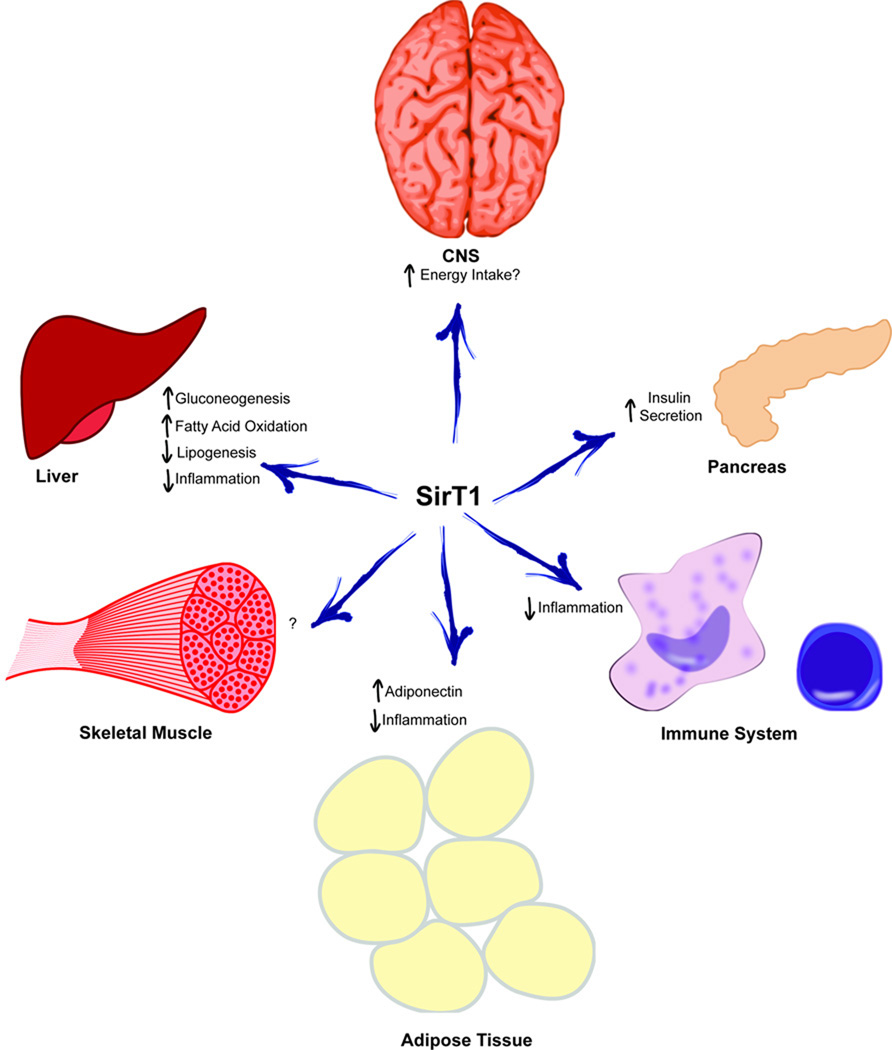
Figure.
Overview of SirT1 regulated processes in metabolically important tissues.
- What is the primary mechanism behind insulin sensitization evoked by whole body overexpression of SirT1?
- What genetic networks does SirT1 regulate at the level of histone deacetylation in metabolic tissues?
- What additional proteins/metabolites control SirT1 abundance and activity?
- Can pharmacological strategies be developed that augment SirT1 activity and/or abundance?
Acknowledgements
The authors would like to thank Maya Kotas for helpful discussions. This work was supported by grants from the United States Public Health Service: R01 DK-40936, R01 DK-49230, U24 DK-59635, P-30 DK-45735.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 3.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci. 2010;31(5):212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2(9):E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boily G, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3(3):e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120(4):473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289(5487):2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 9.Lin SJ, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418(6895):344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 10.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298(5599):1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 12.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7(10):841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 13.Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci U S A. 2001;98(19):10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 15.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 16.Erion DM, et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. (Translated from eng) Cell Metab. 2009;10(6):499–506. doi: 10.1016/j.cmet.2009.10.007. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 18.Nie Y, et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat Cell Biol. 2009;11(4):492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104(31):12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erion DM, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc Natl Acad Sci U S A. 2009;106(27):11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D, et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008;22(13):1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purushotham A, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou X, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283(29):20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto T, et al. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem. 2000;275(37):28918–28928. doi: 10.1074/jbc.M910350199. [DOI] [PubMed] [Google Scholar]
- 26.Banks AS, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8(4):333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escande C, et al. Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J Clin Invest. 2010;120(2):545–558. doi: 10.1172/JCI39319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16(4):400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi T, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 34.Ponugoti B, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010 doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker AK, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24(13):1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hambly C, Speakman JR. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes Res. 2005;13(9):1548–1557. doi: 10.1038/oby.2005.190. [DOI] [PubMed] [Google Scholar]
- 37.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281(52):39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 39.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138(5):961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahlknecht U, Voelter-Mahlknecht S. Chromosomal characterization and localization of the NAD+-dependent histone deacetylase gene sirtuin 1 in the mouse. Int J Mol Med. 2009;23(2):245–252. [PubMed] [Google Scholar]
- 43.Voelter-Mahlknecht S, Mahlknecht U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med. 2006;17(1):59–67. [PubMed] [Google Scholar]
- 44.Yeung F, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawahara TL, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaquero A, et al. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16(1):93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen SB, Olholm J, Paulsen SK, Bennetzen MF, Richelsen B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int J Obes (Lond) 2008;32(8):1250–1255. doi: 10.1038/ijo.2008.78. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizaki T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29(5):1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sequeira J, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314(16):3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119(10):3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu F, et al. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology. 2010;151(6):2504–2514. doi: 10.1210/en.2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schug TT, et al. Myeloid Deletion of SIRT1 Induces Inflammatory Signaling in Response to Environmental Stress. Mol Cell Biol. 2010;30(19):4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290(5494):1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich MO, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30(35):11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cakir I, et al. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4(12):e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramadori G, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28(40):9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillum MP, et al. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135(5):813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramadori G, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bordone L, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4(2):e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moynihan KA, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2(2):105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 62.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Feige JN, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 65.Milne JC, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beher D, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74(6):619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 67.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 68.Pacholec M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai H, et al. SIRT1 activation by small molecules - kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010 doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinciguerra M, Fulco M, Ladurner A, Sartorelli V, Rosenthal N. SirT1 in muscle physiology and disease: lessons from mouse models. Dis Model Mech. 2010;3(5–6):298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshizaki T, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298(3):E419–E428. doi: 10.1152/ajpendo.00417.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28(2):277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Zhao W, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451(7178):587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451(7178):583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- 75.Okazaki M, et al. PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocr J. 2010;57(5):403–413. doi: 10.1507/endocrj.k10e-004. [DOI] [PubMed] [Google Scholar]