Abstract
OBJECTIVES:
Medication management of attention-deficit/hyperactivity disorder (ADHD) is often suboptimal. We examined whether (1) brief physician training plus computer-assisted medication management led to greater reduction in ADHD symptoms and (2) adherence to the recommended titration protocol produced greater symptomatic improvement.
METHODS:
A randomized medication trial was conducted that included 24 pediatric practices. Children who met criteria for ADHD were randomly assigned by practice to treatment-as-usual or a specialized care group in which physicians received 2 hours of didactic training on medication management of ADHD plus training on a software program to assist in monitoring improvement. Parent and teacher reports were obtained before treatment and 4, 9, and 12 months after starting medication.
RESULTS:
Children in both specialized care and treatment-as-usual groups improved on the ADHD Rating Scales and SNAP-IV, but there were no group differences in improvement rates. Brief physician training alone did not produce improvements. When recommended titration procedures were followed, however, outcomes were better for total and inattentive ADHD symptoms on both the ADHD Rating Scales and SNAP-IV parent and teacher scales. Results were not attributable to discontinuation because of adverse effects or failure to find an effective medication dose.
CONCLUSIONS:
Brief physician training alone did not lead to reductions in ADHD symptoms, but adherence to a protocol that involved titration until the child's symptoms were in the average range and had shown a reliable change led to better symptom reduction. Computer-assisted medication management can contribute to better treatment outcomes in primary care medication treatment of ADHD.
Keywords: attention-deficit/hyperactivity disorder, computer-assisted practice, physician education
WHAT'S KNOWN ON THIS SUBJECT:
Medication is effective in reducing attention-deficit/hyperactivity disorder symptoms, but community management is suboptimal. Computer-assisted decision-making can improve quality of care. Structured titration procedures can improve outcomes, but centralized procedures to identify optimal levels may be difficult to implement.
WHAT THIS STUDY ADDS:
Brief physician training alone did not lead to greater clinical improvement, but adherence to a protocol that involved titration until a clinically significant change resulted, by using a medication management software program, led to greater symptom reduction.
Medication management for attention-deficit/hyperactivity disorder (ADHD) in primary care practice is often suboptimal.1 Guidelines for pharmacologic treatment of ADHD have been developed by the American Academy of Pediatrics (AAP)2,3 and others.4–7 Treated children whose physicians were trained in the AAP guidelines improve,8–10 but it is not clear if they improve more than those of untrained physicians. In that study and others,11,12 central committees determined optimal dose, a process difficult to replicate in community practice, and used forced-choice titration procedures, with each child administered each designated dose of study medication.8–10 Reluctance to deploy this approach may have contributed to less than half of diagnosed children receiving a titration trial.10
In the present study, in which the effectiveness is examined of a brief physician training program to improve primary care ADHD medication management, we addressed 2 limitations of previous studies by (1) including a comparison group to assess the effects of physician training on child behavior, (2) providing training in the use of an office-based, computerized program to determine optimal dose, and (3) examining whether adherence to the recommended titration protocol produced greater symptomatic improvement.
METHODS
Study Design and Setting
We enrolled children with ADHD from 24 Chicago-area pediatric practices in 2003 to 2009. Following a cluster randomized trial design, we stratified practices according to demography (3 clinics that serve low-income families; 21 private practices), number of pediatricians, and geography (city, suburb) and, with institutional review board approval, we randomized 12 pairs of practices to specialized care (SC) or treatment-as-usual (TAU) interventions.
Participants
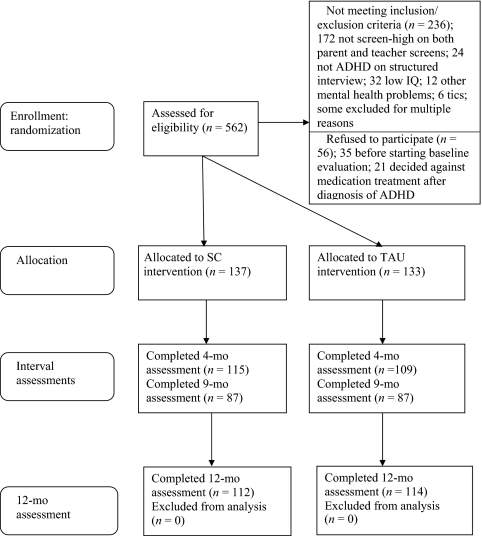
Participating physicians referred patients with suspected ADHD for screening. The screening sample included 562 children aged 5.9 to 11.11 years (mean: 8.3 years), with 419 (74.6%) boys. To be eligible, children had to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for the diagnosis of ADHD. Exclusion criteria were (1) ADHD medication in previous 2 months, (2) child not with primary caretaker for previous 6 months, (3) enrolled in classroom for children with intellectual disabilities or IQ < 70, (4) taking medication incompatible with stimulants, (5) history of intolerance to stimulants, (6) suicidal ideation or autism spectrum disorder, and (7) medical exclusions (antipsychotic medication in previous 2 months, chronic tics/Tourette syndrome, major illness, or abnormally high blood pressure or pulse). Of 562 screened, 236 were excluded, and 56 declined participation (Fig 1) before consenting to treatment or receiving information specific to TAU or SC treatment procedures.
FIGURE 1.
Enrollment flowchart.
Final Sample
There were 270 families who consented to participation in the treatment phase of the study after their child was diagnosed with ADHD. This included 208 boys (77.0%) and 62 girls (23.0%) (mean age: 8.2 years). Self-identified race/ethnicity was white (81.5%), Hispanic (12.2%), black (2.5%), other (2.2%), and not reporting (1.5%).
There were 137 (50.7%) children in the SC and 133 (49.3%) in the TAU condition (Table 1). There were no group differences in child's age, parental marital status, or socioeconomic status, but there was a significant group gender difference, χ2 (1, N = 270) = 4.66 (P = .031), with the SC group having more boys (82.5%) than the TAU group (71.4%). There were more minority (Hispanic, black, or other) children in the SC group, χ2 (1, N = 266) = 8.51 (P = .0041), and whites comprised 75.9% of the SC condition and 89.5% of the TAU condition. Because of these differences, minority status and gender were entered as covariates in analyses.
TABLE 1.
Demographics and Methylphenidate Equivalents for Children Who Enter Treatment
| SC Group (N = 137) | TAU Group (N = 133) | |
|---|---|---|
| Age at study entry, y (±SD) | 8.25 (1.38) | 8.19 (1.62) |
| Gender, n (%) male | 113 (82.5) | 95 (71.4) |
| Race/ethnicity, n (%) | ||
| NonHispanic white | 101 (73.7) | 119 (89.5) |
| Black | 6 (4.4) | 1 (.8) |
| Hispanic | 23 (16.8) | 10 (7.5) |
| Other | 3 (2.2) | 3 (2.3) |
| Missing | 4 (2.9) | 0 |
| Marital status, n (%) | ||
| Married or remarried | 105 (76.6) | 103 (77.4) |
| Not married | 18 (13.1) | 21 (15.8) |
| Not reported | 14 (10.2) | 9 (6.8) |
| Socioeconomic status (Hollingshead class), n (%) | ||
| 1 (highest) | 19 (13.9) | 20 (15.0) |
| 2 | 70 (51.1) | 59 (44.4) |
| 3 | 30 (21.9) | 35 (26.3) |
| 4 | 13 (9.5) | 15 (11.3) |
| 5 (lowest) | 5 (3.6) | 4 (3.0) |
| Mean dose (methyl-phenidate equivalent), n (%) | 27.68 (12.9) | 30.37 (15.41) |
For the total treated sample, there were 120 children with ADHD-combined type (49.0%), 24 with hyperactive/impulsive type (9.8%), and 101 with inattentive type (41.2%). The 2 study groups did not differ on ADHD type.
Measures
ADHD Diagnosis
The Diagnostic Interview Schedule for Children IV-Parent version13 is a structured, parent-report, psychiatric diagnostic interview. Eligible children had to meet criteria for any ADHD type.
Outcome Measures
The ADHD Rating Scales-IV (ADHDRS)14–16 are parent- and teacher-reported scales with items derived from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition ADHD symptom list. The ADHDRS is the only ADHD rating scale with available test-retest reliability and normative data. The SNAP-IV,17,18 used widely in assessment of ADHD, was completed by parents and teachers before treatment and at 4, 9, and 12 months after. SNAP and ADHDRS total and inattention scores were used.
Adverse Effects
The Side Effect Rating Scale,19 which lists common adverse effects of medications for ADHD, was completed by the parent at 4, 9, and 12 months after treatment began. Children completed the Beck Anxiety Inventory for Youth and the Beck Depression Inventory for Youth20 at baseline, 4 months, 9 months, and 12 months to screen for suicidal ideation and high levels of anxiety or depression.
Procedure
Screening and Pretreatment Evaluations
If parent and physician thought screening for ADHD was indicated, parents and teachers completed the ADHDRS and SNAP. Children with scores > 75th percentile on both parent and teacher forms were eligible for the pretreatment evaluation, at which time the Diagnostic Interview Schedule for Children IV-Parent version was administered. Those with a diagnosis of ADHD-combined, inattentive, or hyperactive/impulsive types, regardless of comorbidities, were invited to participate in the medication trial. If families agreed, the physicians were notified of the child's diagnosis and eligibility, and an office visit was scheduled to initiate treatment.
Home visits were conducted at 4 and 12 months after treatment began. At 9 months, questionnaires were mailed home. Of 270 families, 83.0%, 64.4%, and 83.7% completed assessments at 4, 9, and 12 months, respectively.
Interventions
For the SC group, we derived medication management procedures from the ADHD guidelines of the AAP,3 the American Academy of Child and Adolescent Psychiatry,5,21 and Texas Children's Medication Algorithm Project.6,7 Physicians received 2 hours of office-based training in using stimulant medications and atomoxetine. A bachelor's degree-level ADHD specialist provided 1 hour of training to office staff in the use of software (Focus on ADHD Medication Management Program [ie, the Focus program] [Children's Memorial Hospital, Chicago, IL]) designed for monitoring and guiding medication titration, and returned to the office for the first 3 patients per physician to ensure that staff understood program use. Free software for managing ADHD medications per this procedure is available at www.childrensmemorial.org/professionals/focus-on-adhd.aspx.
The SC protocol called for physicians to educate families about ADHD and medications used in its treatment. Blood pressure, pulse, height, and weight were to be obtained. For titration, physicians were encouraged to start children on a short-acting stimulant whenever possible (as in the MTA study11). Because there are no clinical predictors of which stimulant is best, physicians chose to start the child on methylphenidate, dexedrine, or mixed salts amphetamine. Following protocol, the standard regimen was 3 times daily, with adjustments in timing or dose made for the third dose to reduce effects on appetite and sleep onset. Protocol called for medication use during weekends and summer.
Physicians were to encourage parents and teachers to complete the ADHDRS weekly during the mediation titration phase. After receiving and scoring the ADHDRSs, a telephone call or office visit was made with the family to adjust doses or make medication changes. This process was to be repeated until the optimal dose level (see below) was decided on. Subsequently, physicians were encouraged to meet quarterly with families.
The study involved a novel clinically significant change (CSC) titration procedure that draws on mental health research to determine if rating scale improvements represent clinically meaningful change.22,23 CSC required (1) scores on the monitoring measure moved from the dysfunctional to the normal range and (2) amount of change exceeded the amount explained by the reliability of the outcome measure. A reliable change index (RCI)22,24 on the basis of the measure's (ADHDRS) test-retest reliability was calculated by the Focus software; change was considered reliable if the RCI exceeded the 95% confidence interval. The optimal dose for maintenance was the lowest dose resulting in (1) change from clinically elevated pretreatment level to the average range (<68th percentile) for the child's age and gender and (2) a degree of change that exceeded the 95% confidence level for the ADHDRS's RCI.
In each office, ADHDRS scores were entered into the Focus program on a computer located in the office suite at before treatment and for each new dose. Program output (in paper-based format; only 1 practice used an electronic medical chart) indicated whether the child's scores were in the average range and the RCI was significant. If both criteria were met, optimal dose was achieved; if not, the dose was to be increased incrementally and the ADHDRS obtained again. If a CSC was not obtained when reaching the maximum dose for the chosen medication, a second stimulant was tried and the titration plan reapplied. If no CSC resulted with the second stimulant, atomoxetine was prescribed. If that was ineffective, a referral to a child and adolescent psychiatrist was recommended. This occurred with 3 children. Per protocol, pediatricians monitored daytime dose using teacher ADHDRS and evening dose with parent ADHDRS. After deciding on an optimal dose, physicians and families decided whether to change to a long-acting formulation.
For the TAU group, children in TAU practices were identified and evaluated as in the SC practices. Pediatricians then provided treatment per their usual procedure.
Assessing Protocol Adherence
Two research assistants reviewed transcriptions of medical charts of SC physicians and independently rated (1) whether medication was started on the lowest dose, (2) whether medication was prescribed 3 times per day, (3) whether medication was titrated sequentially, and (4) whether CSC titration procedures were followed. Interrater agreement was high (mean κ = .99 [range: .95–1.0]).
Data Analysis
Linear mixed model analyses examined treatment differences using 2 (SC versus TAU) × 4 (before treatment and at months 4, 9, and 12) repeated measures analysis in an intent-to-treat design (previous-observation-carried-forward method). Treatment group and practice were entered as fixed effects; gender and race/ethnicity were entered as covariates to control statistically for their effects. In this design, the trials effect indicates whether there was a significant change over time on the outcome measure for both intervention groups (SC and TAU) combined. The treatment × trials interaction indicates whether there was a significant difference between the 2 groups in the rate of improvement over time. Because of the effect of ADHD on classroom functioning, teacher SNAP and ADHDRS ratings were primary outcome measures. In examining the effects of protocol adherence in the SC group, a similar 2 (achieved a CSC change versus did not achieve a CSC change) × 4 (trials) repeated measures intent-to-treat analysis was conducted. χ2 analyses were conducted to examine whether specific protocol components were associated with achieving a CSC change, and t tests were used to compare differences in total daily medication dose.
RESULTS
Main Effects for Treatment
In Table 2, the trials and treatment condition (SC versus TAU) × trials effects are presented. For both total ADHD symptoms and inattentive symptoms on the ADHDRS and SNAP scales, there were significant trials effects, with large gains noted by the 4-month assessment and continued improvement at 9 and 12 months. This finding is consistent with previous studies that reveal an overall effect of medication on child ADHD symptoms.
TABLE 2.
Trials and Treatment (SC Versus TAU) × Trials Effects
| Measure | Rater | Time (Trials Effect), F, P, ES | Group Differences Over Time (Treatment × Trials Effect), F, P, ES |
|---|---|---|---|
| ADHDRS | |||
| ADHDRS total scale | Parent | 12.00, .001, −.32 | .92, NS |
| Teacher | 15.74, .001, −.39 | 1.9, NS | |
| ADHDRS inattention scale | Parent | 10.20, .001, −.32 | .73, NS |
| Teacher | 13.65, .001, −.48 | 1.64, NS | |
| SNAP | |||
| SNAP total scale | Parent | 22.49, .001, −.28 | .83, NS |
| Teacher | 17.82, .001, −.46 | .94, NS | |
| SNAP inattention scale | Parent | 16.14, .001, −.36 | .39, NS |
| Teacher | 14.86, .001, −.78 | .90, NS |
ES indicates effect size; NS, not significant.
There was, however, no significant difference in the rate of change over time between the 2 treatment conditions for either measure. Thus, the brief training for the SC physicians was not sufficient to produce improvement in child functioning versus the TAU group. These results did not differ when eliminating the 9-month data, where the larger number of missing cases resulted in more previous observations carried forward.
Additional analyses to examine whether treatment effects were specific to ADHD subtype revealed no significant treatment condition × trials differences on parent or teacher total SNAP or ADHDRS scales for the combined ADHD subtype, or ADHD-inattentive subtype on the ADHDRS or SNAP inattention scales (ADHD-hyperactive/impulsive subtype was not examined because of the small n).
Adherence to the SC Protocol
We examined whether outcomes were associated with adherence to the treatment protocol. Although adherence could be viewed as a physician function (ie, did the physician follow protocol), adherence to protocol is also a function of the family's willingness to follow recommendations and teacher's willingness to provide reports. Thus, we viewed adherence as a function of the physician-family teacher team.
Adherence to Titration Procedure
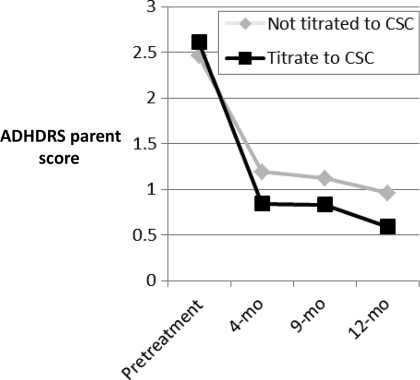
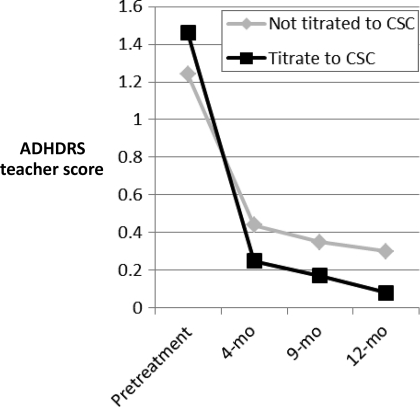
The most critical protocol component involved the titration procedure. For 38 children, the recommended titration procedure (titrating until a CSC change occurred and average-range scores were attained) was followed; for 99, it was not. When followed, children had better outcomes for parent and teacher report on the total and inattentive subscales for both the ADHDRS parent and teacher total scales and inattentive scales, and the SNAP parent and teacher total and inattentive scales (Table 3) (Figs 2 and 3 illustrate change across time for ADHDRS scores, with similar results for SNAP scores).
TABLE 3.
Trials and Adherence Group (Titration Criteria Met Versus Not Met) × Trials Effects
| Measure | Rater | All Children in the SC Group |
Children in the SC Group Without Adverse Effects |
||
|---|---|---|---|---|---|
| Time (Trial Effect), F, P | Adherence × Time (Trial) Effect, F, P | Time (Trial Effect), F, P | Adherence × Time (Trial) Effect, F, P | ||
| ADHDRS | |||||
| ADHDRS total scale | Parent | 14.62, .001 | 3.57, .014 | 10.69, .001 | .18, NS |
| Teacher | 19.15, .001 | 3.92, .009 | 18.86, .001 | 3.89, .009 | |
| ADHDRS inattention scale | Parent | 12.21, .001 | 2.96, .032 | 9.44, .001 | 1.41, NS |
| Teacher | 16.50, .001 | 3.25, .021 | 16.49, .001 | 3.30, .020 | |
| SNAP | |||||
| SNAP total scale | Parent | 26.49, .001 | 4.44, .004 | 20.68, .001 | 2.68, .046 |
| Teacher | 22.15, .001 | 5.24, .001 | 25.72, .001 | 5.44, .001 | |
| SNAP inattention scale | Parent | 18.66, .001 | 2.87, .036 | 14.29, .001 | 1.32, NS |
| Teacher | 17.24, .001 | 2.75, .042 | 19.45, .001 | 3.20, .031 | |
NS indicates not significant.
FIGURE 2.
Results for ADHDRS parent-total score. ADHDS scores are standardized so that 0 is average for child's age and gender; 1.96 = +1 SD.
FIGURE 3.
Change in ADHRS teacher scale. ADHDS scores are standardized so that 0 is average for child's age and gender; 1.96 = +1 SD.
We examined whether adherence to the titration procedures was associated with demographic factors, which raises the possibility that demographic factors accounted for the titration adherence effects. Adherence to titration procedures was unrelated to minority status, gender, socioeconomic status, age at baseline, or ADHD diagnostic type.
It was possible that some children experienced adverse effects that prevented them from achieving scores in the normal range. To examine this possibility, children who were discontinued from medication because of adverse effects were eliminated from analyses. Trials effects (Table 3) were similar to the analyses for all SC children, with significant improvements for both ADHDRS and SNAP measures, parent and teacher raters, and total and inattentive scores. For teacher ratings, the treatment × trials effects were the same for the minimal-adverse effect children as for the total SC sample, with greater improvement for both measures, total and inattentive symptom scales. The results were less consistent for the parent measures. For the total SC group, there were improvements for the good adherence group on each measure. For the children with minimal adverse effects, the parent ratings continued to be significant for the SNAP total score, but not for the SNAP inattention scale or either ADHDRS total or inattention scales.
We also examined whether some children failed to meet the criterion for clinically significant change because medications were ineffective, ie, they received the maximum dose and did not exhibit a clinically significant change. This did not occur; only 6 children in the SC group received the maximum dose of a medication.
Adherence to Other Protocol Components
We examined whether other specific factors contributed to outcome. Nonsignificant elements included starting on a short-acting versus long-acting stimulant; starting on the lowest dose; and proceeding sequentially when titrating evening medications. Significant effects were associated with progressing sequentially, χ2 (1, N = 137) = 4.24 (P = .039) for daytime medications.
Dose Differences
Comparisons between groups were made for the highest daily dose of medication given, with stimulant medications converted to methylphenidate equivalents. The groups did not differ on total daily dose (Table 1). In addition, there was no significant difference (t107 = .30, P = .76) in total daily medication dose between the group adhering to the titration procedure (mean: 27.1 mg/day, SD: 12.6) and the nonadhering group (mean: 27.9 mg/day, SD: 13.1).
Adverse Effects
There were no differences on the Barkley adverse effects scale between SC and TAU groups at 4, 9, or 12 months.
DISCUSSION
In this study we examined the effectiveness of brief didactic training plus training in, and use of, computer-assisted titration for pediatricians in the management of first-line ADHD medications (stimulants and atomoxetine). There were 3 major findings. First, consistent with many previous studies, children treated with medication improved. Second, the brief training provided to the special care group physicians was not sufficient to produce better outcomes for their patients as reported by parents or teachers. Thus, these data highlight the need for including a comparison group in studies that test guideline implementation.
Third, protocol adherence is important for improving clinical outcomes. Previous studies have revealed that computer-assisted decision-making increases adherence to guidelines.25,26 This intervention differed from previous work in using a computer-assisted titration procedure to assess reliable and clinically meaningful change, with optimal dose being the lowest dose producing a reliable change and symptom ratings in the average range (<68th percentile) for the child's age and gender. When the physician/parent/teacher team titrated the medications to the point where a CSC was achieved, there were significant improvements in classroom behavior on parent and teacher measures not used in the titration process. The improvement associated with a CSC could not be attributed to adverse effects interfering with achieving a CSC in some children, or to inability to find a medication dose sufficient to produce desired improvements in others. In addition, improvement associated with titration to a CSC did not result in a higher total daily dose of medication, raising the possibility that some children who did not achieve a CSC did not receive an adequate dose, whereas others may have been medicated beyond the point of achieving a CSC and, thus, were overmedicated.
The titration procedure used in this study is preferable to those in which the maintenance dose moves the child's symptom scores below a clinical cutoff without guidelines for determining optimal dose. The latter procedure may result in unreliable improvement (ie, if the child's scores initially were low in the clinical range), ending the titration when the child's score reaches the average range (<68th percentile) may result in a suboptimal level of symptom reduction because a CSC has not been achieved. Hand-calculation of the RCI and searching manuals to identify average-range scores per age and gender is too time-consuming for clinicians; this is an ideal situation for computer application. In addition, unlike forced titration procedures, the CSC procedure means that children are not required to try a dose that may be higher than necessary to achieve a CSC.
Observations by the ADHD specialist during office visits and comments by office staff and physicians during those visits reveals that some practices were more successful at implementing the use of software and medication management than others. Practices that identified 1 or 2 support staff members who became very comfortable with the program's use were more successful, as were practices that stressed to the parents the importance of obtaining school reports consistently to achieve the best results. In the present study, a research assistant demonstrated the scoring software in person and over the telephone. Its use may be more difficult for community pediatricians lacking that assistance.
Brief physician training with computer-assisted medication management did not result in enhanced treatment gains as assessed by parent or teacher ratings. However, when procedures were followed to titrate medications until a clinically significant change was achieved, improvements resulted compared with children for whom medication was not titrated in that manner. Additional studies of computer-assisted medication management procedures in pediatric care, particularly for managing psychiatric problems, are needed.
ACKNOWLEDGMENT
This study was supported by National Institute of Mental Health grant RO1 MH 066866 (to Dr Lavigne).
Drs Lavigne and LeBailly contributed to the conceptualization of the study, data acquisition, data analysis and interpretation, and manuscript preparation; Dr Dulcan contributed to the conceptualization of the study, interpretation of results, and manuscript preparation; Dr Binns contributed to the conceptualization of the study, data acquisition, interpretation of results, and manuscript preparation; Dr Cummins contributed to the conceptualization of the study and manuscript preparation; and Dr Jha contributed to data acquisition and manuscript preparation.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00179894).
FINANCIAL DISCLOSURE: Dr Dulcan is a consultant for ADHD/LD on the advisory board for Eli Lilly Co, and a consultant for Care Management Technologies Inc; the other authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
Abbreviations:
- ADHD
- attention-deficit/hyperactivity disorder
- AAP
- American Academy of Pediatrics
- SC
- specialized care
- TAU
- treatment as usual
- ADHDRS
- ADHD Rating Scales-IV
- CSC
- clinically significant change
- RCI
- reliable change index
REFERENCES
- 1. Miller A. Appropriateness of psychostimulant prescription to children: theoretical and empirical perspectives. Can J Psychiatry. 1999;44(10):1017–1024 [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105(5):1158–1170 [DOI] [PubMed] [Google Scholar]
- 3. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033–1044 [DOI] [PubMed] [Google Scholar]
- 4. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(10 suppl):85S–121S [DOI] [PubMed] [Google Scholar]
- 5. Greenhill LL, Pliszka S, Dulcan MK. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):26S–49S [DOI] [PubMed] [Google Scholar]
- 6. Pliszka SR, Greenhill LL, Crismon ML, et al. The Texas Children's Medication Algorithm Project: Report of the Texas Consensus Conference Panel on Medication Treatment of Childhood Attention-Deficit/Hyperactivity Disorder. Part I. Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(7):908–919 [DOI] [PubMed] [Google Scholar]
- 7. Pliszka SR, Greenhill LL, Crismon ML, et al. The Texas Children's Medication Algorithm Project: Report of the Texas Consensus Conference Panel on Medication Treatment of Childhood Attention-Deficit/Hyperactivity Disorder. Part II: Tactics. Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(7):920–927 [DOI] [PubMed] [Google Scholar]
- 8. Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pediatr Adolesc Med. 2010;164(2):160–165 [DOI] [PubMed] [Google Scholar]
- 9. Epstein JN, Langberg JM, Lichtenstein PK, Mainwaring BA, Luzader CP, Stark LJ. Community-wide intervention to improve the attention-deficit/hyperactivity disorder assessment and treatment practices of community physicians. Pediatrics. 2008;122(1):19–27 [DOI] [PubMed] [Google Scholar]
- 10. Epstein JN, Rabiner D, Johnson DE, et al. Improving attention-deficit/hyperactivity disorder treatment outcomes through use of a collaborative consultation treatment service by community-based pediatricians: a cluster randomized trial. Arch Pediatr Adolesc Med. 2007;161(9):835–840 [DOI] [PubMed] [Google Scholar]
- 11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073–1086 [DOI] [PubMed] [Google Scholar]
- 12. Nikles CJ, Mitchell GK, Del Mar CB, Clavarino A, McNairn N. An n-of-1 trial service in clinical practice: testing the effectiveness of stimulants for attention-deficit/hyperactivity disorder. Pediatrics. 2006;117(6):2040–2046 [DOI] [PubMed] [Google Scholar]
- 13. Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38 [DOI] [PubMed] [Google Scholar]
- 14. DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. J Clin Child Psychol. 1991;220:245–253 [Google Scholar]
- 15. DuPaul GJ, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent ratings of attention-deficit/hyperactivity disorder symptoms: factor structure and normative data. J Psychopathol Behav Assess. 1998;20:83–102 [Google Scholar]
- 16. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R, McGoey KE, Ikeda MJ. Teacher ratings of attention-deficit/hyperactivity disorder symptoms: factor structure and normative data Psychol Assess. 1997;9(4):436–444 [DOI] [PubMed] [Google Scholar]
- 17. Swanson J. The SNAP-IV teacher and parent rating scale. 2000. Available at: www.adhd.net Accessed September 15, 2003
- 18. Swanson J, Schuck S, Mann M, Carlson C, Hartman K. Categorical and dimensional definitions and evaluations of symptoms of ADHD: the SNAP and the SWAN rating scales. 2000. Available at: www.adhd.net/snap_swan.pdf Accessed September 15, 2003 [PMC free article] [PubMed]
- 19. Barkley RA. Attention-deficit Hyperactivity Disorder: a Clinical Workbook. New York, NY: Guilford Publications; 1991 [Google Scholar]
- 20. Beck JS, Beck AT, Jolly JB. Beck Youth Inventories Manual. San Antonio, TX: Psychological Corporation; 2001 [Google Scholar]
- 21. Pliszka S; AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921 [DOI] [PubMed] [Google Scholar]
- 22. Jacobson N, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19 [DOI] [PubMed] [Google Scholar]
- 23. Kazdin AE. The meanings and measurement of clinical significance. J Consult Clin Psychol. 1999;67(3):332–339 [DOI] [PubMed] [Google Scholar]
- 24. Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J Consult Clin Psychol. 1999;67(3):300–307 [DOI] [PubMed] [Google Scholar]
- 25. Shiffman RN, Freudigman KA, Brandt CA, Liaw Y, Navedo DD. A guidelines implementation system using handheld computers for office management of asthma: effects on adherence and patient outcomes. Pediatrics. 2000;105(4 pt 1):767–773 [DOI] [PubMed] [Google Scholar]
- 26. Hunt DL, Haynes B, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes. JAMA. 1998;280(15):1339–1346 [DOI] [PubMed] [Google Scholar]