Abstract
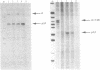
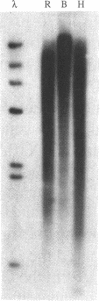
The Xenopus laevis alpha-tubulin gene X alpha T14 produces two mRNAs of 1.7 and 2.15 kb and we have shown that this is due to the use, at approximately equal frequency, of alternative 3' processing sites. Unusually, the hexanucleotide polyadenylation signal responsible for use of the downstream site, pA2, is CAUAAA in contrast to the consensus AAUAAA used at the upstream site, pA1. Since such a variant hexanucleotide would normally be expected to reduce drastically the efficiency of 3' processing, we have examined the 3' flanking sequences involved in pA2 usage in injected oocytes. In deletion mutants with 40 bp or 440 bp of 3' flanking DNA use of pA2 was almost totally abolished whereas when 770 bp of the natural flank was present pA2 was used normally. This polyadenylation signal therefore requires an unexpectedly large amount of flanking DNA and we have identified in the required region a member of a novel family of 450 bp interspersed repeats that we have termed Pir elements. We speculate that because of the variant hexanucleotide efficient use of pA2 has to be potentiated by the Pir element, perhaps through an effect on transcriptional pausing or termination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashfield R., Enriquez-Harris P., Proudfoot N. J. Transcriptional termination between the closely linked human complement genes C2 and factor B: common termination factor for C2 and c-myc? EMBO J. 1991 Dec;10(13):4197–4207. doi: 10.1002/j.1460-2075.1991.tb04998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin C., Nepveu A., Marcu K. B. Molecular requirements for transcriptional initiation of the murine c-myc gene. Oncogene. 1989 May;4(5):549–558. [PubMed] [Google Scholar]
- Bartsch I., Schoneberg C., Grummt I. Purification and characterization of TTFI, a factor that mediates termination of mouse ribosomal DNA transcription. Mol Cell Biol. 1988 Sep;8(9):3891–3897. doi: 10.1128/mcb.8.9.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccari E., Mazzetti P. The nucleotide sequence of the ribosomal protein L14 gene of Xenopus laevis. Nucleic Acids Res. 1987 Feb 25;15(4):1870–1872. doi: 10.1093/nar/15.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connelly S., Manley J. L. RNA polymerase II transcription termination is mediated specifically by protein binding to a CCAAT box sequence. Mol Cell Biol. 1989 Nov;9(11):5254–5259. doi: 10.1128/mcb.9.11.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway L., Wickens M. A sequence downstream of A-A-U-A-A-A is required for formation of simian virus 40 late mRNA 3' termini in frog oocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3949–3953. doi: 10.1073/pnas.82.12.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell. 1990 Nov 16;63(4):655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kislauskis E., Wentworth B. M., Villa-Komaroff L. Alternative 5' exons either provide or deny an initiator methionine codon to the same alpha-tubulin coding region. Nucleic Acids Res. 1987 Jan 12;15(1):199–218. doi: 10.1093/nar/15.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D. Potentiation of a polyadenylylation site by a downstream protein-DNA interaction. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4373–4377. doi: 10.1073/pnas.87.11.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Harris P., Levitt N., Briggs D., Proudfoot N. J. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 1991 Jul;10(7):1833–1842. doi: 10.1002/j.1460-2075.1991.tb07709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Gil A., Proudfoot N. J. Position-dependent sequence elements downstream of AAUAAA are required for efficient rabbit beta-globin mRNA 3' end formation. Cell. 1987 May 8;49(3):399–406. doi: 10.1016/0092-8674(87)90292-3. [DOI] [PubMed] [Google Scholar]
- Good P. J., Richter K., Dawid I. B. The sequence of a nervous system-specific, class II beta-tubulin gene from Xenopus laevis. Nucleic Acids Res. 1989 Oct 11;17(19):8000–8000. doi: 10.1093/nar/17.19.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Weiner A. M. Formation of the 3' end of U1 snRNA requires compatible snRNA promoter elements. Cell. 1986 Oct 24;47(2):249–258. doi: 10.1016/0092-8674(86)90447-2. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Kane C. M. RNA polymerase: regulation of transcript elongation and termination. FASEB J. 1991 Oct;5(13):2833–2842. doi: 10.1096/fasebj.5.13.1916107. [DOI] [PubMed] [Google Scholar]
- Labhart P., Reeder R. H. Ribosomal precursor 3' end formation requires a conserved element upstream of the promoter. Cell. 1987 Jul 3;50(1):51–57. doi: 10.1016/0092-8674(87)90661-1. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Falck-Pedersen E., Darnell J. E., Jr, Shenk T. A poly(A) addition site and a downstream termination region are required for efficient cessation of transcription by RNA polymerase II in the mouse beta maj-globin gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8306–8310. doi: 10.1073/pnas.84.23.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. J., Elkington J. A., Lloyd M. M., Jones M. B., Williams J. G. Mutations downstream of the polyadenylation site of a Xenopus beta-globin mRNA affect the position but not the efficiency of 3' processing. Cell. 1986 Jul 18;46(2):263–270. doi: 10.1016/0092-8674(86)90743-9. [DOI] [PubMed] [Google Scholar]
- Mason P. J., Jones M. B., Elkington J. A., Williams J. G. Polyadenylation of the Xenopus beta 1 globin mRNA at a downstream minor site in the absence of the major site and utilization of an AAUACA polyadenylation signal. EMBO J. 1985 Jan;4(1):205–211. doi: 10.1002/j.1460-2075.1985.tb02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Hart R. P., Wong W. W., Nevins J. R. Sequences capable of restoring poly(A) site function define two distinct downstream elements. EMBO J. 1986 Nov;5(11):2907–2913. doi: 10.1002/j.1460-2075.1986.tb04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay B., Reeder R. H. A DNA-binding protein is required for termination of transcription by RNA polymerase I in Xenopus laevis. Mol Cell Biol. 1990 Jun;10(6):2793–2800. doi: 10.1128/mcb.10.6.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K. M., Morgan G. T. An oocyte-expressed alpha-tubulin gene in Xenopus laevis; sequences required for the initiation of transcription. Nucleic Acids Res. 1989 Jul 11;17(13):5041–5055. doi: 10.1093/nar/17.13.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton K. M., Morgan G. T. Premature termination of transcription can be induced on an injected alpha-tubulin gene in Xenopus oocytes. Mol Cell Biol. 1990 Feb;10(2):727–735. doi: 10.1128/mcb.10.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H., Asselin C., Dufort D., Yang J. Q., Gupta K., Marcu K. B., Nepveu A. A cis-acting element in the promoter region of the murine c-myc gene is necessary for transcriptional block. Mol Cell Biol. 1989 Dec;9(12):5340–5349. doi: 10.1128/mcb.9.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. T., Middleton K. M. Short interspersed repeats from Xenopus that contain multiple octamer motifs are related to known transposable elements. Nucleic Acids Res. 1990 Oct 11;18(19):5781–5786. doi: 10.1093/nar/18.19.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. How RNA polymerase II terminates transcription in higher eukaryotes. Trends Biochem Sci. 1989 Mar;14(3):105–110. doi: 10.1016/0968-0004(89)90132-1. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Ogg S. C., Wickens M. P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5799–5805. doi: 10.1093/nar/18.19.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J. The complete sequence of a frog alpha-tubulin gene and its regulated expression in mouse L-cells. Biochem J. 1988 Jan 15;249(2):465–472. doi: 10.1042/bj2490465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Transcription elongation and eukaryotic gene regulation. Oncogene. 1990 Jun;5(6):777–785. [PubMed] [Google Scholar]
- Villasante A., Wang D., Dobner P., Dolph P., Lewis S. A., Cowan N. J. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986 Jul;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Pettine S. M., Shenk T. Functional analysis of point mutations in the AAUAAA motif of the SV40 late polyadenylation signal. Nucleic Acids Res. 1989 May 25;17(10):3899–3908. doi: 10.1093/nar/17.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S., Mirels L. F., Calayag M. C., Bishop J. M. Premature termination of transcription from the P1 promoter of the mouse c-myc gene. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11383–11387. doi: 10.1073/pnas.88.24.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]