Abstract
Exposure to UVB radiation before antigen delivery at an unirradiated site inhibits functional immunological responses. Mice treated dorsally with suberythemal low-dose UVB and immunized with ova in abdominal skin generated ova-specific CD8 T cells with a significantly decreased activation, expansion, and cytotoxic activity compared with unirradiated mice. UVB also impaired the delayed-type hypersensitivity (DTH) reaction to ova. Transfer of CD4+CD25+ cells from UVB-exposed mice did not suppress the ova-specific CD8 T-cell response or DTH reaction in unexposed mice, confirming that systemic low-dose UVB does not induce long-lived functional regulatory CD4+CD25+ T cells. Repairing cyclobutane pyrimidine dimer–type DNA damage and blocking aryl hydrocarbon receptor signaling also did not reverse the immunosuppressive effect of UVB on ova-specific CD8 T cells and DTH, suggesting that cyclobutane pyrimidine dimers and the aryl hydrocarbon receptor are not required in systemic low-dose UVB-induced immunosuppression. The known UVB chromophore, cis-urocanic acid, and reactive oxygen species triggered the inhibition of DTH caused by UVB, but they were not involved in the modulation of CD8 T cells. These findings indicate that systemic low-dose UVB impedes the primary response of antigen-specific CD8 T cells by a novel mechanism that is independent of pathways known to be involved in systemic suppression of DTH.
UVB radiation (290 to 320 nm) in natural sunlight is a potent immunosuppressant. UVB can inhibit the immune system from generating optimal responses to tumors, contact haptens, and various microbial antigens (Ags; viral, fungal, and parasitic) that can lead to exacerbated disease. Alternatively, immunosuppressive UVB can also be beneficial to control autoimmune diseases, such as psoriasis1 and experimental autoimmune encephalomyelitis.2 The epidermis of skin absorbs UVB through chromophores, including nuclear DNA, cytoplasmic tryptophan, and extracellular trans-urocanic acid (UCA). The molecular processes that follow trigger a cascade of events that cumulates in the phenomenon of UVB-induced immunosuppression. Some of the hallmarks of this immunosuppression include UVB-induced genetic mutations in skin cells, circulation of cis-UCA from the isomerization of trans-UCA, production of reactive oxygen species (ROS), and generation of regulatory T and B cells that can transfer suppression into UVB-naïve mice.3 Because UVB has both detrimental and beneficial effects on the immune system, it is critical to understand the mechanisms regulated by UVB so that effective prophylactic and palliative therapies can be designed for skin diseases, such as skin cancer, and immune-mediated diseases in internal organs, such as multiple sclerosis.
A previous study4 showed that UVB can inhibit CD8 and CD4 T-cell responses to haptens. In a model of contact hypersensitivity (CHS), we demonstrated that a low dose of UVB (approximately 5 minutes of summer sunlight in Sydney, Australia, at midday) is sufficient to inhibit the activation and expansion of effector CD8 and CD4 T cells in skin-draining lymph nodes (sDLNs) after sensitization to a contact hapten at an unirradiated site (systemic, Ag, and UVB at distal sites). However, it was too low to activate functional and durable CD4+CD25+ regulatory T cells. The effector T cells generated in this environment exhibited reduced skin infiltration and interferon-γ production on CHS elicitation. Moreover, this systemic low-dose UVB regimen prevented the development of dermal memory CD8 T cells.
Exposing mice to a low-dose UVB regimen, followed by transcutaneous immunization with ova protein through the same skin site (local, Ag, and UVB at the same site), inhibits the proliferation, cytotoxicity, and interferon-γ production of transgenic and endogenous ova-specific CD8 T cells.5,6 Contrary to what we previously observed, transferable suppression of the ova-specific CD8 T-cell response was correlated to the presence of CD4+CD25+ regulatory T cells in sDLNs in this model.5 Other researchers7,8 have also demonstrated that regulatory T cells derived from mice irradiated with inflammatory high doses of UVB in a systemic Ag model can impede CHS and delayed-type hypersensitivity (DTH) reactions. These UV-activated regulatory T cells may inhibit T cells and skin inflammatory reactions by altering Ag-presenting cells (APCs).9
Several UVB chromophores have been identified that can independently trigger UVB-induced immunosuppression, as shown by various studies that have repaired DNA damage, neutralized cis-UCA, and prevented ROS production. The downstream processes initiated by UVB chromophores include production of immunosuppressive mediators [ie, IL-4, IL-10, and platelet-activating factor (PGE2)], migration of skin APCs into sDLNs, aberrant Ag presentation, mast cell activation, and induction of regulatory T cells.10 However, it is unknown whether these pathways are involved during systemic low-dose UVB, which is representative of daily nonrecreational sunlight exposure. Given that a previous investigation showed that UVB has a long-term deleterious influence on CD8 T-cell immunity, we wanted to further examine what UVB-stimulated mechanisms contribute to the inhibition of primary CD8 T-cell responses in the absence of CD4+CD25+ regulatory T cells. In addition, we assessed the ability of systemic low-dose UVB to modulate a second separate inflammatory reaction in the skin at an unirradiated site. DTH reactions are complex skin immune reactions that are primarily driven by type 1 helper CD4 T cells and various innate cells.11,12 For this study, we used the model protein Ag, ova, that allowed us to study the endogenous Ag-specific CD8 T-cell response and a DTH reaction after UVB irradiation and immunization. UVB significantly decreased the activation, expansion, and cytotoxic activity of splenic ova-specific CD8 T cells, but this was not attributed to known UVB chromophores considered to be critical in UVB-induced immunosuppression. However, DTH reactions were modulated by cis-UCA and ROS. These findings indicate that short-term UVB can inhibit Ag-specific CD8 T-cell responses and DTH in vivo via different mechanisms and, therefore, that a novel unknown mechanism is responsible for regulating the effects of UVB on CD8 T-cell activation in secondary lymphoid organs.
Materials and Methods
Mice
C57BL/6J female mice were used at the age of 8 weeks (Animal Resource Centre, Perth, Australia). Mice were given food and water ad libitum. All experiments were conducted under the approval of The University of Sydney Animal Ethics Committee.
UVB Source
A 1000-W xenon arc lamp solar simulator (Oriel, Stanford, CT), filtered with two 200- to 400-nm dichroic mirrors and a 310-nm narrow-band interference filter (CVL Laser, Albuquerque, NM), was used to produce the UVB spectra that had a peak irradiance of 4.20 × 10-5 mW/cm2 at a wavelength of 312 nm and a half band width of approximately 15 nm. UVA (>320 nm) and UVC (<290 nm) contaminated the spectra by approximately 23% and 0.61%, respectively. This spectrum was previously published.13 Spectral output and intensity were measured with a spectroradiometer (OL-754; Optronics Laboratories, Orlando, FL), and a broadband radiometer (International Light Technologies, Inc., Peabody, MA) calibrated against the source was used continuously to monitor fluctuations in output. The timing of UVB delivery was accurately maintained using an automated timing device.
UVB Protocol and Immunization
Mouse dorsal hair was shaved using animal clippers (Oster, McMinnville, TN) and an electric razor (Remington, Vienna, Austria) 24 hours before irradiation. During irradiation, mice were restrained in a black poly(methyl methacrylate) (Perspex) box with a quartz lid. Ears and heads were protected from UVB with black poly(methyl methacrylate). Mouse dorsums were irradiated with 150 mJ/cm2 UVB daily for 3 consecutive days, which is approximately half of a minimal erythemal dose. Three days after the last UVB irradiation, mice were immunized on their abdomens s.c. with 200 μg ova (Sigma-Aldrich, St Louis, MO) and 40 μg saponin (Sigma-Aldrich) in saline.
Application of Biological Modifiers
cis-UCA (20 μg; Sigma-Aldrich) and trans-UCA (40 μg; Sigma-Aldrich) were also applied to dorsal skin for 3 consecutive days in an innocuous cosmetic oil-in-water base lotion instead of UVB.14,15 α-Naphthoflavone (100 pmol; Sigma-Aldrich) was topically administered to dorsal skin 30 minutes before UVB in base lotion.16 Base lotion alone was applied to all control mice. Ketanserin (500 nmol; Sigma-Aldrich) was delivered in PBS i.p. 30 minutes before UVB, whereas control mice were injected with PBS alone.17 A mixture of the antioxidants, vitamin E (0.05 mg; Sigma-Aldrich) and butylated hydroxytoluene (0.75 mg; Sigma-Aldrich), was injected i.p. 2 hours before UVB in olive oil (Sigma-Aldrich).18 Olive oil alone was given to control mice. Immediately after UVB, the dorsal skin of mice was topically treated with 125 ng liposomes (a gift from Daniel B. Yarosh, AGI Dermatics, Freeport, NY) containing the DNA repair enzyme, T4 endonuclease V (T4N5), in 1% hydrogel. Control mice received 125 ng of empty liposomes.19
Transfer of Regulatory CD4+CD25+ T Cells
Mice were irradiated with 150 mJ/cm2 UVB daily for 3 days and immunized with ova 3 days after the last UVB irradiation; 7 days after immunization, the sDLNs (brachial, axillary, and inguinal) were removed. Cells from unirradiated but immunized mice were also harvested. CD4+CD25+ regulatory T cells were isolated using a microbead CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and a separator (autoMACS Pro Separator; Miltenyi Biotec GmbH) by following the manufacturer's instructions. Purity was determined to be >92% on a flow cytometer (FACSCanto; Becton Dickinson, San Jose, CA) based on CD4, CD25, and β T-cell receptor expression. Naïve mice were i.v. injected with 0.5 × 106 CD4+CD25+ cells and were immunized the next day.
DTH Reaction
Mice were assessed for a DTH reaction 7 days after immunization by intradermally injecting 50 μg ova in saline into each ear. Ears were measured before ear challenge and at 24-hour points for 3 days after challenge using micrometer calipers (Interapid, Rolte, Switzerland). Any increase due to nonspecific ear inflammation from the injection was assessed in naïve unimmunized mice. The DTH reaction was determined by the difference between before and after ear thickness measurements after accounting for nonspecific inflammation.
Tetramer Staining of Ova-Specific CD8 T Cells
Single-cell suspensions of spleens were first blocked with anti-CD16/CD32 antibody (clone 93; eBioscience, San Diego, CA) before staining with H-2K(b)–APC tetramers (ACRF Biomolecular Resource Facility, Australian National University, Canberra, Australia) bound with SIINFEKL peptide (GL Biochem, Shanghai, China) for 1 hour at room temperature. Cells were washed in FACS buffer (5% fetal calf serum, 10 mmol/L EDTA, and 0.05% sodium azide in PBS) before labeling with anti–CD8α-PerCP-Cy5.5 (clone 53–6.7; Becton Dickinson), anti–CD44-phycoerythrin (PE) (clone IM7; Becton Dickinson), anti–CD4-PECy7 (clone RM4-5; Becton Dickinson), anti–CD19-PECy7 (clone 1D3; Becton Dickinson), and β T-cell receptor–APC–AlexaFluor750 (clone H57-597; eBioscience) for 30 minutes at 4°C. After washing, the cells were stained using a kit (LIVE/DEAD Fixable Green Dead cell stain kit; Invitrogen, Carlsbad, CA), per the manufacturer's instructions. Cells were analyzed using a flow cytometer (FACSCanto). At least 500,000 events were acquired per sample.
In Vivo Cytotoxicity Assay
Splenocytes from naïve C57BL/6J female mice were incubated with target peptide, SIINFEKL, or irrelevant peptide [ie, tyrosine-related protein 2 (SVYDFFVWL, GL Biochem)] for 90 minutes at 37°C. Cells were washed and then differentially stained with carboxyfluorescein diacetate, succinimidyl ester at 2 μmol/L (irrelevant peptide) and 0.2 μmol/L (target peptide) using a kit (CellTrace carboxyfluorescein diacetate, succinimidyl ester kit; Invitrogen). Equal proportions of irrelevant peptide (5 × 106 cells) and target peptide (5 × 106 cells) were i.v. injected into mice at day 7 after immunization. Four hours after transfer, single-cell suspensions of spleens were analyzed on a flow cytometer (FACSCanto). The percentage of specific lysis was determined as follows:
Statistics
DTH responses were evaluated by a two-way repeated-measures analysis of variance. CD8 T-cell responses between unirradiated and irradiated or between treated and untreated groups of mice were compared with an unpaired 2-tailed Student's t-test (Prism version 4.0; GraphPad Software, La Jolla, CA).
Results
Systemic Low-Dose UVB Impedes Primary Splenic Ova-Specific CD8 T-Cell Responses and DTH Reactions Independently of Regulatory CD4+CD25+ T-Cell Activity
Systemic low-dose UVB can interfere with a normal CD8 T-cell response to a hapten during sensitization of a CHS reaction.4 Because the Ag-processing requirements for stimulating naïve CD8 T cells to exogenous protein are different from hapten Ag, we first assessed whether systemic low-dose UVB can also dampen responses to ova. Mouse dorsums were irradiated daily for 3 days with low-dose UVB (ie, 150 mJ/cm2), which is approximately equivalent to half of a minimal erythemal dose or 15 minutes of summer sunlight in Sydney.20 Mice were then immunized 3 days after the last UVB irradiation with ova protein and saponin injected s.c. in the abdomen. The expansion and cytotoxic activity of ova-specific CD8 T cells were investigated 7 days after immunization during the peak of the primary CD8 T-cell response to ova in the spleen, which is a major secondary lymphoid organ. Compared with unirradiated mice, UVB significantly reduced (approximately 55%) both the number and percentage of activated splenic ova-specific CD8 T cells (Figure 1A: P = 0.003 and P = 0.0051, respectively). The in vivo cytotoxic killing ability of ova-specific CD8 T cells was also significantly decreased (ie, twofold) in UVB-irradiated mice (Figure 1B: P = 0.0004). To examine the effect of UVB on a systemic and skin inflammatory reaction, we performed a DTH test during which mouse ears were challenged with ova 7 days after immunization. Mice irradiated with UVB before immunization exhibited a decreased DTH response throughout the days observed after challenge compared with unirradiated mice (Figure 1C: P = 0.0039).
Figure 1.
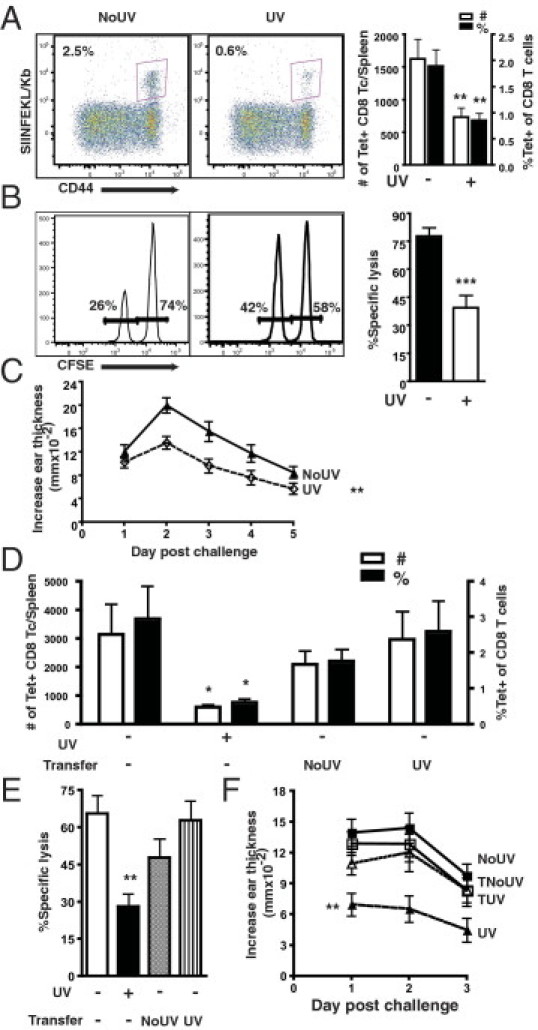
Systemic low-dose UVB impedes primary ova-specific CD8 T-cell (Tc) responses and DTH independently of regulatory CD4+CD25+ T-cell activation. A: Mice were irradiated with 150 mJ/cm2 UVB daily for 3 days on the dorsum and then s.c. immunized with 200 μg ova and 40 μg saponin in saline on the abdomen 3 days after the last irradiation. Seven days after immunization, ova-specific CD8 T cells in the spleen were examined by SIINFEKL-tetramer (Tet) staining. Representative dot plots of activated CD44hi Tet-positive CD8 T cells are gated on CD8+ β T-cell receptor–positive T cells from immunized unirradiated (NoUV) and UV-irradiated mice. The total splenic number and the percentage of CD44hi ova-specific CD8 T cells of total CD8 T cells are presented. B: On day 7 after immunization, mice were injected with SIINFEKL–CFSE-lo– and irrelevant–CFSE-hi–labeled cells, and the extent of in vivo cytotoxic killing was determined from spleens at 4 hours after injection. Representative histograms from NoUV and UV-irradiated mice are shown. The percentage-specific lysis from in vivo cytotoxic killing is presented. CFSE, carboxyfluorescein diacetate, succinimidyl ester. C: On day 7 after immunization, mice were challenged with ova intradermally in the ear skin; and the DTH reaction was determined at 24-hour intervals for 5 days after challenge. The increase in ear thickness is shown in NoUV and UV-irradiated mice after controlling for nonspecific increases due to the injection in unimmunized mice. D: CD4+CD25+ cells were isolated from the sDLNs of donor mice that had been irradiated with 150 mJ/cm2 UVB daily for 3 days on the dorsum and immunized. Cells, 0.5 × 106, were adoptively transferred into naïve mice i.v.; the next day, recipient mice were immunized with ova. Seven days after immunization, SIINFEKL-Tet–positive cells were examined in the spleens. The total number and the percentage of CD44hi Tet–positive cells of CD8 T cells are presented in mice that were not transferred and in mice that were transferred with cells from NoUV or UV-irradiated donors. E: The percentage-specific lysis from an in vivo cytotoxic killing assay at 7 days after immunization in transferred and not transferred mice. F: The DTH reaction at 7 days after immunization in NoUV, UV irradiated and not transferred (UV), transferred with cells from unirradiated donors (TNoUV), and transferred with cells from UV-irradiated donors (TUV) mice. Data represent a pool of three to four repeat experiments (n = 9 to 12 mice per group) and are given as mean ± SEM. Comparisons between not transferred UV-irradiated and unirradiated are shown. *P < 0.05, **P < 0.01, and ***P < 0.001.
Other researchers5 have shown that adoptive transfer of CD4+CD25+ cells derived from mice irradiated with local low-dose UVB and ova immunized inhibits the activation of transgenic ova-specific T cells in vivo. However, a previous investigation4 indicated that systemic low-dose UVB inhibits primary CD8 T cells independently of adaptive regulatory CD4+CD25+ T cells. Therefore, we wanted to investigate whether systemic low-dose UVB was again inhibiting CD8 T-cell activation and function in the absence of functional adaptive regulatory CD4+CD25+ T cells derived from UVB- and ova-exposed mice. Donor mice were irradiated with our low-dose UVB regimen and were immunized at a distant site with ova, before the CD4+CD25+ cells were isolated from sDLNs 7 days after immunization. CD4+CD25+ cells were transferred into naïve mice; the next day, the mice were immunized. The splenic ova-specific CD8 T-cell response and DTH were determined 7 days later. There was significantly decreased ova-specific CD8 T-cell expansion [Figure 1D: P = 0.0229 (number) and P = 0.0174 (percentage)], cytotoxicity (Figure 1E: P = 0.0018), and DTH response (Figure 1F: P = 0.0030) in untransferred mice that were UVB irradiated compared with unirradiated, as previously described. Transfer of CD4+CD25+ cells from unirradiated mice did not significantly affect the splenic ova-specific CD8 T-cell response and DTH compared with unirradiated nontransferred mice (Figure 1, D-F). However, transfer of CD4+CD25+ cells from UVB-irradiated mice also did not alter the splenic activated ova-specific CD8 T-cell response and DTH reaction from those of unirradiated nontransferred mice. These findings indicate that systemic low-dose UVB, followed by ova immunization, does not generate durable functional CD4+CD25+ regulatory T cells able to impede subsequent endogenous Ag-specific CD8 T-cell responses to a protein or systemic inflammatory skin reactions.
cis-UCA Can Inhibit DTH but Not Splenic Ova-Specific CD8 T-Cell Responses
We then wanted to ascertain, at our low UVB dose, whether any of the known UVB chromophores and the subsequent pathways they activate are critical for regulating Ag-specific CD8 T cells and skin inflammatory reactions. The stratum corneum of skin is lined with the deaminated histidine, trans-UCA, that can absorb UVB, resulting in its isomerization to cis-UCA.21 cis-UCA may mediate UVB-induced immunosuppression through the serotonin receptor.17 Topical application with cis-UCA did not significantly alter the expansion and cytotoxic activity of ova-specific CD8 T cells from those of unirradiated mice treated with base lotion (Figure 2, A and B). Similarly, treatment with trans-UCA also did not influence the ova-specific CD8 T-cell response. In contrast, cis-UCA, but not trans-UCA, reduced the DTH reaction compared with unirradiated base-treated mice (Figure 2C: P = 0.0050), close to the levels observed in UVB-irradiated mice.
Figure 2.
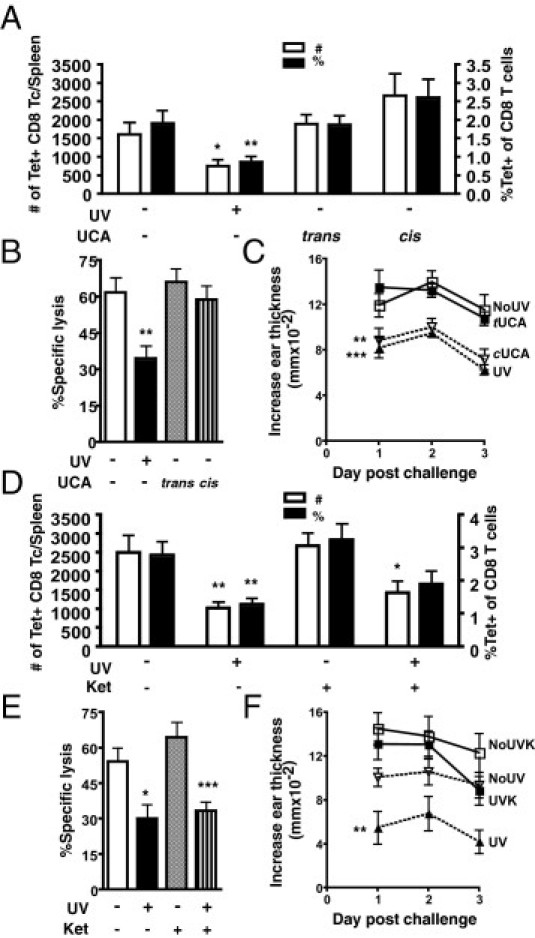
Topical cis-UCA can mimic UVB, and blocking serotonin receptor (5-HT2AR) signaling can rescue the UVB-suppressed DTH; however, neither can modulate the ova-specific CD8 T-cell (Tc) response. A: Mice were topically treated with cis-UCA or trans-UCA daily for 3 days; 3 days after the last application, mice were immunized with ova on the abdomen. Control mice were given base lotion only. Splenic CD44hi tetramer (Tet)–positive CD8 T cells were examined 7 days after immunization. The total number and the percentage of CD8 T cells are shown. B: The percentage-specific lysis from an in vivo cytotoxicity assay was determined 7 days after immunization. C: The DTH reaction from mice 7 days after immunization; mice were unirradiated with base lotion (NoUV), UV irradiated with base lotion (UV), unirradiated with trans-UCA (tUCA), and unirradiated with cis-UCA (cUCA). D: Mice were administered the specific 5-HT2AR antagonist, ketanserin (Ket), 30 minutes i.p. before each exposure to 150 mJ/cm2 UVB. Control mice were given PBS only. The total number and the percentage of CD44hi Tet-positive CD8 T cells at 7 days after immunization are shown. E: The percentage-specific lysis from an in vivo cytotoxicity assay was determined 7 days after immunization. F: The DTH reaction from mice 7 days after immunization; mice were unirradiated with PBS (NoUV), UV irradiated with PBS (UV), unirradiated with Ket (NoUVK), and UV irradiated with Ket (UVK). Data represent a pool of three to four repeat experiments (n = 9 to 12 mice per group) and are given as mean ± SEM. Comparisons between control UV irradiated versus unirradiated, cis UCA treated unirradiated versus control unirradiated, ketanserin treated irradiated versus unirradiated are shown. *P < 0.05, **P < 0.01, and ***P < 0.001.
To confirm our observations seen with cis-UCA, we administered the serotonin receptor–specific antagonist, ketanserin, to mice before UVB irradiation to block cis-UCA. Consistent with cis-UCA–treated mice, ketanserin treatment did not rescue mice from the inhibitory effects of UVB on ova-specific CD8 T cells in the spleen (Figure 2, D and E). In ketanserin-treated mice, the number of activated ova-specific CD8 T cells was still reduced by UVB (Figure 2D: P = 0.0183), and the cytotoxic activity was impaired (Figure 2E: P = 0.0008), compared with unirradiated mice. However, ketanserin prevented UVB irradiation from significantly decreasing the DTH reaction compared with unirradiated mice (Figure 2F). This is in contrast to the UVB-induced inhibition of the DTH reaction observed in mice given PBS only (Figure 2F: P = 0.0041). Therefore, although cis-UCA can suppress DTH reactions and blocking serotonin receptor signaling can rescue DTH responses from UVB-induced inhibition, low-dose UVB-induced cis-UCA was not responsible for inhibiting the CD8 T-cell response to ova.
Repair of Cyclobutane Pyrimidine Dimers Does Not Prevent the UVB-Induced Inhibition of CD8 T Cells and DTH
Absorption of UVB by the DNA of epidermal skin cells causes genetic damage. One of these types of photolesions is cyclobutane pyrimidine dimers (CPD). The use of liposomes containing T4N5, which initiates repair of CPD, has prevented systemic UVB-induced suppression of a DTH to fungal Ag.19 Therefore, immediately after each irradiation, mice were topically administered T4N5 liposomes. However, we found that T4N5 liposomes did not rescue mice from the inhibitory effects of this low-dose UVB on CD8 T-cell activation. Compared with unirradiated mice treated with T4N5 liposomes, the number (P = 0.0097), percentage (P = 0.0063), and cytotoxic activity (P = 0.0085) of splenic ova-specific CD8 T cells remained significantly decreased after UVB exposure (Figure 3, A and B). The DTH reaction also remained suppressed in T4N5-treated mice. T4N5 did not abrogate the reduced response in UVB-irradiated mice compared with unirradiated mice (Figure 3C). Controls using empty liposomes showed the same results as T4N5-containing liposomes. The activity of the T4N5 liposomes to repair CPD was verified in irradiated mouse skin by immunostaining for CPD lesions (data not shown). Thus, these results indicate that CPD formation is not critical to initiate the processes by which UVB suppresses the activation of splenic ova-specific CD8 T cells and DTH reactions.
Figure 3.
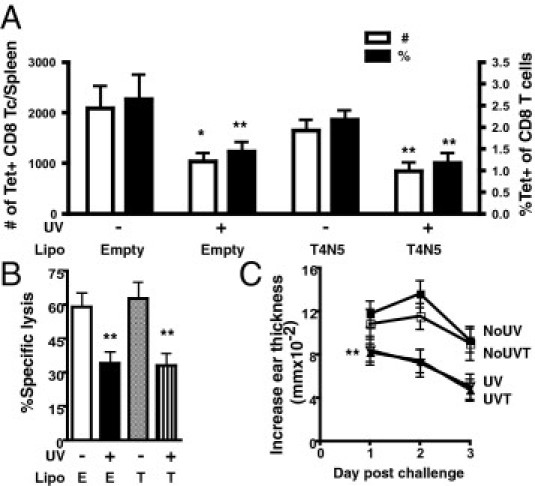
Treatment with T4N5 liposomes to repair CPD DNA damage does not reverse the inhibitory effects of UVB on CD8 T-cell (Tc) activation and DTH. A: Immediately after each UV irradiation, mice were topically treated with liposomes (Lipo) containing T4N5 repair enzymes or empty Lipo. Splenic CD44hi tetramer (Tet)–positive CD8 T cells were examined 7 days after immunization. The total number and the percentage of CD8 T cells are shown. B: The percentage-specific lysis from an in vivo cytotoxicity assay was determined 7 days after immunization in mice given empty (E) or T4N5 (T) Lipo. C: The DTH reaction from mice 7 days after immunization; mice were unirradiated with empty (NoUV), UV irradiated with empty (UV), unirradiated with T4N5 (NoUVT), and UV irradiated with T4N5 (UVT). Data represent a pool of three to four repeat experiments (n = 9 to 12 mice per group) and are given as mean ± SEM. Comparisons between empty UV irradiated versus unirradiated or T4N5 UV irradiated versus unirradiated are shown. *P < 0.05 and **P < 0.01.
Blocking the Aryl-Hydrocarbon Receptor Does Not Protect against UVB-Induced Inhibition of CD8 T Cells and DTH
When cytoplasmic tryptophan in skin cells absorbs UVB, it degrades into the photoproduct, 6-formylindolo[3,2-b]carbazole, a natural high-affinity ligand for the aryl-hydrocarbon receptor (AhR). The activation of AhR leads to the transcription of genes known to be involved in UVB-induced immunosuppression, including cyclooxygenase-2.22 Thus, we investigated whether AhR activation is orchestrating the inhibition of CD8 T-cell activation in the spleen and DTH after systemic low-dose UVB. Mice were administered the AhR antagonist, α-naphthoflavone, to prevent AhR signaling on UVB exposure. Mice treated with α-naphthoflavone and UVB exhibited decreased activated ova-specific CD8 T-cell responses [Figure 4A: P = 0.0070 (number) and P = 0.0073 (percentage)] and cytotoxic activity (Figure 4B: P = 0.0160) compared with unirradiated α-naphthoflavone–treated mice. Similarly, α-naphthoflavone did not prevent UVB from reducing DTH responses (Figure 4C: P = 0.0295). Thus, UVB activation of AhR does not appear to mediate the inhibition of these immune responses.
Figure 4.
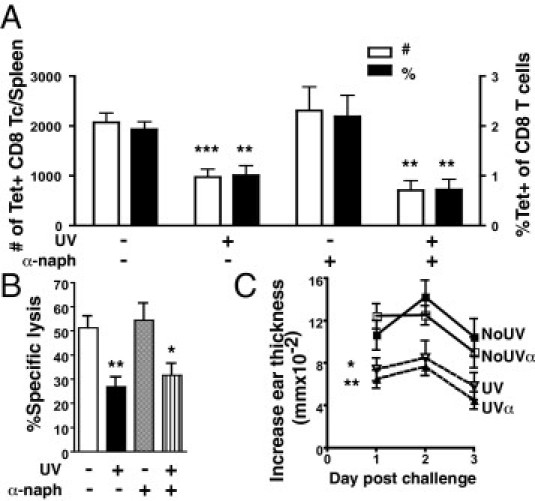
Treatment with an AhR antagonist does not prevent UVB-induced suppression of ova-specific CD8 T-cell (Tc) responses and DTH. A: Mice were topically treated with the AhR antagonist, α-naphthoflavone (α-naph), 30 minutes before each UV irradiation. Splenic CD44hi tetramer (Tet)–positive CD8 T cells were examined 7 days after immunization. The total number and the percentage of CD8 T cells are shown. B: The percentage-specific lysis from an in vivo cytotoxicity assay was determined 7 days after immunization in mice given base lotion or α-naph. C: The DTH reaction from mice 7 days after immunization; mice were unirradiated with base (NoUV), UV irradiated with base (UV), unirradiated with α-naph (NoUVα), and UV irradiated with α-naph (UVα). Data represent a pool of three to four repeat experiments (n = 9 to 12 mice per group) and are given as mean ± SEM. Comparisons between control UV irradiated versus unirradiated or α-naph UV irradiated versus unirradiated are shown. *P < 0.05, **P < 0.01, and ***P < 0.001.
Antioxidants Can Restore DTH but Fail to Protect Ova-Specific CD8 T Cells from UVB
Tryptophan, alongside other UVB chromophore candidates, including NADPH, flavins, and extracellular lipids, contributes to the production of harmful ROS in response to UVB. Antioxidant treatment can prevent UVB-induced immunosuppression23; therefore, we pretreated mice with antioxidants (ie, vitamin E and butylated hydroxytoluene) before exposing them to UVB. Antioxidant treatment did not protect the number, percentage, or cytotoxic activity of activated splenic ova-specific CD8 T cells from UVB irradiation because they were reduced compared with unirradiated and antioxidant-treated mice (Figure 5, A and B: P = 0.0259, P = 0.0262, and P = 0.0001, respectively). On the other hand, antioxidants did prevent UVB from suppressing a DTH reaction. The DTH reaction in antioxidant-treated UVB-irradiated mice was not significantly different from that in unirradiated mice (Figure 5C). Moreover, UVB-irradiated antioxidant-treated mice did show an improved DTH reaction compared with UVB-irradiated mice treated with base (P = 0.0225). Although these findings do indicate that ROS produced in response to low-dose UVB is involved in decreasing DTH, ROS do not inhibit the activation of splenic ova-specific CD8 T cells.
Figure 5.
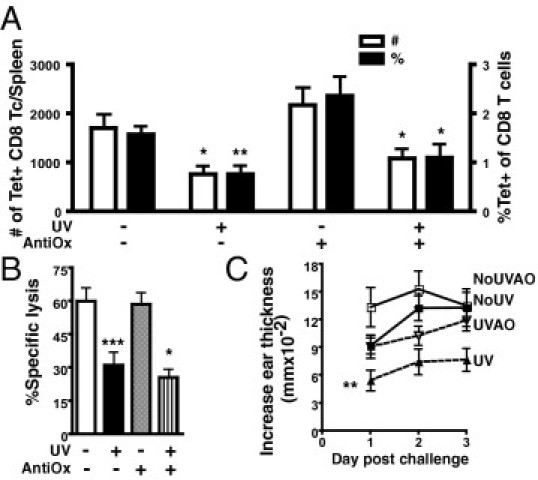
Antioxidant treatment can protect a DTH but does not reverse the inhibition of ova-specific CD8 T-cell (Tc) activation caused by UVB. A: Two hours before each UV irradiation, mice were treated with vitamin E and butylated hydroxytoluene (AntiOx) in olive oil i.p. Control mice were given olive oil only. The total number and the percentage of CD44hi tetramer (Tet)–positive CD8 T cells in spleens 7 days after immunization are shown. B: The percentage-specific lysis from an in vivo cytotoxicity assay in mice administered antioxidants or olive oil before UVB. C: The DTH reaction from mice 7 days after immunization; mice were unirradiated with olive oil (NoUV), UV irradiated with olive oil (UV), unirradiated with antioxidants (NoUVAO), and UV irradiated with antioxidants (UVAO). Data represent a pool of three to four repeat experiments (n = 9 to 12 mice per group) and are given as mean ± SEM. Comparisons between control UV irradiated versus unirradiated or antioxidant UV irradiated versus unirradiated are shown. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
Exposure to systemic low-dose UVB before immunization is detrimental to T-cell responses by inhibiting the activation of effector T cells and memory T-cell development.4 Although some UVB regimens can activate natural regulatory T cells or induce Ag-specific regulatory CD4+CD25+ T cells to suppress CD8 T-cell immunity,5,24 we found that suberythemal doses of UVB, delivered at a systemic site from Ag, do not induce functional CD4+CD25+ regulatory T cells that inhibit subsequent Ag-specific CD8 T-cell responses and DTH reactions. These experiments cannot exclude the activation of short-lived regulatory T cells at earlier times. Further examination of the key known UVB chromophores and the primary pathways they activate also demonstrated no involvement in UVB-induced inhibition of Ag-specific CD8 T cells in this model. However, cis-UCA and the ROS pathway modulated DTH reactions. More important, these findings provide evidence that systemic low-dose UVB modulates CD8 T-cell immunity and DTH by different mechanisms and that a novel pathway inhibits CD8 T-cell responses in the spleen.
This study investigated the effects of UVB on Ag-specific CD8 T-cell responses in the spleen at low UVB doses, attainable during daily sun exposure and when Ag was applied at sites not directly exposed to UVB. In comparison, most other studies examining mechanisms of UVB-induced immunosuppression have used local low-dose systems (irradiation regimens of 100 to 120 mJ/cm2 for 4 days, similar to this study) or systemic high-dose systems (single erythemal doses of 5000 to 15,000 mJ/cm2). Only a few studies have explored the mechanisms of systemic low-dose UVB; therefore, it is not unexpected that recognized pathways were not involved in this low-dose model.
The absence of UVB-induced adaptive regulatory CD4+CD25+ T cells in this study parallels a previous finding in another systemic low-dose UVB system with a hapten Ag.4 Studies by McGlade et al25 support this observation because no participation of regulatory CD4+CD25+ T cells was found in the inhibition of CD4 effector T cells in lung-draining lymph nodes during ova-induced allergic airway disease after systemic high-dose UVB. In contrast, Ghoreishi and Dutz5 demonstrated that local low-dose UVB-induced adaptive regulatory CD4+CD25+ T cells were responsible for suppressing the proliferation and interferon-γ production of transgenic ova-specific CD8 T cells in sDLNs. At low doses of UVB, the activation of regulatory CD4+CD25+ T cells may be dependent on local Ag at the UVB-exposed site. This may be because of the unknown mechanism by which UVB-induced vitamin D3 in skin can activate regulatory CD4+CD25+ T cells in sDLNs.26 Ghoreishi et al27 observed that regulatory T cells expanded by vitamin D3 can only inhibit ova-specific CD8 T cells in a local, not a systemic, model. In addition, regulatory T cells may require stimulation by CPD DNA-damaged Langerhans cells migrating into sDLNs,28 whereas this study found no requirement of CPD formation as a trigger for UVB-induced immunosuppression. Instead, it is possible that other suppressor cell types, such as UVB-induced suppressor B cells, could be indirectly inhibiting the responses of ova-specific CD8 T cells by interfering with Ag presentation in secondary lymphoid organs. Exposure to systemic low-dose solar-stimulated UV containing 140 mJ/cm2 UVB generated activated B cells in sDLNs, which were able to inhibit dendritic cells from stimulating T-cell–mediated skin immune reactions.29
Pioneering work by Kripke et al19 demonstrated that CPD formation and transferable suppressor cells are responsible for DTH suppression by systemic UVB, which is unlike this study. Notably, our investigation differed in the Ag and source of UV used. Kripke et al used killed Candida, which, although inert like ova, still expresses pathogen-associated molecular patterns, making it differentially recognizable and processed by the immune system compared with ova. Other researchers30 have shown that the nature of the Ag in the investigation is a determinant for the molecular pathway by which UVB suppresses the immune response, possibly because of varying requirements for Ag presentation. This study also used a 310-nm UVB interference filter to ensure maximal UVB-only exposure without any UVC. In comparison, most other investigations31 concerning CPD formation have used unfiltered FS40 sunlamps, which emit contaminating UVC. The enhanced potency of UVC in causing DNA damage may account for the variable importance of CPD at systemic low UVB doses. Nghiem et al 32 found CPD to be involved in solar-simulated UV suppression of DTH using a source that did not contain UVC. However, their study used 8000 mJ/cm2 UV, which is considerably higher than the 150 mJ/cm2 used in the current study. The magnitude of the dose differences between these studies suggests that higher doses of UV or spectra more efficient at causing CPD are required for these photolesions to be responsible for systemic UV immunosuppression. However, we did not examine the effects of repairing other types of DNA damage (eg, double-stranded breaks and oxidative photoproducts); therefore, it is possible that alternative forms of genetic damage may be the critical proponent of systemic low-dose UVB-induced immunosuppression in this model.
The importance of cis-UCA in mediating systemic low-dose UVB-induced immunosuppression depended on the immune reaction being examined. This finding is consistent with previous findings by Moodycliffe et al33 showing that neutralization with anti–cis-UCA antibody can prevent the generation of suppressor cells after systemic high-dose UVB, but it could not protect CHS reactions to a hapten. Evidently, cis-UCA targets specific immune processes in its mediation of UVB-induced immunosuppression, but our study found that this does not include the modulation of ova-specific CD8 T-cell responses in the spleen. Rather, cis-UCA alters components of a DTH reaction to ova, perhaps by directly stimulating CD4 T cells to secrete IL-10.34 DTH reactions are driven by type 1 helper CD4 T cells that activate local mast cells to release inflammatory mediators.35,36 The presence of type 2 helper CD4 T cells inhibits type 1 helper CD4 T cells, thereby dampening DTH reactions.37 Similarly, antioxidant treatment also failed to protect ova-specific CD8 T cells from UVB; however, it did protect DTH. Recent evidence38 demonstrates that ROS stimulated by cis-UCA on UVB irradiation can contribute to CPD and 8-oxo-deoxyguanosine DNA damage in skin. Although it is unknown if 8-oxo-deoxyguanosine in skin after UVB can regulate in vivo cellular immune processes, it may be possible that cis-UCA and ROS induced 8-oxo-deoxyguanosine after systemic low-dose UVB to trigger the inhibition of DTH.
Activation of the AhR is part of the UVB stress response; to our knowledge, this is the first study that has examined whether it mediates UVB-induced immunosuppression in vivo. No evidence indicated that UVB-induced activation of the AhR results in the inhibition of splenic Ag-specific CD8 T cells and DTH. One of the molecular pathways triggered by UVB-activated AhR is cyclooxygenase-2 up-regulation and PGE2 production.22 Because systemic low-dose UVB inhibits the activation of effector CD8 and CD4 T cells independently of the PGE2 pathway,4 it is, therefore, unlikely that AhR signaling initiates UVB-induced suppression of CD8 T cells in this model. Similarly, although platelet-activating factor was outside the scope of this study, it may be surmised that this UVB-induced inflammatory mediator is not a mechanistic factor because it suppresses DTH and CHS via PGE2.39,40 However, further examination is required to elucidate a role for platelet-activating factor in this model.
Systemic low-dose UVB significantly inhibited the activation, expansion, and cytotoxic activity of endogenous ova-specific CD8 T cells in the spleen through a process that appears to be distinct from the recognized pathways that regulate DTH and CHS reactions (namely, regulatory CD4 T cells, cis-UCA, CPD formation, and ROS). Compelling evidence from local low-dose UVB models indicates that the proliferation of Ag-specific CD8 T cells to ova protein can be suppressed by regulatory CD4+CD25+ T cells activated by vitamin D3.27 Modulation of skin dendritic cells by vitamin D3 is proposed to be important in this process; however, these cells are also prone to UVB-induced CPD.28 Skin dendritic cells take up exogenous Ag, which they then process and present to CD4 T cells in sDLNs. To an extent, they can also cross present to CD8 T cells; however, stimulation of CD8 T cells mostly occurs after Ag transfer to CD8α+ dendritic cells in secondary lymphoid organs, which then cross present to CD8 T cells.41 Ghoreishi and Dutz5 observed that proliferation of transgenic ova-specific CD8 T cells is not inhibited by local low-dose UVB when SIINFEKL peptide is the immunogen. This observation indicates that direct stimulation of CD8 T cells may not be affected by UVB and that UVB primarily impairs CD8 T-cell responses by interfering with CD4 T-cell help or cross presentation. In this systemic model, although migrating skin APCs carrying ova protein are not directly exposed to UVB, they may have become altered by changes in the sDLNs because of UVB, including the presence of UVB-modulated skin dendritic cells and inflammatory mediators that can activate suppressor B cells.42 Certainly, systemic low-dose UVB does reduce the proliferation and migration of effector CD4 T cells to a hapten, lending support to the possibility that ova-specific CD4 T cells have impaired activation.4 Interruption during the activation of ova-specific CD4 T cells or Ag transfer and cross presentation would considerably reduce the activation of naïve and endogenous ova-specific CD8 T-cell precursors, resulting in decreased CD8 T-cell immunity to ova.
The fact that novel UVB chromophores or mechanisms must be regulating CD8 T-cell immunity in the spleen has important implications for therapies that are being investigated to promote CD8 T-cell responses to tumors and skin viral infections in the presence of physiological UVB. Although they enhance CPD repair in skin to prevent the generation of cancerous cells, sunscreens containing T4N5 liposomes do little to protect CD8 T-cell responses from systemic low-dose UVB. This is particularly important when the Ag is inert and fails to activate innate immunity, such as a tumor Ag. Notably, this study shows that DTH reactions are not an appropriate measure of CD8 T-cell immunity in models of systemic UVB-induced immunosuppression because the mechanisms involved are different. The demonstration that UVB regulates immune reactions in different organs by alternative pathways importantly opens the avenue for the development of therapies that can prevent the immunosuppressive nature of low-dose UVB in the skin without compromising UV regulation of immunity in internal organs. For example, low doses of UVB could be used to control the activity of autoreactive T cells in a major reservoir organ, such as the spleen, during multiple sclerosis, alongside antioxidant or anti–cis-UCA combination therapy, to preserve the skin immune system against new Ag challenges. Further investigation of the molecular and cellular changes triggered by UVB in lymphoid organs in vivo will be required to optimize therapies to aid the eradication of tumors and viruses by cytotoxic CD8 T cells.
Acknowledgments
We thank Vivienne Reeve, Ph.D. (University of Sydney, Sydney, Australia), for constructive discussions regarding cis-UCA and for providing the base lotion and Dan Yarosh, Ph.D. (AGI Dermatics, Freeport, NY), for supplying T4N5 and empty liposomes. Tetramers were synthesized by the ACRF Biomolecular Resource Facility, John Curtin Medical School, Australian National University, Canberra, Australia.
Footnotes
Supported by a National Health and Medical Research Council grant (457405 to G.M.H.) and by Epiderm.
References
- 1.Johnson-Huang L.M., Suárez-Fariñas M., Sullivan-Whalen M., Gilleaudeau P., Krueger J.G., Lowes M.A. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol. 2010;130:2654–2663. doi: 10.1038/jid.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becklund B.R., Severson K.S., Vang S.V., Deluca H.F. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci U S A. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliday G.M., Rana S. Waveband and dose dependency of sunlight-induced immunomodulation and cellular changes. Photochem Photobiol. 2008;84:35–46. doi: 10.1111/j.1751-1097.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 4.Rana S., Byrne S.N., MacDonald L.J., Chan C.Y., Halliday G.M. Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am J Pathol. 2008;172:993–1004. doi: 10.2353/ajpath.2008.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghoreishi M., Dutz J.P. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 6.Wang L., Jameson S.C., Hogquist K.A. Epidermal Langerhans cells are not required for UV-induced immunosuppression. J Immunol. 2009;183:5548–5553. doi: 10.4049/jimmunol.0900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorman S., Tan J.W.Y., Yerkovich S.T., Finlay-Jones J.J., Hart P.H. CD4+ T cells in lymph nodes of UVB-irradiated mice suppress immune responses to new antigens both in vitro and in vivo. J Invest Dermatol. 2007;127:915–924. doi: 10.1038/sj.jid.5700600. [DOI] [PubMed] [Google Scholar]
- 8.Kripke M.L., Morison W.L. Studies on the mechanism of systemic suppression of contact hypersensitivity by ultraviolet B radiation. Photodermatology. 1986;3:4–14. [PubMed] [Google Scholar]
- 9.Schwarz A., Schwarz T. UVR-induced regulatory T cells switch antigen-presenting cells from a stimulatory to a regulatory phenotype. J Invest Dermatol. 2010;130:1914–1921. doi: 10.1038/jid.2010.59. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich S.E. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 11.Black C.A. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5:7. [PubMed] [Google Scholar]
- 12.Miller S.D., Jenkins M.K. In vivo effects of GK1.5 (anti-L3T4a) monoclonal antibody on induction and expression of delayed-type hypersensitivity. Cell Immunol. 1985;92:414–426. doi: 10.1016/0008-8749(85)90022-x. [DOI] [PubMed] [Google Scholar]
- 13.Matthews Y.J., Halliday G.M., Phan T.A., Damian D.L. A UVB wavelength dependency for local suppression of recall immunity in humans demonstrates a peak at 300 nm. J Invest Dermatol. 2010;130:1680–1684. doi: 10.1038/jid.2010.27. [DOI] [PubMed] [Google Scholar]
- 14.Hart P.H., Jaksic A., Swift G., Norval M., El-Ghorr A.A., Finlay-Jones J.J. Histamine involvement in UVB- and cis-urocanic acid-induced systemic suppression of contact hypersensitivity responses. Immunology. 1997;91:601–668. doi: 10.1046/j.1365-2567.1997.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeve V.E., Bosnic M., Boehm-Wilcox C., Ley R.D. Differential protection by two sunscreens from UV radiation-induced immunosuppression. J Invest Dermatol. 1991;97:624–628. doi: 10.1111/1523-1747.ep12483006. [DOI] [PubMed] [Google Scholar]
- 16.Merchant M., Safe S. In vitro inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced activity by alpha-naphthoflavone and 6-methyl-1,3,8-trichlorodibenzofuran using an aryl hydrocarbon (Ah)-responsive construct. Biochem Pharmacol. 1995;50:663–668. doi: 10.1016/0006-2952(95)00180-8. [DOI] [PubMed] [Google Scholar]
- 17.Walterscheid J.P., Nghiem D.X., Kazimi N., Nutt L.K., McConkey D.J., Norval M., Ullrich S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci U S A. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos G., Kazimi N., Nghiem D.X., Walterscheid J.P., Ullrich S.E. Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol Appl Pharmacol. 2004;195:331–338. doi: 10.1016/j.taap.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Kripke M.L., Cox P.A., Alas L.G., Yarosh D.B. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne S.N., Spinks N., Halliday G.M. Ultraviolet A irradiation of C57BL/6 mice suppresses systemic contact hypersensitivity or enhances secondary immunity depending on dose. J Invest Dermatol. 2002;119:858–864. doi: 10.1046/j.1523-1747.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Noonan F.P., De Fabo E.C., Morrison H. Cis-urocanic acid, a product formed by ultraviolet B irradiation of the skin, initiates an antigen presentation defect in splenic dendritic cells in vivo. J Invest Dermatol. 1988;90:92–99. doi: 10.1111/1523-1747.ep12462045. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche E., Schäfer C., Calles C., Bernsmann T., Bernshausen T., Wurm M., Hübenthal U., Cline J.E., Hajimiragha H., Schroeder P., Klotz L.O., Rannug A., Fürst P., Hanenberg H., Abel J., Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliday G.M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz A., Maeda A., Wild M.K., Kernebeck K., Gross N., Aragane Y., Beissert S., Vestweber D., Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 25.McGlade J.P., Strickland D.H., Lambert M.J., Gorman S., Thomas J.A., Judge M.A., Burchell J.T., Zosky G.R., Hart P.H. UV inhibits allergic airways disease in mice by reducing effector CD4 T cells. Clin Exp Allergy. 2010;40:772–785. doi: 10.1111/j.1365-2222.2010.03469.x. [DOI] [PubMed] [Google Scholar]
- 26.Gorman S., Kuritzky L.A., Judge M.A., Dixon K.M., McGlade J.P., Mason R.S., Finlay-Jones J.J., Hart P.H. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 27.Ghoreishi M., Bach P., Obst J., Komba M., Fleet J.C., Dutz J.P. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol. 2009;182:6071–6078. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz A., Maeda A., Kernebeck K., van Steeg H., Beissert S., Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne S.N., Halliday G.M. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol. 2005;124:570–578. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim T.H., Moodycliffe A.M., Yarosh D.B., Norval M., Kripke M.L., Ullrich S.E. Viability of the antigen determines whether DNA or urocanic acid act as initiator molecules for UV-induced suppression of delayed-type hypersensitivity. Photochem Photobiol. 2003;78:228–234. doi: 10.1562/0031-8655(2003)078<0228:votadw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Kim T.H., Ullrich S.E., Ananthaswamy H.N., Zimmerman S., Kripke M.L. Suppression of delayed and contact hypersensitivity responses in mice have different UV dose responses. Photochem Photobiol. 1998;68:738–744. [PubMed] [Google Scholar]
- 32.Nghiem D.X., Kazimi N., Mitchell D.L., Vink A.A., Ananthaswamy H.N., Kripke M.L., Ullrich S.E. Mechanisms underlying the suppression of established immune responses by ultraviolet radiation. J Invest Dermatol. 2002;119:600–668. doi: 10.1046/j.1523-1747.2002.01845.x. [DOI] [PubMed] [Google Scholar]
- 33.Moodycliffe A.M., Bucana C.D., Kripke M.L., Norval M., Ullrich S.E. Differential effects of a monoclonal antibody to cis-urocanic acid on the suppression of delayed and contact hypersensitivity following ultraviolet irradiation. J Immunol. 1996;157:2891–2899. [PubMed] [Google Scholar]
- 34.Holan V., Kuffova L., Zajicova A., Krulova M., Filipec M., Holler P., Jancarek A. Urocanic acid enhances IL-10 production in activated CD4+ T cells. J Immunol. 1998;161:3237–3241. [PubMed] [Google Scholar]
- 35.Cher D.J., Mosmann T.R. Two types of murine helper T cell clone, II: delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:3688–3694. [PubMed] [Google Scholar]
- 36.van Loveren H., Meade R., Askenase P.W. An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med. 1983;157:1604–1617. doi: 10.1084/jem.157.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo L.V., DeKruyff R.H., Umetsu D.T., Caspi R.R. Regulation of the interaction between Th1 and Th2 T cell clones to provide help for antibody production in vivo. Eur J Immunol. 1995;25:708–716. doi: 10.1002/eji.1830250312. [DOI] [PubMed] [Google Scholar]
- 38.Sreevidya C.S., Fukunaga A., Khaskhely N.M., Masaki T., Ono R., Nishigori C., Ullrich S.E. Agents that reverse UV-induced immune suppression and photocarcinogenesis affect DNA repair. J Invest Dermatol. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walterscheid J.P., Ullrich S.E., Nghiem D.X. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Q., Yao Y., Konger R.L., Sinn A.L., Cai S., Pollok K.E., Travers J.B. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Dermatol. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., Brooks A.G., Heath W.R. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 42.Matsumura Y., Byrne S.N., Nghiem D.X., Miyahara Y., Ullrich S.E. A role for inflammatory mediators in the induction of immunoregulatory B cells. J Immunol. 2006;177:4810–4817. doi: 10.4049/jimmunol.177.7.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]


