Abstract
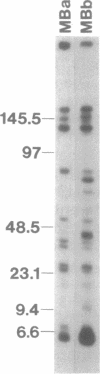
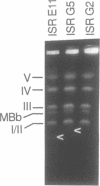
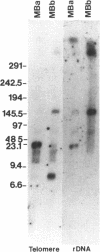
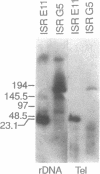
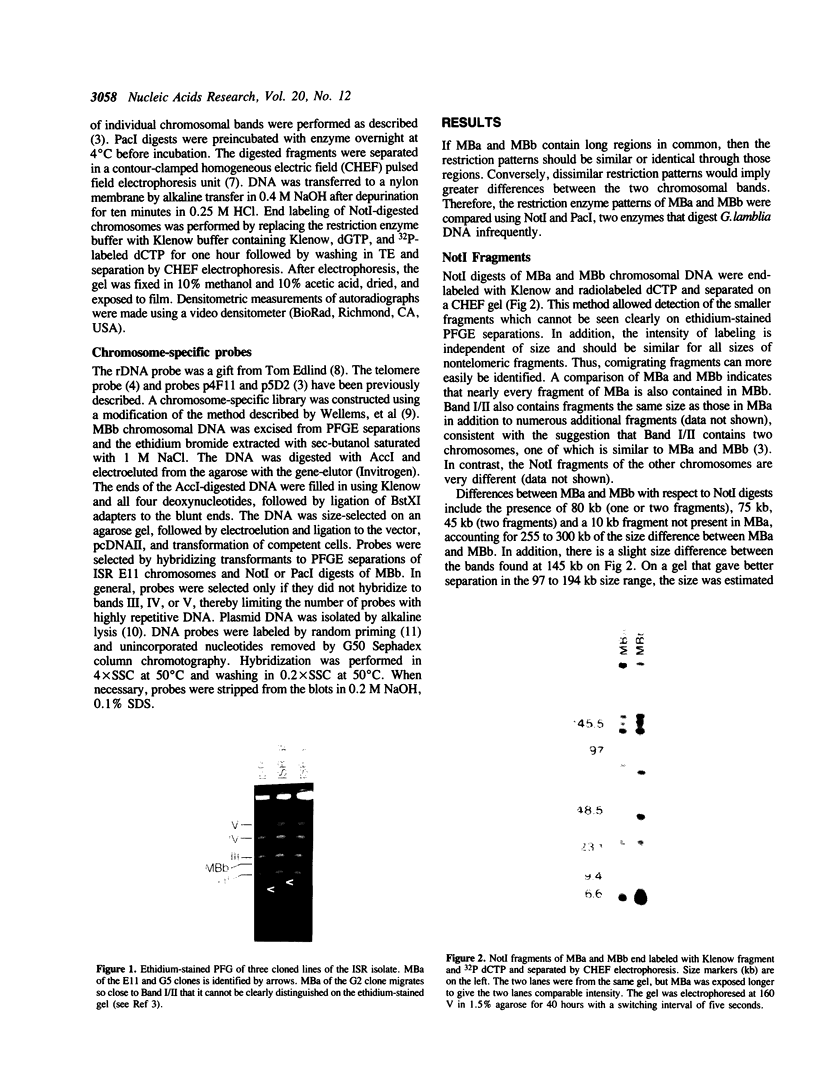
Giardia lamblia trophozoites contain at least five sets of chromosomes that have been categorized by chromosome-specific probes. Pulsed field separations of G. lamblia chromosomes also demonstrated minor bands in some isolates which stained less intensely with ethidium than the major chromosomal bands. Two of the minor bands of the E11 clone of the ISR isolate, MBa and MBb, were similar to each other and to chromosomal band I by hybridization to total chromosomal DNA and by hybridization of specific probes. In order to determine the extent of this similarity, I have developed a panel of probes for many of the Pacl restriction fragments and have shown that most of the Pacl and Notl fragments found in MBa are also present in MBb. The differences are found in both telomeric regions. At one end, MBb contains a 300 kb region not found in MBa. At the other end of MBb is a 160 kb region containing the rDNA repeats which is bounded on one end by the telomeric repeat and on the other by sites for multiple enzymes that do not digest the rDNA repeats. The corresponding region of MBa is 23 kb in size. The size difference is consistent with the eightfold greater number of rDNA repeats in MBb than MBa and suggests that 30% of the size difference is accounted for by different numbers of copies of the rDNA repeat. MBa of another ISR clone (ISR G5) is 150 kb larger in size than MBa of ISR E11. The data suggest that MBa and MBb are homologous chromosomes of different sizes and that a portion of the size difference is accounted for by different copy numbers of the rDNA repeat.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Nash T. E., Wellems T. E. Telomeric location of Giardia rDNA genes. Mol Cell Biol. 1991 Jun;11(6):3326–3330. doi: 10.1128/mcb.11.6.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R. D., Nash T. E., Wellems T. E. The Giardia lamblia trophozoite contains sets of closely related chromosomes. Nucleic Acids Res. 1988 May 25;16(10):4555–4567. doi: 10.1093/nar/16.10.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai R., van Schravendijk M. R., Anders R. F., Peterson M. G., Thomas L. M., Cowman A. F., Kemp D. J. Expression of the RESA gene in Plasmodium falciparum isolate FCR3 is prevented by a subtelomeric deletion. Mol Cell Biol. 1989 Aug;9(8):3584–3587. doi: 10.1128/mcb.9.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T. D., Chakraborty P. R. Unusual ribosomal RNA of the intestinal parasite Giardia lamblia. Nucleic Acids Res. 1987 Oct 12;15(19):7889–7901. doi: 10.1093/nar/15.19.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. B., Korman S. H., Cantor C. R., Smith C. L. Giardia lamblia: haploid genome size determined by pulsed field gel electrophoresis is less than 12 Mb. Nucleic Acids Res. 1991 Apr 25;19(8):1905–1908. doi: 10.1093/nar/19.8.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., Schittini M., Keithly J. S., Warburton P. W., Cantor C. R., Van der Ploeg L. H. Karyotype analysis of Leishmania species and its use in classification and clinical diagnosis. Science. 1986 May 9;232(4751):762–765. doi: 10.1126/science.3961502. [DOI] [PubMed] [Google Scholar]
- Gottesdiener K., Garciá-Anoveros J., Lee M. G., Van der Ploeg L. H. Chromosome organization of the protozoan Trypanosoma brucei. Mol Cell Biol. 1990 Nov;10(11):6079–6083. doi: 10.1128/mcb.10.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey A., Mitchell R., Upcroft J. A., Boreham P. F., Upcroft P. Complete nucleotide sequence of the ribosomal RNA tandem repeat unit from Giardia intestinalis. Nucleic Acids Res. 1990 Jul 11;18(13):4006–4006. doi: 10.1093/nar/18.13.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick K. S., Peattie D. A. In situ analyses reveal that the two nuclei of Giardia lamblia are equivalent. J Cell Sci. 1990 Mar;95(Pt 3):353–360. doi: 10.1242/jcs.95.3.353. [DOI] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Le Blancq S. M., Kase R. S., Van der Ploeg L. H. Analysis of a Giardia lamblia rRNA encoding telomere with [TAGGG]n as the telomere repeat. Nucleic Acids Res. 1991 Oct 25;19(20):5790–5790. doi: 10.1093/nar/19.20.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blancq S. M., Korman S. H., Van der Ploeg L. H. Frequent rearrangements of rRNA-encoding chromosomes in Giardia lamblia. Nucleic Acids Res. 1991 Aug 25;19(16):4405–4412. doi: 10.1093/nar/19.16.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Sinnis P., Wellems T. E. Long-range restriction maps of Plasmodium falciparum chromosomes: crossingover and size variation among geographically distant isolates. Genomics. 1988 Nov;3(4):287–295. doi: 10.1016/0888-7543(88)90117-6. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Walker-Jonah A., Panton L. J. Genetic mapping of the chloroquine-resistance locus on Plasmodium falciparum chromosome 7. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3382–3386. doi: 10.1073/pnas.88.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesehahn G. P., Jarroll E. L., Lindmark D. G., Meyer E. A., Hallick L. M. Giardia lamblia: autoradiographic analysis of nuclear replication. Exp Parasitol. 1984 Aug;58(1):94–100. doi: 10.1016/0014-4894(84)90024-9. [DOI] [PubMed] [Google Scholar]