Abstract
Background
Vitamin D deficiency has been associated with markers for allergy and asthma severity in children with asthma. However, its association with Chinese adult asthmatics has not been studied.
Objective
To examine whether vitamin D status is associated with lung function and total serum IgE in Chinese adults with newly diagnosed asthma.
Methods
We conducted a cross-sectional study including 435 Chinese patients aged >18 years with newly diagnosed asthma. Vitamin D status was assessed by measuring serum 25 hydroxyvitamin D (25OHD) concentrations. The primary outcomes included airflow limitation, as measured by the forced expiratory volume in 1 s (FEV1), FEV1 % predicted, and FEV1/forced vital capacity (FVC), and serum total IgE concentration.
Results
Vitamin D deficiency was prevalent in Chinese adults with asthma, with 88.9% of the subjects having 25OHD <50 nmol/l. Serum 25OHD concentration was positively correlated with FEV1 % predicted (p = 0.02, r = 0.12). After adjusting for age, sex, body mass index, smoking, month of blood collection, and symptom duration, we found significant positive associations between 25OHD concentrations and FEV1 (in liters), FEV1 % predicted, and FEV1/FVC (p for trend < 0.05 for all). The adjusted odds ratios for the highest versus the lowest 25OHD quartile were 0.50 (0.26–0.96) for FEV1 <75% predicted and 0.44 (0.20–0.95) for FEV1/FVC% <0.75. There was no significant association between 25OHD concentrations and total IgE.
Conclusions
Vitamin D deficiency was highly prevalent in Chinese asthma patients, and vitamin D status was associated with lung function.
Key Words: Vitamin D, Asthma, Lung function, FEV1, IgE
Introduction
Asthma has been recognized as a typical complex disease with a high number of factors modulating the expression of asthma-related phenotypes and/or outcomes [1,2]. It has been suggested that decreasing sun exposure may in part play a potential role in asthma's etiology [3]. Vitamin D has been thought to mediate the observed association between sun exposure and asthma [3]. Vitamin D is a nutrient and hormone that can be obtained from a few natural foods (e.g. fatty fish and fish liver oils) and fortified food (e.g. milk and cereal), and it can be generated endogenously from sunlight exposure via a photosynthetic mechanism in the skin [4]. Vitamin D deficiency has been reported in many populations, even in those living in areas with abundant sun exposure [4,5].
25-Hydroxyvitamin D (25OHD) is the major circulating form of vitamin D, and its concentration in serum has been thought to reflect the status of vitamin D [4]. A low 25OHD concentration has been recognized as a possible risk factor for several chronic lung diseases, including asthma and other respiratory disorders [2,6,7]. Epidemiologic studies have also suggested that higher maternal vitamin D intakes during pregnancy play a protective role against wheezing illnesses in young children [8,9,10]. Recently, lower vitamin D concentrations have been found to be associated with higher risks of asthma exacerbations (e.g. hospitalization and use of anti-inflammatory medications) and increased levels of serum total IgE and peripheral eosinophil count in Costa Rican children with asthma [2]. In a cross-sectional study based on the US Third National Health and Nutrition Examination Survey, a dose-dependent association between lower serum 25OHD and reduced pulmonary function, as assessed by the forced expiratory volume in 1 second (FEV1) and the forced vital capacity (FVC), was observed among participants, most of whom did not have asthma [11]. Results from in vitro studies also showed that vitamin D had the capability of reversing steroid resistance in individuals with asthma [12]. Taken together, these results suggest that vitamin D may play a role in the pathogenesis of asthma and may also influence asthma-related phenotypes.
However, there has been no epidemiological study to date examining the association between vitamin D status and asthma-related phenotypes in a large cohort of adults with asthma. We, therefore, conducted a cross-sectional study to examine whether serum 25OHD concentrations were associated with airflow limitation, as measured by FEV1 (in liters), FEV1 % predicted, and FEV1/FVC, and the markers representing allergy, including serum total IgE and blood eosinophil count, in 435 Chinese adult patients with newly diagnosed asthma.
Subjects and Methods
Diagnosis of Asthma
We included 435 unrelated adult asthma patients (268 women and 167 men) aged >18 years who are of Han ethnicity from Beijing and neighboring regions. Participants were recruited at the Pulmonary Clinic of Peking Union Medical College Hospital, one of the major referral centers in Northern China. The study protocol was reviewed and approved by the Peking Union Medical College Hospital human research ethics committee, and all subjects gave their written informed consent to participate in the study.
All cases had current asthma symptoms including wheeze, cough, waking up at night, and shortness of breath, and all participants reported no regular use of anti-inflammatory medications in the previous 6 months. Asthma diagnosis was made based on the patients’ symptoms plus objective evidence from lung function tests according to the criteria defined by the American Thoracic Society [13].
Measurement of Lung Function and IgE
Pulmonary function tests were performed in all participants according to American Thoracic Society guidelines [14] by an experienced technician in a blind manner using the MasterScreen system (Jaeger Co., Höchberg, Germany) at the Peking Union Medical College Hospital lung function test laboratory. The following parameters were documented: FEV1, FVC, peak expiratory flow rate, and flow-volume loop. These parameters were repeated until 3 measurements with a variation in FEV1 values <5% were obtained; the highest FEV1 value was used for further analysis. The patients with <70% of FEV1 % predicted underwent airway reversibility testing, with positive airway reversibility defined by both a 12% improvement in FEV1% and a ≥200-ml increase in the absolute FEV1 value after β2-agonist inhalation. The cases with ≥70% of FEV1 % predicted underwent methacholine challenge testing, and positive airway hyperresponsiveness was defined by a 20% fall in FEV1 at a dose of inhaled methacholine ≤16 mg/ml [15].
Serum total IgE was assessed using the UniCAP system (Pharmacia, Uppsala, Sweden) as per the manufacturer's instructions. High total IgE was defined as >100 kU/l. The peripheral blood eosinophil count was measured using Coulter counter techniques.
Assessment of Serum 25OHD Concentrations and Covariates
Fasting blood samples were collected, processed, and frozen within 2 h of the drawing of blood and stored at −70°C until analyzed. The serum 25OHD concentration, the major circulating form of vitamin D, was measured in 435 patients using commercial ELISA kits (Immunodiagnostic Systems Limited, Bolden, UK) according to the manufacturer's recommendations. The limit of detection was 5 nmol/l. Laboratory personnel were blinded to asthma severity. The coefficients of variation were 4.6% when the average concentration of 25OHD was 40.3 nmol/l and 6.4% when the average concentration was 72 nmol/l.
Information on age, sex, and smoking status was collected via a questionnaire. Symptom duration was determined by a review of medical records. Weight and height were measured during the interview by trained field workers. The body mass index (BMI) was calculated as weight (kg)/height (m)2.
Statistics
Statistical analyses were performed with SAS version 9.1 (SAS Institute, Inc., Cary, N.C., USA). Logarithmic transformations were performed for serum 25OHD and total IgE to normalize the distribution of data. Participants were also divided into quartile categories of serum vitamin D concentration. Means were compared using the General Linear Models procedure in SAS with the Dunnett adjustment for multiple comparisons. We also conducted secondary analyses using multiple logistic regressions to estimate the odds ratios (ORs) of the likelihood of having abnormal lung function (FEV1 % predicted and FEV1/FVC) and the total IgE values. Analyses were adjusted for age (years), sex, BMI (<23, 23–26.9, or ≥27), smoking (current, former, or never), season of blood collection, and symptom duration (years). Linear trends were tested for significance by regressing log-transformed 25OHD levels on lung function measures, with adjustment for the covariates listed above. In a sensitivity analysis, we regressed the 25OHD levels on the periodic function –sin(2πx/12)–cos(2πx/12), where x is the month of sample collection, the age at sample collection, and sex. The residuals from this model were added to the sex-specific 25OHD means derived from the model to create an adjusted 25OHD measurement [16]. We tested whether the associations based on this adjusted level were consistent with what we observed using the original 25OHD values.
We also examined the interaction of vitamin D concentration with other important risk factors for lung function and total IgE, including age (<42 vs. ≥42 years, based on the median value), sex, smoking status (ever vs. never), and symptom duration (<5 vs. ≥5 years, based on the median value).
Results
Characteristics of the Subjects
None of participants included in the current study had received regular treatment recommended by the current guideline, particularly anti-inflammatory medication use [1]. Among these patients, 75.2% reported allergic rhinitis and 51.8% of subjects were allergic to at least 1 common allergen defined by a skin prick test. No patient reported skin covering. An airway challenge test was performed in the asthma subjects with ≥70% of baseline FEV1 % predicted (33% of the asthma patients), and all of these subjects were documented to have positive airway hyperresponsiveness. Participants with higher 25OHD were less likely to be women (table 1). No clear relationships were observed between serum vitamin D status and age, BMI, smoking status, the presence of allergic rhinitis, and symptom duration (table 1). As expected, we observed a clear pattern in the association between the month of blood collection and 25OHD. The lowest level of 25OHD was seen from January to March, while the highest level was seen from June to October (data not shown).
Table 1.
Characteristics of participants according to serum 25OHD concentrations
| Quartile of serum 25OHD concentration |
||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| n | 108 | 109 | 109 | 109 |
| Median, nmol/l | 16.3 | 23.9 | 32.5 | 49.3 |
| Age, years | 43.8 ± 1.2 | 41.0 ± 1.2 | 42.4 ± 1.2 | 43.4 ± 1.2 |
| Females, % | 67.6 | 67.0 | 62.4 | 49.5* |
| BMI | 23.2 ± 0.3 | 23.7 ± 0.2 | 23.5 ± 0.3 | 23.6 ± 0.3 |
| Past smokers, % | 17.5 | 10.9 | 16.4 | 20.2 |
| Current smokers, % | 8.7 | 8.9 | 8.7 | 12.1 |
| Symptom duration, years | 10.8 ± 0.8 | 9.7 ± 0.6 | 8.8 ± 0.8 | 8.2 ± 0.9 |
| Presence of allergic rhinitis, % | 75.0 | 73.2 | 76.9 | 76.2 |
Age- and sex-adjusted means ± SE.
p < 0.05 relative to the lowest quartile.
Prevalence of Vitamin D Deficiency in Patients
The concentration of 25OHD ranged from 9 to 85 nmol/l. Of the 435 participants, 89.0% (387 participants) had serum 25OHD <50 nmol/l (referred to as a deficiency) while only 2.8% (12 participants) had a concentration >75 nmol/l (referred to as a sufficiency). Only 1.5% of women had sufficient vitamin D status, relative to 4.8% of men (p = 0.05).
Association of Serum 25OHD Concentrations with Lung Function and Serum Total IgE
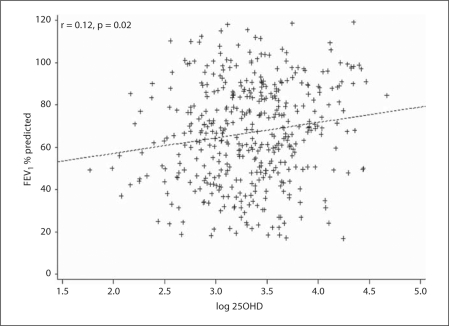
Participants with a higher 25OHD concentration had a higher FEV1 % predicted (p = 0.02, r = 0.12) (fig. 1). After adjusting for age, smoking, sex, symptom duration, BMI, and the season of blood collection, there remained a significant association between a higher 25OHD concentration and a higher FEV1 (l) (p = 0.02, r = 0.11). There was a significant association between higherserum 25OHD concentrations and better lung function as evaluated by the absolute FEV1 value, FEV1 % predicted, and FEV1/FVC (p for trend <0.05 for all) (table 2) after adjusting for smoking, symptom duration, the season of blood collection, and other covariates. Furthermore, the adjusted ORs comparing 2 extreme 25OHD concentration quartiles were 0.50 (95% CI 0.26–0.96; p = 0.007) for FEV1 <75% of predicted and 0.44 (95% CI 0.20–0.95; p = 0.007) for FEV1/FVC% <0.75 (table 2). However, there were no significant associations between vitamin D status and either total IgE or the peripheral blood eosinophil count.
Fig. 1.
Plot of a linear association between log-transformed serum 25OHD (nmol/l) concentrations and FEV1 % predicted (%) (p = 0.02, adjusted r = 0.12). Linear trends were tested for significance by regressing log-transformed 25OHD levels on FEV1 % predicted, with adjustment for age (years), sex, BMI (<23, 23–26.9, or ≥27), smoking (current, former, or never), season of blood collection, disease duration (years), and other confounders.
Table 2.
Association between serum 25OHD concentrations and lung function, total IgE, and peripheral eosinophil count
| Q uartile of serum 25OHD concentration |
p trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| FEV1, liters | 2.0 ± 0.07 | 2.0 ± 0.07 | 2.0 ± 0.07 | 2.1 ± 0.07 | 0.03 |
| FEV1 % predicted, % | 62.9 ± 3.8 | 65.1 ± 3.7 | 72.8 ± 3.7 | 72.9 ± 3.7 | 0.02 |
| Participants with FEV1 <75% predicted, % | 81.5 | 75.0 | 79.6 | 72.5 | |
| Adjusted OR | 1 (ref.) | 0.57 (0.31–1.07) | 0.53 (0.28–1.03) | 0.50 (0.26–0.96)* | 0.007 |
| FEV1/FVC, % | 60.9 ± 1.3 | 63.2 ± 1.3 | 63.0 ± 1.3 | 66.2 ± 1.3* | 0.0005 |
| Participants with FEV1/FVC <0.75, % | 70.4 | 57.4 | 57.9 | 54.1 | |
| Adjusted OR | 1 (ref.) | 0.67 (0.32–1.39) | 0.76 (0.35–1.68) | 0.44 (0.20–0.95)* | 0.007 |
| Total IgE, kU/l | 230 ± 1.2 | 174 ± 1.2 | 234 ± 1.2 | 192 ± 1.2 | 0.50 |
| Participants with total IgE >100 kU/l, % | 81.3 | 77.8 | 81.5 | 77.9 | |
| Adjusted OR | 1 (ref.) | 0.99 (0.53–1.83) | 1.18 (0.62–2.23) | 0.91 (0.48–1.71) | 0.59 |
| Eosinophil count, × 109/l | 0.59 ± 0.06 | 0.39 ± 0.06 | 0.45 ± 0.06 | 0.46 ± 0.06 | 0.17 |
| Eosinophils, % | 7.3 ± 0.5 | 6.3 ± 0.5 | 7.0 ± 0.5 | 6.6 ± 0.5 | 0.92 |
Adjusted means ± SE. Adjusted for age (years), sex, smoking (current, past, or never), BMI (<23, 23–27, ≥27), season of blood collection, and symptom duration (years). ref. = Reference.
p < 0.05 relative to the lowest quartile.
When we restricted to never-smokers, we observed significant associations of serum vitamin D with the absolute FEV1 value, FEV1 % predicted, and FEV1/FVC (p < 0.05 for all), as in our primary analyses. Likewise, there was no association between 25OHD concentrations and total IgE among never-smokers. Using the adjusted 25OHD as the exposure generated similar results; vitamin D concentrations were significantly associated with a higher FEV1 % predicted (p for trend = 0.007) and FEV1/FVC% (p for trend = 0.006). We also observed a positive trend between a higher vitamin D concentration and a higher FEV1 % predicted among subjects with >75% FEV1 predicted (p for trend = 0.25).
We did not observe significant interactions between vitamin D concentration and age, smoking status, overweight, or symptom duration in relation to measures of lung function (p interaction > 0.1 for all).
Discussion
In this sample of Chinese adults with asthma, vitamin D deficiency was highly prevalent (approximately 90%). Low serum vitamin D concentrations were significantly associated with airway obstruction, as evaluated by FEV1 and FEV1/FVC. These associations were independent of several potential confounding factors, such as smoking, body mass index, sex, and age. Because all participants are Han Chinese living in Beijing, China, and immediate surrounding areas (latitude 39°54′ north), this homogeneous population has relatively fewer unmeasured or uncontrolled (residual) confounding factors by ethnicity (i.e. skin color) and other variables, such as sun exposure level. The diagnosis of asthma was defined by current asthmatic symptoms plus explicit diagnostic tests such as airway hyperresponsiveness to methacholine or positive airway reversibility to short-acting β2-agonist. Further, we included only newly diagnosed adult patients and thus, to a greater extent, minimized the possibility of phenotypic heterogeneity which may cause a spurious association.
Our observations of a positive association between vitamin D status and lung function in these Chinese asthma patients extend the findings of a previous study in which higher serum vitamin D concentrations were associated with better values of FEV1 and FVC in a representative sample of the general population of US adults [11]. Our findings are also consistent with findings from a recent study of 54 US adult asthmatics, where vitamin D levels were also positively associated with lung function [17].
Elevated serum total IgE is a risk factor for asthma independently of the allergen-specific IgE [18]. In a British birth cohort including 9,377 participants born in 1958, a significantly nonlinear relationship between serum vitamin D and total IgE was observed [19]. Although in our primary analysis we did not observe a significant association between serum 25OHD and total IgE, we found that participants with a sufficient vitamin D concentration (≥75 nmol/l) had a lower risk of having an elevated IgE level (OR = 0.3; 95% CI 0.10–1.18) relative to those with a severe vitamin D deficiency (<20 nmol/l), but this did not achieve statistical significance.
Vitamin D could be involved in asthma pathogenesis through several mechanisms. Vitamin D receptor and vitamin D metabolic enzymes have been found in many cells including immune cell types [20,21], and vitamin D receptor gene polymorphisms have been shown to be associated with asthma risk in both Caucasians and Chinese [22,23]. It has been shown that vitamin D inhibits IL-4 and the IL-4-induced expression of IL-13 in both CD4+ and CD8+ human cord blood cells [24]. Further, vitamin D, in a dose-dependent manner, induces Fox3+ regulatory T cells via its anti-inflammatory capability and prompts a tolerogenic phenotype in human dendritic cells [25]. However, it is noted that a large dose of vitamin D supplementation during infancy has been thought to be able to enhance Th2 responses [26] and, therefore, increases the risk of having allergy and asthma in later life [27,28]. These seemingly contradictory findings regarding immune cell functioning may be due to the timing of exposure to vitamin D, i.e. prenatal versus postnatal exposure. Further studies are needed to clarify the role of vitamin D in immune cell development and function. In a recent study, Sutherland et al. [17] found a significant inverse association between BMI and serum vitamin D among inhaled corticosteroid-untreated asthmatic patients. Although we observed that obese participants tended to have lower vitamin D concentrations, the association was not significant (data not shown).
It has been shown that, in addition to affecting immune cells, vitamin D affects smooth muscle function and proliferation, which has a direct relevance for lung function in asthma and in airway remodeling [29]. Airway remodeling is an important feature of asthma and is correlated with airflow limitation [30]. Vitamin D influences airway remodeling by affecting smooth muscle cell movement, growth, and contractility and by inhibiting transforming growth factor-β and matrix metalloproteinase as well as fibroblast proliferation [29,31]. Furthermore, in animal models, vitamin D has been shown to affect lung development and maturation in utero and in the immediate postpartum period [32,33].
Previous studies have consistently shown that vitamin D deficiency (serum 25OHD <50 nmol/l) is prevalent in general Chinese populations; the prevalence ranges from 69 to 94% depending on the latitude and age [34,35]. These studies, together with our findings, suggest that vitamin D deficiency may be rather common in the Chinese population and understanding its adverse impact on health outcomes, therefore, has important public health implications.
Our study has several limitations. Its cross-sectional design precludes the ability to discern temporal associations. A longitudinal study in both children and adults may elucidate a clearer relationship. It is possible that patients with severe asthma symptoms reduced their outdoor activities which thus led to a reduced exposure to sunlight. However, given that these are all newly-diagnosed asthmatics, this likely has a negligible effect on our results. Further, the use of anti-inflammatory drugs could confound the association between vitamin D and lung function [2]. However, the patients included in the current study had never received any regular inhaled or systemic glucocorticosteroids before blood samples were collected after careful review of the medical records. We cannot exclude the possibility that a few of the participants used anti-inflammatory medications without a prescription. However, as the number of such participants should be rather small, its effect on the observed association between vitamin D and lung function would thus also be small. Additionally, we did not have detailed information on the socioeconomic status and lifestyle details (e.g. rural vs. urban) of the participants. Finally, we did not collect information on the use of vitamin D supplements. However, there are no foods fortified with vitamin D in China. The prevalence of vitamin D supplement use has also been shown to be rather low in Chinese adults [34].
In summary, we found a high prevalence of vitamin D deficiency in a sample of Chinese asthma patients. Low serum vitamin D was associated with lower lung function. Prospective studies are warranted to clarify the direction of the observed relationship between vitamin D status and asthma severity.
Financial Disclosure and Conflicts of Interest
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this paper.
Acknowledgements
This study was in part supported by grants from the Education Ministry of China (NCET 2006-0156), the Natural Sciences Foundation of China (No. 30470767), and NIH 1R01 NS062879-01A2.
We acknowledge Professors Xueying Zhou and Xiaoping Xing for their helpful discussions and valuable suggestions. We thank Yi Ma for the help in the measurement of lung function.
References
- 1.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 2.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 6.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 8.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 10.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39:875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 11.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 12.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma – this official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 14.Standardization of spirometry – 1987 update: official statement of American Thoracic Society. Respir Care. 1987;32:1039–1060. [PubMed] [Google Scholar]
- 15.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, et al. Guidelines for methacholine and exercise challenge testing – 1999: this official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 16.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherrill DL, Stein R, Halonen M, Holberg CJ, Wright A, Martinez FD. Total serum IgE and its association with asthma symptoms and allergic sensitization among children. J Allergy Clin Immunol. 1999;104:28–36. doi: 10.1016/s0091-6749(99)70110-7. [DOI] [PubMed] [Google Scholar]
- 19.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE – a significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 20.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol. 2009;9:202–207. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittke A, Chang A, Froicu M, Harandi OF, Weaver V, August A, Paulson RF, Cantorna MT. Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch Biochem Biophys. 2007;460:306–313. doi: 10.1016/j.abb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med. 2004;170:1057–1065. doi: 10.1164/rccm.200404-447OC. [DOI] [PubMed] [Google Scholar]
- 23.Saadi A, Gao G, Li H, Wei C, Gong Y, Liu Q. Association study between vitamin D receptor gene polymorphisms and asthma in the Chinese Han population: a case-control study. BMC Med Genet. 2009;10:71. doi: 10.1186/1471-2350-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 26.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 27.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann NY Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 29.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol. 1999;104:509–516. doi: 10.1016/s0091-6749(99)70315-5. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee A, Damera G, Bhandare R, Gu S, Lopez-Boado Y, Panettieri R, Jr, Tliba O. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br J Pharmacol. 2008;155:84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besancon F, Cayre YE, Garabedian M. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89–90:93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297:L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo J, Lam CW, Leung J, Lau WY, Lau E, Ling X, Xing X, Zhao XH, Skeaff CM, et al. Very high rates of vitamin D insufficiency in women of child-bearing age living in Beijing and Hong Kong. Br J Nutr. 2008;99:1330–1334. doi: 10.1017/S0007114507844382. [DOI] [PubMed] [Google Scholar]