Abstract
This pilot study describes a relationship between insulin resistance and µ-opioid neurotransmission in limbic appetite and mood-regulating regions in women with polycystic ovary syndrome, suggesting that insulin-opioid interactions may contribute to behavioral and reproductive pathologies of PCOS. We found that 1) insulin resistant PCOS patients (n=7) had greater limbic µ-opioid receptor availability (non-displaceable binding potential) than controls (n=5), 2) receptor availability was correlated with severity of insulin resistance, and 3) receptor availability normalized after insulin-regulating treatment.
Keywords: Positron emission tomography, glucose regulation, mu-opioid receptors, neuroimaging, beta-endorphins, PCOS, insulin resistance
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in young women(1), affects up to 18% of reproductive-aged women(2). Reproductive and metabolic disruptions include hyperandrogenism and infertility, insulin resistance (IR), and a higher prevalence of impaired glucose tolerance and type 2 diabetes(3–9).The endogenous opioid system may contribute to PCOS pathogenesis, through central effects on gonadotropin secretion and peripheral effects on glucose metabolism(10–16). Endogenous opioidsmediate insulin’s neuromodulatory effects, and almost 90% of cells containing insulin receptors are immunoreactive for the µ-opioid receptor (µOR) agonistβ-endorphin, suggesting interactions between insulin and opioid neurotransmission(17, 18).
Opioid abnormalities in PCOS and the impact of potential modulation by insulin are presently unknown, but evidence suggests decreased central opioid sensitivity in PCOS patients.Luteal phase luteinizing hormone (LH) pulsatility slowing by progesterone, which is largely absent in PCOS, is mediated by GnRH release inhibition by β-endorphin(19, 20). CNS opioid system changesin PCOS patientshave not been reported.
We evaluated IR effects on µORs in women with insulin resistant PCOS (IR-PCOS), and used positron emission tomography (PET) with the µ-receptor selective radiotracer [11C] carfentanil(21, 22) to compare receptor availabilityto non-IR controls, and to assess the ability of the hypoglycemic agent metformin to restore µOR concentrations. We hypothesized decreased opioid activity in IR-PCOS, reflected by unbound µORs, and increased opioid activity after metformin treatment, in the β-endorphin projection regions nucleus accumbens/ventral pallidum and amygdala(23).
Women aged 21 – 40 years were recruited intoIR-PCOS patients (n=7) andnon-IRcontrols (n=5) groups. PCOS criteriawere irregular menstrual cycles/amenorrhea and hyperandrogenism(24). IR was estimated using the homeostatic model (HOMA2-IR; glycemia(mmol/1) × insulinemia(µU/ml)/22.5)(25), using HOMA2-IR sensitivity ≤60%(26). Controls had regular menstrual cycles, normal androgens and glucose tolerance,no hirsutism or acne, and HOMA2-IR sensitivity ≥80%.
Participants were healthy, right-handed, nonsmokers, excluded forsignificant illness including diabetes, hormones within 2 months, pregnancy within 6 months, centrally-acting medications, substance abuse,MRI contraindications, corticosteroid, cimetidine use, and opioid allergy.
Women underwent medical histories, physical exam, and fasting glucose, insulin, 2 hour 75g dextrose oral glucose tolerance test (OGTT), free and total testosterone, dehydroepiandrosterone sulfate (DHEAS), lipids, blood count, TSH, electrolytes, and liver function screening.
Procedures were approved by the University of Michigan Institutional Review Board and Radiation Safety Review Committee. Written informed consent was obtained.
MRI and [11C]carfentanil PET scansoccurredduring the follicular phase, if cycling.. IR-PCOS patients subsequently began metformin (500mg titrated to1500mg daily). PET scan and OGTT were repeated after four months of treatment.
Subjects underwent one (controls) or two (PCOS patients) 70 minute PET scans (HR+ scanner;Siemens, Knoxville) in 3-dimensional mode (reconstructed full-width/half-maximum resolution, ≈5.5mm in plane, 5.0mm axially), with septa retracted and scatter correction, collecting 28 increasing duration frames. Tracer quantity [11C]carfentanil was administered (10–15mCi, ≤0.03µg/kg) via intravenous line (50% in initial bolus and remainder continuously infused for constant concentrations). [11C]carfentanil was synthesized athigh specific activity by the reactionof [11C]methyl iodide and a normethyl precursor(27).
Images were decay-corrected and reconstructed, and the dynamic frames coregistered to each other and transformed into tracer transport (K1 ratio) and receptor-related(BPND, binding potential) measures, using the occipital cortex as reference region, which lacks µORs,calculated using a modified Logan graphical analysis(28). After 5–7 minutes of radiotracer administration, the Logan plot becomes linear with slope=BPND+1, which is proportional to µORconcentration (Bmax)/receptor radiotracer affinity (Kd) (Bmax/Kd ≈ BPND).
Anatomical MR images were acquired axially with a 3T scanner (GE, Milwaukee) with a spoiled gradient recalled 3D volumetric acquisition (repetition time= 9.6, echo time=3.3, inversion recovery preparation=200ms, flip angle=17°, bandwidth=15.63, 24-cm field-of-view, 1.5mm slice thickness, 106–110 slices, 256×256 matrix, 2 excitations). T1-weighted MR and PET images were coregistered to each other and the International Consortium for Brain Mapping/Montreal Neurological Institute (ICBM/MNI) template(29).
PET images were analyzed usingSPM2(Wellcome Cognitive Neurology, London) and SPSS (SPSS Inc., Chicago). Groupcomparisons were performed using unpaired (control versus PCOS) or paired (pre- versus post-metformin) T tests on µOR BPND data extracted from the nucleus accumbens/ventral pallidum and amygdala. Significance was set at p<0.001 uncorrected with a priori hypotheses. Relationships between µORBPND and IRwere determined using Pearson correlations at p<0.05.
Demographic and clinical information is provided in Table 1A. Mean (SD)age was 26.1 (3.5). PCOS women had oligomenorrhea (cycle length>35 days), and had higher BMI, weight, waist circumference, total and free testosterone, insulin, and HOMA-IR compared to controls. Improvements following metformin treatment did not reach statistical significance.
| A. Demographic and clinical measures | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | PCOS | pa | PCOS | pb | ||||
| n: 5 controls & 7 PCOS patients |
pre- metformin |
control vs. pre- metformin |
post- metformin |
pre- vs. post- metformin |
||||
| Median (IQR) | Median (IQR) | Median (IQR) | ||||||
| Age (yr) | 26 | (8) | 25 | (6) | 0.742 | |||
| Education (yr) | 16 | (4) | 17 | (4) | 0.743 | |||
| Weight (lb) | 123.7 | (35.3) | 203.9 | (85.8) | 0.007 | 187.1 | (98.6) | 0.499 |
| BMI | 23.0 | (3.1) | 35.3 | (16.2) | 0.019 | 31.7 | (15.4) | 0.108 |
| Waist circumference (cm) | 72.0 | (13.5) | 102.0 | (27.6) | 0.004 | 94.5 | (27.6) | 0.176 |
| Free testosterone (pg/ml) | 0.5 | (0.2) | 1.5 | (0.9) | 0.006 | |||
| Total testosterone (ng/ml) | 0.36 | (0.32) | 0.70 | (0.51) | 0.073 | |||
| DHEAS (ug/dL) | 189 | (88) | 200 | (88) | 0.927 | |||
| Total cholesterol (mg/dl) | 179 | (60) | 157 | (46) | 0.073 | |||
| HDL (mg/dl) | 76 | (24) | 47 | (17) | 0.073 | |||
| Triglycerides (mg/dl) | 72 | (37) | 74 | (42) | 0.416 | |||
| Fasting insulin (uU/ml) | 8.1 | (0.9) | 21.4 | (5.1) | 0.004 | 18.7 | (11.5) | 0.398 |
| Fasting glucose (mg/dl) | 89 | (7) | 87 | (15) | 0.683 | 94 | (14) | 0.400 |
| HOMA % sensitivity | 95.4 | (12.8) | 36.6 | (9.3) | 0.004 | 43.6 | (42.2) | 0.398 |
| HOMA insulin resistance | 1.0 | (0.2) | 2.7 | (0.6) | 0.004 | 2.4 | (1.5) | 0.225 |
| B. In vivo µ-opioid receptor availability before and after metformin treatment | ||||
|---|---|---|---|---|
| PCOS pre-metformin > | 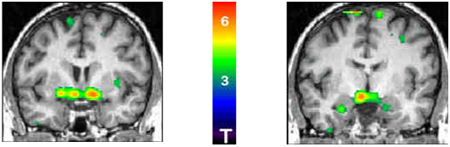 |
|||
| PCOS post-metforminc | ||||
|
Left Nucleus Accumbens |
Right Nucleus Accumbens |
Left Amygdala |
Right Amygdala |
|
| µ-Opioid binding potential (mean ± SD)d | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| PCOS (pre-metformin) | 1.95 ± 0.17 | 2.40 ± 0.11 | 2.28 ± 0.17 | 2.28 ± 0.23 |
| PCOS (post-metformin) | 1.65 ± 0.27 | 2.11 ± 0.21 | 2.08 ± 0.19 | 2.11 ± 0.22 |
| Control | 1.53 ± 0.28 | 2.05 ± 0.28 | 2.03 ± 0.07 | 2.06 ± 0.26 |
| Binding potential correlation (R(p))e | R (p) | R (p) | R (p) | R (p) |
| Fasting insulin (mU/mL) | .633 (.027) | .706 (.010) | .618 (.032) | .303 (.339) |
| HOMA % sensitivity | −.689 (.013) | −.737 (.006) | −.610 (.035) | −.315 (.319) |
| HOMA insulin resistance | .643 (.024) | .720 (.008) | .634 (.027) | .306 (.333) |
Mann-Whitney test between controls and PCOS patients pre- -metformin treatment
Wilcoxon signed ranks test between PCOS patients before and after metformin treatment
Regions with greater µ-opioid receptor binding potential in pre-treated patients (T test, compared to post-treatment)
µ-Opioid binding potential expressed as Bmax/Kd; Bmax = receptor concentration and Kd = receptor affinity for radiotracer
Pearson correlation coefficient and 2-tailed significance level across controls and PCOS patients pre-insulin regulation
Baseline µ OR BPND was greater in IR-PCOS women than controls in the nucleus accumbens/ventral pallidum (coordinates, x,y,z (mm), left −6,−4,−12, cluster size 3088mm3, p=0.009; right 10,0,−10, cluster size 2912mm3, p=0.012) and amygdala, bilaterally (left −26,−8,−24, cluster size 896mm3, p=0.045; right 18,−4,−22, cluster size 416mm3, p=0.161).
After 4 months of metformin treatment, BPND in IR-PCOS women was reduced in the nucleus accumbens/ventral pallidum by15.2(10.5)% (left, t=3.95, p=0.008) and 12.2(7.7)% (right, t=4.26, p=0.005) and in the amygdala by 8.7(7.2)% (left, t=3.07, p=0.022) and 7.1(7.7)% (right, t=2.66, p=0.038). BPND values were not statistically different from controls.
Baseline regional µORBPND was correlated with IR (fasting insulin, HOMA% sensitivity and HOMA-IR) for the nucleus accumbens/ventral pallidum bilaterally and the left amygdala (table 1b). The change in µORBPND after treatment was not significantly correlated withIR improvements.
This is the first study to evaluate the link betweenopioid neurotransmission and IR in PCOS.We focused our analysis on the amygdala and nucleus accumbens, limbicβ-endorphin neurotransmission projection areaswith reproductive, metabolic, appetite, and mood function, all of which can be disordered in PCOS(8, 30, 31). We found that IR-PCOS patientsshowed greater µORavailability in these regions thannon-IRcontrols. Four months of metformin treatment decreased receptor availability to levels similar to controls. These resultssupport insulin modulation of central opioid activity in PCOS.
Insulin receptorsare expressed in proopiomelanocortin (POMC) containing neurons, the precursor of β-endorphin. Arcuate nucleus POMC neurons project to the amygdala, nucleus accumbens and hypothalamus, where they regulate mood, reward processing, including food reward, and the hypothalamic-adrenal and hypothalamic-gonadal axes(32–35). Detection of altered opioid receptor measures in IR-PCOS patients in these regions provides information about PCOS pathophysiology, and suggests a mechanism to explain neuroendocrine dysregulation, increased incidence of depression, and appetite dysregulation and obesity associated with PCOS(8, 30, 36).
A classic neuroendocrine feature of PCOS is rapid and high amplitude LH pulses(37, 38). Opioids are believed to mediate sex steroids negative feedback on gonadotropin release via the hypothalamus and amygdala(21, 39).Although we did not test for presynaptic opioid release, altered µORavailability in the IR-PCOS subjects may indicate reduced central opioid activity and compensatory receptor upregulation. Consistent with the hypothesized relationships between µORavailability and insulin, we observed positive correlations between baseline µORBPND andIR.Metformin treatment normalized µORBPND in IR-PCOS patients, suggesting that improved insulin sensitivity restores dysregulated opioid neurotransmission, consistent with research showing that chronic opioid receptor antagonism has metabolic and reproductive effects only in PCOS patients with IR(10–12, 40–43).
Altered opioid function in IR-PCOS may contribute to appetite and mood difficulties observed in PCOS(8, 30, 36, 44). Opioid transmission in the ventral pallidum/nucleus accumbens regulates reward processing and hedonic function/motivation, and µORagonistsstimulate food-intake and appetite for high-fat/high-glucose foods through amygdala and nucleus accumbens reward circuitry(34, 35, 45–47). The ventral basal ganglia and amygdala’s rich population of µORsregulate affective states(32). In women, major depression is associated with opioid system dysregulation in the amygdala(33). Abnormal glucose metabolism is also linked to depression, and diabetesprevalence is 2–3 times higher in depressed individuals (48–50).
Study strengths include intrasubject design and control comparison.Limitationsare sample size, lack of placebo, and no comparison with non-IR PCOS women.Hypothalamic nucleiare not easily assessed with PET because of size, limitedresolution,and averaging of activity with surrounding areas. In future studies, a challenge intervention to induce endogenous opioid release would allow presynaptic function evaluation, and distinguish isolated changes in receptor availability from alterations in presynaptic endogenous opioid tone(22, 29, 51).
The endogenous opioid systemmay represent an essential link in the interface between the reproductive and metabolic systems. While further study is required to understand the complex relationship between insulin function and opioid transmission in PCOS, this pilot study provides strong initial evidence of altered metabolic-opioid system interactions in IR women with PCOS. We found altered µORavailability prior to metformin treatment in regions that regulate appetite and mood, and modulation of receptor sites after metformin treatment. Our results suggest that insulin-opioid interactions contribute to behavioral and reproductive pathologies of PCOS, which should be explored in comprehensive larger-scale studies.
ACKNOWLEDGEMENTS
We thank the University of Michigan PET Center and fMRI laboratory, Anne Tkaczyk for study coordination, and especially the participants of our study.
Grants and fellowships supporting the writing of this paper:
This work was supported by the National Center for Research Resources (UL1RR024896), the University of Michigan Office of the Vice President for Research and the fMRI Laboratory Pilot Program, and for investigator support, by the National Institute for Child Health and Human Development (5T32HD007048), the University of Michigan Postdoctoral Translational Scholars Program,and the Phil F. Jenkins Research Fund. This work utilized Chemistry Laboratory Core of the Michigan Diabetes Research and Training Center funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: the authors have nothing to disclose
REFERENCES
- 1.Franks S. Polycystic ovary syndrome: a changing perspective. Clin Endocrinol (Oxf) 1989;31(1):87–120. doi: 10.1111/j.1365-2265.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 2.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 3.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2006;91(1):48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22(1):141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. The Journal of clinical endocrinology and metabolism. 2005;90(6):3236–3242. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. The Journal of clinical endocrinology and metabolism. 1999;84(1):165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 7.Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod. 2001;16(9):1995–1998. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- 8.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. 2008;14(4):367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 10.Fulghesu AM, Lanzone A, Cucinelli F, Caruso A, Mancuso S. Long-term naltrexone treatment reduces the exaggerated insulin secretion in patients with polycystic ovary disease. Obstet Gynecol. 1993;82(2):191–197. [PubMed] [Google Scholar]
- 11.Guido M, Romualdi D, Lanzone A. Role of opioid antagonists in the treatment of women with glucoregulation abnormalities. Curr Pharm Des. 2006;12(8):1001–1012. doi: 10.2174/138161206776055895. [DOI] [PubMed] [Google Scholar]
- 12.Lanzone A, Apa R, Fulghesu AM, Cutillo G, Caruso A, Mancuso S. Long-term naltrexone treatment normalizes the pituitary response to gonadotropin-releasing hormone in polycystic ovarian syndrome. Fertility and sterility. 1993;59(4):734–737. doi: 10.1016/s0015-0282(16)55851-8. [DOI] [PubMed] [Google Scholar]
- 13.Lanzone A, Fulghesu AM, Fortini A, Cutillo G, Cucinelli F, Di Simone N, et al. Effect of opiate receptor blockade on the insulin response to oral glucose load in polycystic ovarian disease. Hum Reprod. 1991;6(8):1043–1049. doi: 10.1093/oxfordjournals.humrep.a137482. [DOI] [PubMed] [Google Scholar]
- 14.Cumming DC, Reid RL, Quigley ME, Rebar RW, Yen SS. Evidence for decreased endogenous dopamine and opioid inhibitory influences on LH secretion in polycystic ovary syndrome. Clin Endocrinol (Oxf) 1984;20(6):643–648. doi: 10.1111/j.1365-2265.1984.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 15.Barnes RB, Lobo RA. Central opioid activity in polycystic ovary syndrome with and without dopaminergic modulation. The Journal of clinical endocrinology and metabolism. 1985;61(4):779–782. doi: 10.1210/jcem-61-4-779. [DOI] [PubMed] [Google Scholar]
- 16.Eyvazzadeh AD, Pennington KP, Pop-Busui R, Sowers M, Zubieta JK, Smith YR. The role of the endogenous opioid system in polycystic ovary syndrome. Fertility and sterility. 2009;92(1):1–12. doi: 10.1016/j.fertnstert.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Kleinrok Z, Sieklucka-Dziuba M, Sek A, Rodziewicz E, Latus U. The effect of repeated administration of insulin on pain threshold and gamma-aminobutyric acid level in mouse brain. Acta Physiol Pol. 1987;38(6):477–482. [PubMed] [Google Scholar]
- 18.Unger JW, Lange W. Insulin receptors in the pituitary gland: morphological evidence for influence on opioid peptide-synthesizing cells. Cell Tissue Res. 1997;288(3):471–483. doi: 10.1007/s004410050833. [DOI] [PubMed] [Google Scholar]
- 19.Berga SL, Yen SS. Opioidergic regulation of LH pulsatility in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 1989;30(2):177–184. doi: 10.1111/j.1365-2265.1989.tb03739.x. [DOI] [PubMed] [Google Scholar]
- 20.Khoury SA, Reame NE, Kelch RP, Marshall JC. Diurnal patterns of pulsatile luteinizing hormone secretion in hypothalamic amenorrhea: reproducibility and responses to opiate blockade and an alpha 2-adrenergic agonist. The Journal of clinical endocrinology and metabolism. 1987;64(4):755–762. doi: 10.1210/jcem-64-4-755. [DOI] [PubMed] [Google Scholar]
- 21.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, et al. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. The Journal of clinical endocrinology and metabolism. 1998;83(12):4498–4505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- 22.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26(21):5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain research. 1994;643(1–2):245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 24.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, editor. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Mohlig M, Spranger J, Ristow M, Pfeiffer AF, Schill T, Schlosser HW, et al. Predictors of abnormal glucose metabolism in women with polycystic ovary syndrome. Eur J Endocrinol. 2006;154(2):295–301. doi: 10.1530/eje.1.02095. [DOI] [PubMed] [Google Scholar]
- 27.Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nucl Med Biol. 2001;28(6):733–734. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 30.Farrell K, Antoni MH. Insulin resistance, obesity, inflammation, and depression in polycystic ovary syndrome: biobehavioral mechanisms and interventions. Fertility and sterility. 2010;94(5):1565–1574. doi: 10.1016/j.fertnstert.2010.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25(4):235–251. [PubMed] [Google Scholar]
- 32.Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Archives of general psychiatry. 2003;60(11):1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Archives of general psychiatry. 2006;63(11):1199–1208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 34.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rassi A, Veras AB, Dos Reis M, Pastore DL, Bruno LM, Bruno RV, et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr Psychiatry. 2010;51(6):599–602. doi: 10.1016/j.comppsych.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Balen AH. Hypersecretion of luteinizing hormone and the polycystic ovary syndrome. Hum Reprod. 1993;8 Suppl 2:123–128. doi: 10.1093/humrep/8.suppl_2.123. [DOI] [PubMed] [Google Scholar]
- 38.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12(4):351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 39.Parvizi N, Ellendorff F. Gonadal steroids in the amygdala--differential effects on LH. Brain research. 1980;195(2):363–372. doi: 10.1016/0006-8993(80)90072-4. [DOI] [PubMed] [Google Scholar]
- 40.Hadziomerovic D, Rabenbauer B, Wildt L. Normalization of hyperinsulinemia by chronic opioid receptor blockade in hyperandrogenemic women. Fertility and sterility. 2006;86(3):651–657. doi: 10.1016/j.fertnstert.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 41.Lanzone A, Fulghesu AM, Cucinelli F, Ciampelli M, Caruso A, Mancuso S. Evidence of a distinct derangement of opioid tone in hyperinsulinemic patients with polycystic ovarian syndrome: relationship with insulin and luteinizing hormone secretion. The Journal of clinical endocrinology and metabolism. 1995;80(12):3501–3506. doi: 10.1210/jcem.80.12.8530590. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A. Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod. 2008;23(11):2564–2569. doi: 10.1093/humrep/den273. [DOI] [PubMed] [Google Scholar]
- 43.Fruzzetti F, Bersi C, Parrini D, Ricci C, Genazzani AR. Effect of long-term naltrexone treatment on endocrine profile, clinical features, and insulin sensitivity in obese women with polycystic ovary syndrome. Fertility and sterility. 2002;77(5):936–944. doi: 10.1016/s0015-0282(02)02955-2. [DOI] [PubMed] [Google Scholar]
- 44.Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertility and sterility. 2006;86(2):411–417. doi: 10.1016/j.fertnstert.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 45.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Denbleyker M, Nicklous DM, Wagner PJ, Ward HG, Simansky KJ. Activating mu-opioid receptors in the lateral parabrachial nucleus increases c-Fos expression in forebrain areas associated with caloric regulation, reward and cognition. Neuroscience. 2009;162(2):224–233. doi: 10.1016/j.neuroscience.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Napier TC, Mitrovic I. Opioid modulation of ventral pallidal inputs. Annals of the New York Academy of Sciences. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- 48.Adriaanse MC, Bosmans JE. Diabetes prevalence, diabetes regimen and co-morbidity in depressed patients compared with non-depressed patients in primary care in the Netherlands. Diabet Med. 2010;27(6):718–722. doi: 10.1111/j.1464-5491.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- 49.Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010;87(3):302–312. doi: 10.1016/j.diabres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev. 2007;3(4):252–259. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- 51.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


