Abstract
CD4+ T cells are critical for host defense but are also major drivers of immune-mediated diseases. The classical view of Th1 and Th2 subtypes of CD4+ T cells was recently revised by the identification of the Th17 lineage of CD4+ T cells that produce IL-17, which have been found to be critical in the pathogenesis of autoimmune and other diseases. Mechanisms controlling the differentiation of Th17 cells have been well described, but few feasible targets for therapeutically reducing Th17 cells are known. The generation of Th17 cells requires IL-6 and activation of STAT3. During polarization of CD4+ T cells to Th17 cells, we found that inhibition of glycogen synthase kinase-3 (GSK3) blocked IL-6 production, STAT3 activation, and polarization to Th17 cells. Polarization of CD4+ T cells to Th17 cells increased by 10-fold the expression of GSK3β protein levels in Th17 cells, whereas GSK3β was unaltered in regulatory T cells. Diminishing GSK3 activity either pharmacologically or molecularly blocked Th17 cell production, and increasing GSK3 activity promoted polarization to Th17 cells. In vivo inhibition of GSK3 in mice depleted constitutive Th17 cells in intestinal mucosa, blocked Th17 cell generation in the lung after Francisella tularensis infection, and inhibited the increase in spinal cord Th17 cells and disease symptoms in the experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. These findings identify GSK3 as a critical mediator of Th17 cell production and indicate that GSK3 inhibitors provide a potential therapeutic intervention to control Th17-mediated diseases.
CD4+ T cells are critical for host defense but are also major drivers of immune-mediated diseases. The classical division of CD4+ Th cells into IFN-γ–producing Th1 and IL-4–producing Th2 subtypes was recently revised by the identification of the IL-17–producing lineage of Th17 cells (1, 2). Th17 cells have been found to be critical in the pathogenesis of autoimmune diseases and to be essential in clearing foreign pathogens (3). The transcription factors retinoid-related orphan receptor γ T (RORγT) (4) and STAT3 (5, 6) direct Th17 differentiation from naive CD4+ T cells upon stimulation with TGF-β and inflammatory cytokines IL-6 (7) or IL-21 (8, 9). Th17 cells are expanded and stabilized by IL-23 (10, 11) and predominantly produce the cytokines IL-17A, IL-17F, IL-21, and IL-22. In healthy mammals, Th17 cells are rarely detected except in the intestinal lamina propria, where they constitute a considerable proportion of the CD4+ T cells (12, 13). Pathogenic yeast, fungi, and bacteria can elicit expansion of Th17 cells, which induces the production of proinflammatory cytokines, chemokines, and met-alloproteinases (14, 15). Increased numbers of Th17 cells also occur during autoimmune diseases, such as multiple sclerosis (16) and its model in rodents, experimental autoimmune encephalomyelitis (EAE), where Th17 cells appear to be critical for disease pathogenesis (4, 17).
Thus, mechanisms mediating the production of Th17 cells have been identified, and Th17 cells are widely thought to be critical mediators of autoimmune diseases. However, less is known about intracellular signaling mechanisms regulating Th17 cell production that may be targeted by therapeutic interventions to control their pathogenic actions. In this study, we show that glycogen synthase kinase-3 (GSK3) (18) is required for the production of Th17 cells and that in vivo inhibition of GSK3 reduces Th17 cells in the intestinal lamina propria in healthy mice, in mouse lung after infection with the bacteria Francisella tularensis, and in mouse spinal cord during EAE.
Materials and Methods
Mice
C57BL/6 and SJL/J (6–8 wk) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and GSK3S21A/S21A/S9A/S9A knock-in mice were provided by Dr. D. Alessi (University of Dundee, Dundee, U.K.). Mice were housed in a light- and temperature-controlled room and treated in accordance with National Institutes of Health and University of Alabama at Birmingham Institutional Animal Care and Use Committee regulations.
Bone marrow-derived dendritic cell cultures
Bone marrow-derived dendritic cells (BMDCs) were prepared as described (19) and were used on day 10 of culture. BMDCs were stimulated for 4 h with 100 ng/ml LPS (20) and washed prior to culture with T cells. For CD4+ T cell activation, purified CD4+ T cells were cultured with LPS-stimulated BMDCs at a ratio of 5:1, and cells were stimulated with 2.5 µg/ml anti-CD3 (clone 145-2C11) and 5 ng/ml TGF-β with 10 µg/ml anti–IFN-γ (clone XMG1.2) and anti–IL-4 (clone 11B11).
ELISA
CD4+ T cells were differentiated for 5 d, washed with medium, and restimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml) for 4 h or BMDCs were stimulated with 100 ng/ml LPS for 4 h, and IL-6 and IL-17A levels were measured by ELISA in the supernatant according to the manufacturer’s instructions (eBioscience).
CD4+ T cell isolation and activation
Spleens and lymph nodes were collected, and single-cell suspensions were prepared by mechanical disruption in RPMI 1640 medium supplemented with 10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin, 1× nonessential amino acids, 1 µM sodium pyruvate, 2.5 µM2-mercaptoethanol, and 2 mM l-glutamine (R-10). CD4+ T cells were isolated by magnetic sorting with Dynal beads mouse CD4+ beads according to the manufacturer’s instructions (Invitrogen). Irradiated (3000 rads) splenic feeder cells were used as APCs, except where indicated otherwise, and were cultured with purified CD4+ T cells at a ratio of 5:1. CD4+ T cells were activated with 2.5 µg/ml anti-CD3 (clone 145-11), and Th17 cells were differentiated by addition of IL-6 (20 ng/ml; Peprotech), TGF-β (5 ng/ml; Peprotech), anti–IL-4 (10 µg/ ml; clone 11B11), and anti–IFN-γ (10 µg/ml; clone XMG1.2) and regulatory T cells (Tregs) with TGF-β (5 ng/ml), anti–IL-4 (10 µg/ml; clone 11B11), and anti–IFN-γ (10 µg/ml; clone XMG1.2). Where indicated, cultures were supplemented with recombinant mouse IL-23 (10 ng/ml; eBioscience), IL-21 (75 ng/ml; eBioscience), or GSK3 inhibitors 10 µM SB415286 (Tocris), 1 µM TDZD-8 (Calbiochem), 10 mM LiCl (Sigma), or 1 µM CHIR99021 (University of Dundee, U.K.).
Intracellular cytokine staining
CD4+ T cells were collected and were stimulated for 4 h with PMA (50 ng/ml; Alexis) and ionomycin (750 ng/ml; Sigma) in the presence of Golgiplug (IFN-γ and IL-17) at the recommended concentrations (BD Pharmingen). Standard intracellular cytokine staining was carried out as described (7). Cells were first stained extracellularly with allophycocyanin-or FITC-conjugated anti-CD4 (eBioscience) and where indicated with a live-dead dye (Invitrogen), fixed and permeabilized with permeabilization solution (eBioscience), and then were stained intracellularly with Pacific blue-conjugated anti–IFN-γ, PE-conjugated anti–IL-17A, allophycocyanin-conjugated anti–IL-17F, PE-conjugated anti-RORγT, or FITC–Foxp3 (eBioscience). Samples were acquired on an LSRII (BD), and data were analyzed with FlowJo software (Tree Star).
Small interfering RNA transfection
CD4+ T cells were transfected using liposome-mediated transfection reagent Lipofectamine RNAiMAX (Invitrogen) with 50 nM small interfering RNA (siRNA) according to the manufacturer’s instructions with silencer negative control (Ambion) or GSK3α and GSK3β siRNA (Smart Pool; Dharmacon).
Immunoblotting
Western blots were carried out as described previously (21) using Abs to phospho-Tyr705-STAT3, STAT3, phospho-Tyr701-STAT1, STAT1, phospho-Ser9-GSK3β (Cell Signaling Technology), total GSK3α/β (Millipore), and β-actin (Sigma).
Quantitative RT-PCR
Purified CD4+ T cells were differentiated toward Th17 for 4 d, and RNA was isolated by TRIzol extraction (Invitrogen). RNA was retro-transcripted using ImProvII reverse transcriptase (Promega) according to the manufacturer’s instructions. Quantitative RT-PCR was carried out using a Taq-Man with gene expression assay primers for IL-17A, GSK3β, and β2-microglobulin according to the manufacturer’s instructions.
Chromatin immunoprecipitation
CD4+ nuclei from cross-linked cells were resuspended in Tris–EDTA buffer and sonicated. The soluble chromatin was adjusted into immunoprecipitation assay (RIPA) buffer (0.1% NaDodSO4, 1% Triton X-100, 0.1% sodium deoxycholate, 140 mM NaCl) and precleared. Immunoprecipitation was performed with 2 µl anti-STAT3 (Cell Signaling), and the immune complexes were absorbed with protein A beads (Millipore) blocked with BSA and salmon sperm DNA. Immunoprecipitated DNA was subjected to semiquantitative PCR. The PCR products were resolved in 2.0% agarose gels in 1× Tris/borate/EDTA buffer, and the gels were stained with ethidium bromide. Quantitative PCR was used to quantify the chromatin immunoprecipitation results, and all results were normalized by respective input values. The mouse IL-17A promoter was analyzed using the following primers: IL-17A forward, 5′-GGA GAG ATG GCT CAG CAG TTA AG-3′; IL-17A reverse, 5′-TGG TTT CTG GGA ATT GAA CTC A-3′.
TUNEL assay
The APO-DIRECT kit (eBioscience) was used according to the manufacturer’s instructions for the TUNEL assay to detect apoptosis. CD4+ T cells, differentiated or not toward Th17 for 5 d, where indicated in the presence of GSK3 inhibitors, were fixed in 1% paraformaldehyde for 1 h at 4°C and then were permeabilized in ice-cold 70% ethanol overnight. Samples were then incubated at 37°C for 1 h in the dark in a TUNEL reaction mix containing terminal deoxynucleotidyl transferase and FITC-conjugated dUTP to label DNA strand breaks. The fluorescence of cells carrying DNA labeled with FITC-dUTP (TUNEL-positive cells) was analyzed by flow cytometry.
CFSE labeling
CD4+ T cells were suspended at a density of 107 cells per milliliter in PBS. CFSE (Molecular Probes) diluted in PBS was added to an equal volume of prewarmed cell suspension at a final concentration of 5 µM, and the suspension was mixed rapidly. Cells were incubated at room temperature for 7 min, and the reaction was stopped with FBS. Cells were centrifuged and resuspended in culture medium.
Intestinal lymphocytes preparation
One group of mice was provided ad libitum pelleted food containing 0.2% lithium carbonate (Harlan-Teklad) for 4 wk. Intestines were removed, and Peyer’s patches were dissected from the small intestines. Intestines were opened longitudinally and then were cut into strips 1 cm in length. Tissues were washed in cold 1× HBSS supplemented with 2% (v/v) FBS with penicillin (100 IU/ml) and streptomycin (100 µg/ml; H2 solution). Intraepithelial lymphocytes were isolated as follows: gut pieces were incubated for 30 min at 37°C with gentle magnetic stirring in H2 solution containing L-DTT (154 mg/l). Cell suspensions were passed through a hand-held sieve, and tissues were washed with H2 solution. Tissues were then incubated for 30 min at 37°C with gentle magnetic stirring in H2 solution containing 2 mM EDTA. After being strained and washed, the remaining gut tissues were digested completely for 45 min at 37°C with gentle magnetic stirring in R-10 medium with collagenase D (100 U/ml) and DNase (20 mg/ml; Sigma) for the isolation of lamina propria lymphocytes (LPLs). LPLs were purified on a 40%/75% Per-coll gradient by centrifugation for 20 min at 25°C and 600 × g with no brake.
Infection by F. tularensis live vaccine strain
F. tularensis live vaccine strain (LVS) was grown in supplemented Mueller– Hinton broth or agar, as previously described (22). For pulmonary infections, mice were anesthetized and infected by intranasal administration of 5 × 104 CFU F. tularensis LVS. One group of mice was given LiCl (100 µg/g body weight, i.p.) 1 h postinfection and provided lithium diet ad libitum. Mice were humanely euthanized on day 6 after infection, and tissues were collected for in vitro analysis.
Induction of EAE
Pretreated mice were fed lithium-containing food ad libitum for 4 wk. Posttreated mice were given LiCl injections (100 µg/g body weight, i.p.) the first 2 d of treatment and were fed lithium-containing food. EAE was induced in SJL/J female mice with s.c. injection of 150 µg PLP139–151 peptide in CFA containing 1.5 mg/ml heat-killed Mycobacterium tuberculosis H37Ra (Difco Laboratories). On the day of immunization and 48 h later, mice were injected i.p. with 500 ng pertussis toxin in PBS. Mice were examined daily for clinical signs of EAE. Mice were assigned clinical symptom scores as follows: 0, no paralysis; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind legs; 4, complete hind limb paralysis; 5, moribund (animals were humanly euthanized); 6, death. To compare the time course of disease development in different groups of mice, the daily average of the clinical scores was calculated for each group.
Statistical analysis
Statistical significance was analyzed with a one-way multiple range ANOVA for multiple comparisons or with an unpaired t test using Prism software. A p value <0.05 was considered significant.
Results
GSK3 is required for IL-6 production-dependent Th17 polarization
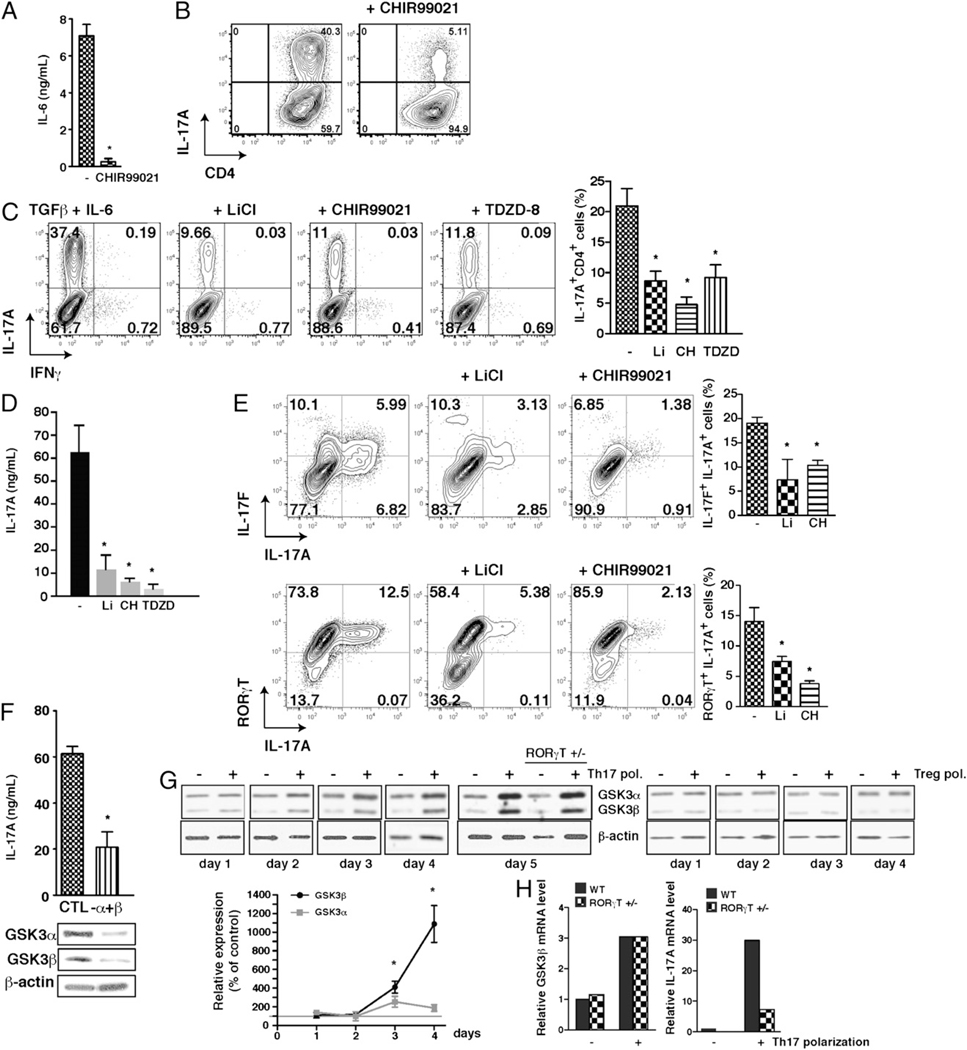
CD4+ T cell polarization to Th17 cells requires costimulation with IL-6 and TGF-β (7, 23). We tested if this is dependent on GSK3 because IL-6 production by inflammatory cells requires active GSK3 (20, 24). CD4+ T cells were polarized toward the Th17 lineage with TGF-β and BMDCs primed with LPS to produce IL-6 (25). Treatment with CHIR99021 (26), the most specific GSK3 inhibitor available (27), reduced LPS-induced IL-6 production by BMDCs by >90% (Fig. 1A). Consistent with decreased IL-6, inhibition of GSK3 with CHIR99021 during 5 d of polarization reduced by 87% the generation of IL-17A–producing CD4+ T cells (Fig. 1B). Thus, IL-6 production and Th17 cell polarization were largely blocked by CHIR99021.
FIGURE 1.
GSK3 is required for Th17 commitment. A, CD4+ T cells were polarized toward Th17 cells in medium supplemented with 5 ng/ml TGF-β with or without 1 µM CHIR99021 during culture on BMDCs that had been stimulated with LPS for 4 h to induce IL-6 production. Supernatants were assessed by ELISA for IL-6 production after 24 h of culture. n = 4. *p < 0.05. B, GSK3 inhibition with 1 µM CHIR99021 blocked the production of IL-17A–producing CD4+ T cells measured by flow cytometry after 5 d of polarization on BMDCs. n = 4. Data are gated on the CD4+ cells. C and D, CD4+ T cells were polarized toward Th17 cells for 5 d cultured on irradiated lymph node cells and splenocytes in medium supplemented with 20 ng/ml IL-6 and 5 ng/ml TGF-β with the GSK3 inhibitor 10 mM LiCl, 1 µM CHIR99021 (CH), or 1 µM TDZD-8. Cells were washed and stimulated for 4 h with PMA/ ionomycin (D) and brefeldin A (C), and IL-17A–producing CD4+ T cells were analyzed by flow cytometry (C), or IL-17A in the media was measured by ELISA (D). n = 5. Flow cytometry analyses were quantified and expressed as mean ± SEM. n = 5 to 10. *p < 0.05 (ANOVA). E, CD4+ T cells were polarized toward Th17 cells for 5 d cultured on irradiated lymph node cells and splenocytes in medium supplemented with 20 ng/ml IL-6 and 5 ng/ml TGFβ with the GSK3 inhibitor 10 mM LiCl, 1 µM CHIR99021 (CH), or 1 µM TDZD-8. n = 5. Cells were washed and stimulated for 4 h with PMA/ionomycin/ brefeldin A. IL-17A–producing and IL-17F–producing (top panel) or RORγT-expressing (bottom panel) CD4+ T cells were analyzed by flow cytometry. Quantitation of flow cytometry data is expressed as mean ± SEM. n = 3 to 10. *p < 0.001 (ANOVA). F, GSK3α and GSKβ were knocked down by siRNA for 48 h, and after 5 d of polarization, IL-17A production during the 4 h of restimulation with ionomycin/PMA was measured by ELISA (top). GSK3α and GSK3β levels were measured by immunoblot after 48 h of knockdown (bottom). Blots were reblotted for the loading control β-actin. n = 3. *p < 0.05. G, GSK3α and GSK3β levels were measured by immunoblotting after 1, 2, 3, 4, and 5 d of polarization, or not, toward Th17 cells or Tregs in cells from wild-type mice and after 5 d in cells from RORγT+/− mice. Blots were reblotted for the loading control β-actin. Quantitation of immunoblot data is expressed as mean ± SEM. n = 3 to 6. *p < 0.05 (compared with initial levels). H, GSK3β and IL-17A mRNA levels in cells from wild-type and RORγT+/− mice after 4 d of polarization, or not, toward Th17 cells.
GSK3 is upregulated and required for Th17 differentiation independently of IL-6
To test if reducing IL-6 production was the only mechanism by which GSK3 inhibition blocked the generation of Th17 cells, exogenous IL-6 was added with TGF-β to polarize CD4+ T cells cultured on feeder cells toward Th17 cells. Using a panel of selective GSK3 inhibitors to ensure blocked Th17 cell production was due to inhibition of GSK3, flow cytometry after restimulation with PMA/ionomycin demonstrated 60–88% reductions of IL-17A–producing CD4+ T cells caused by treatment with each of three structurally diverse, selective GSK3 inhibitors, lithium (28, 29), CHIR99021, or TDZD-8 (30) (Fig. 1C). ELISA measurements of IL-17A released from Th17 polarized cells confirmed 71–92% reductions by GSK3 inhibitors (Fig. 1D). This was also confirmed by a large reduction of IL-17F+ and RORγT+ IL-17A+ CD4+ T cells after treatment with GSK3 inhibitors (Fig. 1E). GSK3 inhibitors did not markedly alter survival or proliferation of CD4+ T cells or Th17 cells under these conditions (Supplemental Fig. 1A–F). Furthermore, siRNA-mediated knockdown of both GSK3α/β isoforms in ~56% of CD4+ T cells (Supplemental Fig. 1G) decreased the production of IL-17A by ~65% (Fig. 1F). IL-17A production was inhibited concentration-dependently by CHIR99021 (Supplemental Fig. 2A) and time-dependently by lithium (Supplemental Fig. 2B). IL-17A produced after Th17 polarization was not reduced by GSK3 inhibitors CHIR99021 or lithium added only during the 4-h restimulation with PMA/ ionomycin after the 5 d of polarization (Supplemental Fig. 2C), indicating their effect was on Th17 polarization rather than a direct effect on IL-17A production. GSK3 inhibitors also reduced by 67–83% Th17 cells after polarization of CD4+ T cells cultured on anti-CD3/anti-CD28–coated beads in place of feeder cells (Supplemental Fig. 2D), confirming that GSK3 inhibitors acted on CD4+ T cells to block the generation of Th17 cells. Together, these results demonstrate that GSK3 inhibitors not only block IL-6 production but also block polarization to Th17 cells independently of reducing IL-6 production.
The dependence of Th17 cell generation on GSK3 prompted us to examine the cellular levels of the two GSK3 isoforms. The levels of both GSK3 isoforms, but particularly GSK3β, increased during the polarization of CD4+ T cells toward Th17 cells but not toward Tregs (Fig. 1G). RT-PCR analyses confirmed that this was due to increased expression of GSK3β, which was blocked in conjunction with reduced Th17 cell production by inhibiting GSK3 with CHIR99021 (Supplemental Fig. 2E). To determine if increased expression of GSK3 is dependent on RORγT, GSK3 levels were measured in RORγT+/− and wild-type CD4+ T cells after Th17 polarization. Upregulation of GSK3α/β protein levels (Fig. 1G) and of GSK3β mRNA (Fig. 1H) during Th17 polarization were independent of RORγT, although Th17 differentiation was reduced by ~60% in RORγT+/− CD4+ T cells (Fig. 1H, Supplemental Fig. 2F). This raised the possibility that increased GSK3 activity amplifies CD4+ T cell polarization to Th17 cells. This was tested using CD4+ T cells isolated from GSK3 knock-in mice (31). These mice express GSK3α and GSK3β at normal levels but with serine-to-alanine mutations at serine-21 in GSK3α and serine-9 in GSK3β to abrogate inhibitory serine phosphorylation of GSK3, the major mechanism for inhibition of GSK3 (18), so GSK3 is constitutively maximally active, but within its normal physiological range. After Th17 polarization, CD4+ T cells from GSK3 knock-in mice exhibited an increased percentage of IL-17A–producing CD4+ T cells (Supplemental Fig. 2C) compared with that of wild-type CD4+ T cells (Fig. 1C), and this was inhibited by the GSK3 inhibitor SB415286.
GSK3 is required for STAT3 activation by IL-6, IL-21, and IL-23
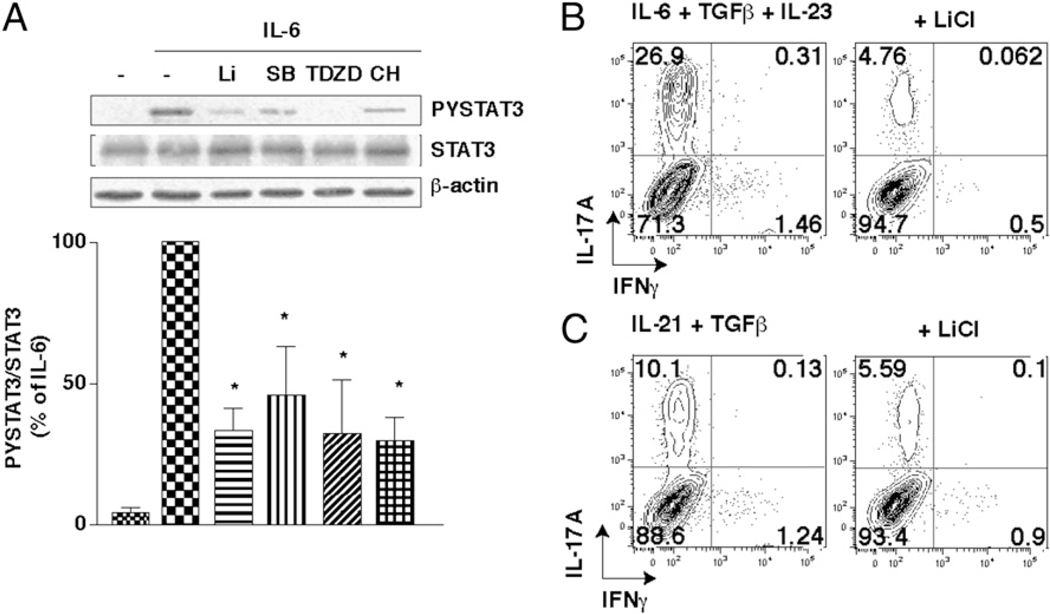
A crucial initiating step in Th17 polarization is the activation of STAT3 by IL-6 (5, 6), which is blocked by inhibition or knock-down of GSK3 in astrocytes (21). Treatment of CD4+ T cells with GSK3 inhibitors reduced IL-6–induced STAT3 activation (Fig. 2A) and the recruitment of STAT3 to the IL-17A promoter (Supplemental Fig. 3A). Th17 differentiation can be achieved independently of IL-6 by the addition of alternative cytokines, IL-23 (10, 11) or IL-21 (8, 9), both of which also act via STAT3 signaling. Consistent with the results obtained with IL-6, polarization in the presence of IL-23 or IL-21 did not overcome the reduction of Th17 cells by GSK3 inhibitors (Fig. 2B, 2C; Supplemental Fig. 3B, 3C), and GSK3 inhibitors also reduced STAT3 activation induced by IL-23 or by IL-21 (Supplemental Fig. 3D, 3E). Thus, GSK3 is required for both IL-6 production and STAT3 activation, each an essential process in the production of Th17 cells, which raised the question of whether in vivo inhibition of GSK3 could impede Th17 cell production.
FIGURE 2.
GSK3 inhibitors reduce STAT3 activation. A, CD4+ T cells were pretreated for 2 h with 10 mM LiCl (Li), 10 µM SB415286 (SB), 1 µM TDZD-8 (TDZD), or 1 µM CHIR99021 (CH) and then stimulated for 30 min with 10 ng/ml IL-6. Cell lysates were immunoblotted for phospho-Tyr705-STAT3, total STAT3, and β-actin. The ratio of phospho-Tyr705-STAT3 to total STAT3 was quantitated and is expressed as the percentage of stimulation obtained with IL-6 alone. Data are mean ± SEM. n = 3. *p < 0.05. B and C, CD4+ T cells were polarized toward Th17 cells for 5 d cultured on irradiated lymph node cells and splenocytes in medium supplemented with10 ng/ml IL-23, 20 ng/ml IL-6, and 0.25 ng/ml TGF-β (B) or 75 ng/ml IL-21 and 5 ng/ml TGF-β (C), each with or without the GSK3 inhibitor 10 mM LiCl. n = 5. IL-17A–producing CD4+ T cells were analyzed after 4-h restimulation with PMA/ionomycin/brefeldin A by flow cytometry (n = 2–3).
GSK3 is required for in situ lamina propria Th17 cells
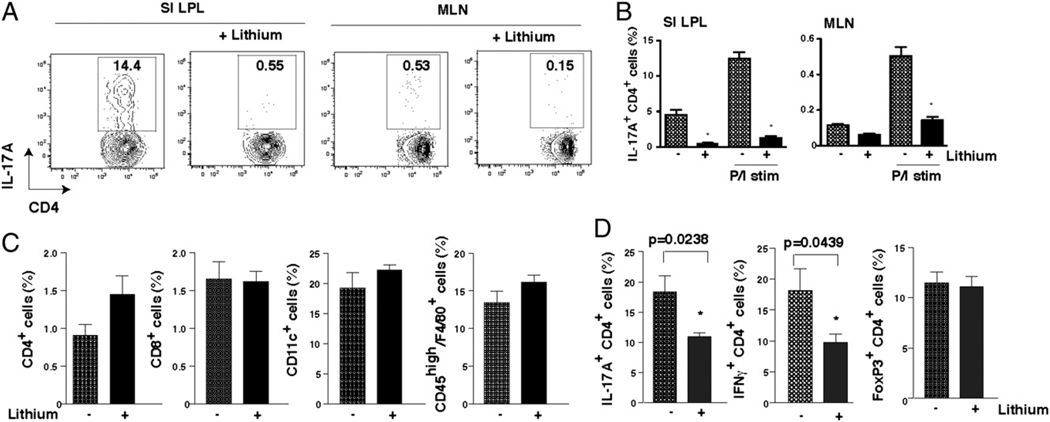
Th17 cells constitute a considerable proportion of CD4+ T cells in the intestinal lamina propria in healthy mice in pathogen-free conditions (4). Therefore, we tested if in vivo inhibition of GSK3 reduced the number of constitutive Th17 cells in the mouse intestinal mucosa. Lithium administration was used for this because lithium is the only GSK3 inhibitor extensively used in vivo in rodents or humans (32). In vivo administration of lithium for 4 wk to mimic its chronic therapeutic use in humans (27–29) dramatically reduced the Th17 cell population in mouse LPLs, as well as in mesenteric lymph nodes (Fig. 3A, 3B). Thus, a therapeutically relevant concentration of lithium effectively reduced the generation of Th17 cells in vivo.
FIGURE 3.
GSK3 inhibitors reduce IL-17A–producing CD4+ T cells in vivo. A, IL-17A–producing CD4+ T cells in the small intestine lamina propria lymphocyte (SI LPL) or mesenteric lymph node (MLN) cell preparations from untreated mice and mice treated with lithium for 4 wk, after restimulation with PMA/ionomycin/brefeldin A for 4 h. B, Quantification of the percentage of IL-17A–producing CD4+ T cells in LPLs and MLNs after restimulation with PMA/ionomycin/brefeldin A for 4 h (P/I stim) or no restimulation. Mean ± SEM; n = 4. *p < 0.001 (compared with no lithium treatment; ANOVA). C, Mice were infected with F. tularensis LVS and 1 h later injected i.p. with 100 mg/kg LiCl in PBS and then kept on a diet with 0.2% lithium. Six days postinfection, CD4+ T cells, CD8+ T cells, CD11c+ dendritic cells, and activated macrophages (CD45highF4/80+) from lungs were quantified by flow cytometry. Mean ± SEM; n = 4 to 5 mice/group. D, CD4+ T cells from lungs of F. tularensis-infected mice were restimulated with ionomycin/PMA/brefeldin A for 4 h, and the percentage of IL-17A+, IFN-γ+, or Foxp3+ (non-restimulated) CD4+ T cells was analyzed by flow cytometry. Mean ± SEM; n = 4–5 mice/group. *p < 0.05 (compared with no lithium treatment; ANOVA).
GSK3 is required for Th17 cells induced by infection or EAE
To test if GSK3 also regulates Th17 cells in pathological conditions, we examined the effects of lithium administration on Th17 cells induced during respiratory infection by F. tularensis LVS, which primarily affects pulmonary function by upregulating Th17 cells (33). IL-17A is induced during F. tularensis LVS infection and is required for the induction of Th1 cell responses (33). Lithium treatment concomitant with the infection did not affect the recruitment of CD4+ and CD8+ T cells, CD45highF4/80+ activated macrophages, or CD11c+ dendritic cells to the lungs or spleen (Fig. 3C, Supplemental Fig. 4A) but significantly decreased IL-17A+ CD4+ and IFN-γ+ CD4+ T cells, without affecting Tregs, in the lungs (Fig. 3D). Th17 cells, Th1 cells, and Tregs in the spleen were not modulated by lithium administration (Supplemental Fig. 4B). These results demonstrate that GSK3 inhibition controls infection-induced Th17 cells.
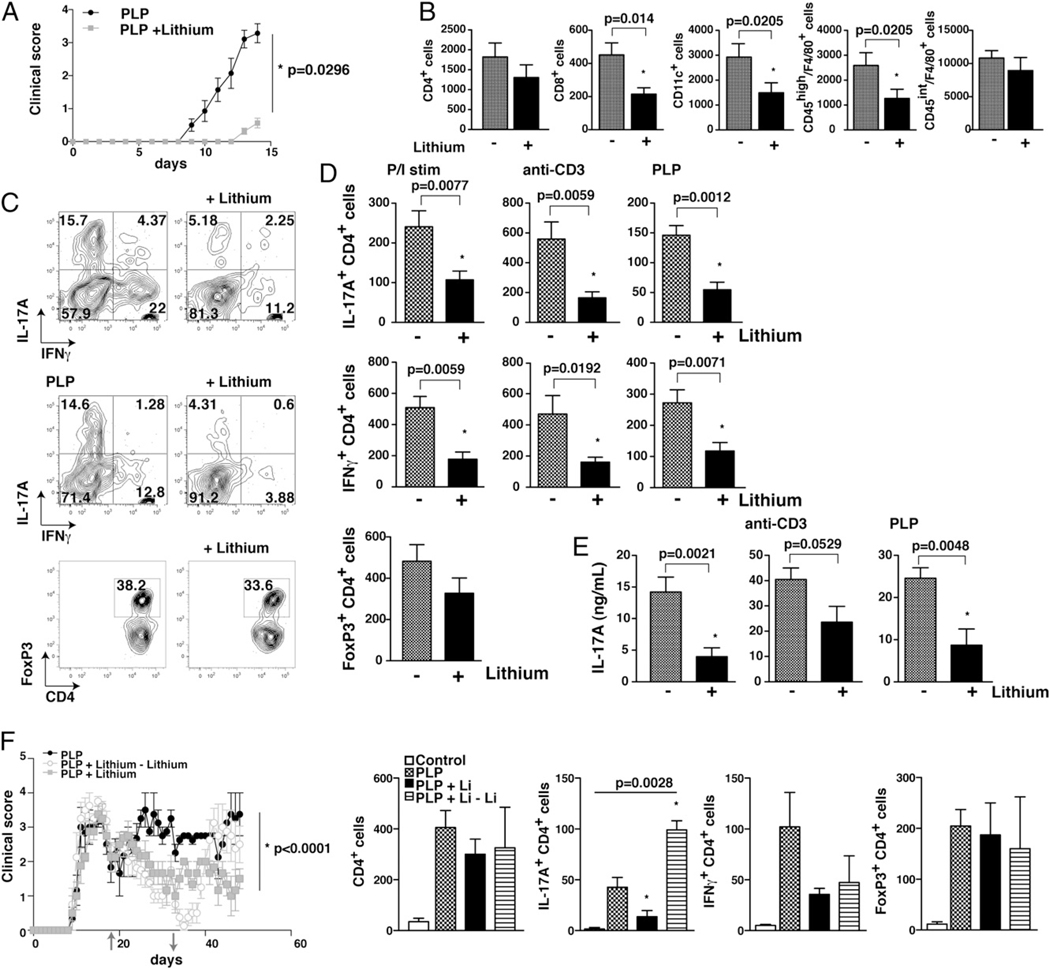
Th17 cells are critical in the pathogenesis of EAE, a mouse model of multiple sclerosis (34–37). The onset of clinical symptoms of EAE in female SJL mice immunized with myelin proteolipid protein (PLP) peptide were largely prevented by pretreatment with lithium (Fig. 4A), extending our previous report that lithium pretreatment blocks MOG peptide-induced EAE (38). Two weeks after immunization, during the initial peak of the disease, lithium treatment did not alter the number of infiltrating CD4+ T cells or CD45intF4/80+-activated microglia in the spinal cord but reduced CD8+ T cells, CD45highF4/80+-activated macrophages, and CD11c+ dendritic cells (Fig. 4B). Examination of T cell subtypes in the spinal cord demonstrated that lithium pretreatment reduced the number of IL-17A+ CD4+ T cells and IFN-γ+ CD4+ T cells, but not Foxp3+ CD4+ T cells, compared with that in lithium-free EAE mice (Fig. 4C, 4D). These results indicate that lithium treatment reduced the production of Th17 cells, while also IFN-γ production was inhibited, as has been reported previously after treatment with GSK3 inhibitors (20, 38). IL-17A production by restimulated CD4+ T cells from the spinal cord was also reduced in lithium-treated mice (Fig. 4E), and there was a corresponding reduction in activation of both STAT1 and STAT3 in spinal cord of lithium-treated mice (Supplemental Fig. 4C). Lithium’s inhibition of GSK3 in the spinal cord was evident by the increased phospho-Ser9-GSK3β (18) (Supplemental Fig. 4C). Treatment with lithium after the first clinical episode of EAE greatly attenuated clinical scores, and mice subsequently withdrawn from lithium relapsed rapidly to reach clinical scores comparable with those of mice never treated with lithium (Fig. 4F). Consistent with results obtained with lithium pretreatment, lithium-posttreated mice had reduced IL-17A–producing and IFNγ– producing CD4+ T cells in the spinal cord, with no change in Foxp3+ CD4+ T cells (Fig. 4F). After lithium withdrawal, there was a large increase in the IL-17A–producing CD4+ T cells in the spinal cord, but no changes in IFN-γ–producing or Foxp3+ CD4+ T cells, indicating a rapid induction of Th17 cells after lithium withdrawal concurrent with disease progression.
FIGURE 4.
GSK3 inhibitors reduce IL-17A–producing CD4+ T cells in EAE. A, Four weeks of lithium pretreatment significantly attenuated EAE clinical scores in PLP-immunized SJL mice. n = 7 to 8. B, In the mice shown in A, the numbers of CD4+ T cells, CD8+ T cells, CD11c+ dendritic cells, activated macrophages (CD45highF4/80+), or microglia (CD45intF4/80+) were measured in spinal cords 14 d after induction of EAE, when lithium-free mice reached maximum scores. C–E, The percentages (C) and numbers (D) of spinal cord IL-17A–producing, IFN-γ–producing, or Foxp3-expressing CD4+ T cells as well as the production of IL-17A (E) were analyzed after restimulation with PMA/ionomycin (E) and brefeldin A (C, D) for 4 h or 1 µg/ml anti-CD3, or 10 µg/ml PLP for 16 h, or no restimulation for Foxp3 F, Lithium treatment initiated 18 d after PLP immunization (see arrow ↑) during remission after the first clinical episode significantly attenuated clinical scores in PLP-immunized EAE mice. n = 3 to 4. Mice subsequently withdrawn from lithium (see arrow ↓) rapidly relapsed to reach clinical scores comparable with those of mice never treated with lithium. The numbers of CD4+ T cells, IL-17A–producing, IFN-γ–producing, and Foxp3 expressing CD4+ T cells were analyzed by flow cytometry. n = 3–4; two-way ANOVA.
Discussion
Recognition that Th17 cells constitute a distinct subset of effector T cells spurred a great deal of progress during the past 5 years, particularly in identifying mechanisms underlying autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis (14). Although the pathogenic actions of Th17 cells in autoimmune diseases and other conditions are in some cases controversial and continue to be clarified, interventions that block Th17 cell production based on signaling pathways regulating Th17 differentiation should be valuable both for defining the actions of Th17 cells and, if feasible, for therapeutic intervention. In this study, we identified the signaling kinase GSK3 as a critical factor in Th17 cell production and found that in vivo inhibition of GSK3 reduces constitutive and pathological populations of Th17 cells. Identification of the GSK3 inhibitor lithium as an effective means for reducing Th17 cells in vivo provides a feasible strategy for translation to therapeutic intervention because lithium is already approved for use in humans.
Th17 cells are generated by the costimulation of CD4+ T cells with TGF-β and IL-6 (7), the latter acting through the STAT3 transcription factor (5, 6). In other types of cells, inhibitors of GSK3 are well established to block two of these signals that are required for the generation of Th17 cells: IL-6 production and STAT3 activation. In this study, we demonstrated that these two critical signals for Th17 cell production also are blocked by GSK3 inhibitors in conjunction with blockade of Th17 cell production, and GSK3 inhibitors also block Th17 polarization induced by the alternative cytokines IL-21 or IL-23, which also signal through STAT3. Differentiation, rather than IL-17A synthesis, was dependent on GSK3 because exposure of polarized Th17 cells to GSK3 inhibitors did not reduce IL-17A production, and lithium treatment after 2 d of in vitro polarization did not reduce IL-17A– producing cells. These findings and the requirement for active GSK3 in IL-6–induced STAT3 DNA binding on the IL-17 promoter demonstrate that GSK3 is a determining regulator at an early stage of Th17 differentiation. This conclusion was further supported by the finding that the level of GSK3β was highly upregulated during Th17 polarization, which is particularly notable considering that GSK3β levels are generally very stable in numerous cell types and conditions (18). This upregulation of GSK3β, and to a lesser extent GSK3α, was not induced by TGF-β alone during Treg polarization and was independent of RORγT but occurred in response to combined stimulation with IL-6 and TGF-β during Th17 polarization. Thus, GSK3 upregulation is an early event, and GSK3 is required, in the generation of Th17 cells.
Inhibition of GSK3 in vivo reduced constitutive Th17 cells in healthy mouse intestine and Th17 cell induction in target pathogenic tissues in response to infection and autoimmune disease. For these studies, we used in vivo administration of lithium because lithium is the only GSK3 inhibitor that has been used extensively in vivo in rodents, and it can be administered chronically to model its use in humans (32). Lithium administration dramatically reduced the Th17 cell population in the lamina propria of healthy mice (demonstrating that GSK3 does not only regulate pathologically driven Th17 production) and in mouse lungs after F. tularensis LVS infection. EAE-induced increases in Th17 cells in the spinal cord, as well as disease symptoms, also were effectively controlled by in vivo lithium administration. Subsequent withdrawal of lithium led to increases in spinal cord Th17 cells, without affecting Th1 or Tregs and worsening of clinical symptoms, results that extend previous links between Th17 cells and autoimmune diseases (34–37). These results demonstrate that inhibition of GSK3 with lithium reduces Th17 cells in EAE but do not exclude other potential beneficial actions of lithium during EAE, such as indicated by the reduced IFN-γ levels in lithium-treated EAE mice. This effect may signify reduced synthesis of IFN-γ, as has been reported previously to be caused by inhibition of GSK3 (20, 38); or diminished Th1 cells, which could be caused by the reported reduction by GSK3 inhibitors of IL-12 (20) that is necessary for the differentiation of Th1 cells; reduced promotion of Th1 cell production by diminished Th17 cells (33, 39); or reduced Th1 cell survival, as GSK3 inhibitors promote death receptor-induced apoptosis (40) and Th1 cells secrete Fas ligand (41).
Taken together, these results demonstrate that GSK3 is required for the generation of Th17 cells, and the GSK3 inhibitor lithium provides a feasible intervention to limit Th17 cell production in vivo. This application can be used to clarify the contributions of Th17 cells in experimental models of autoimmune diseases and may alleviate Th17-mediated diseases in humans. Moreover, GSK3 inhibitors are advantageous compared with other means of reducing Th17 cells, such as anti–IL-17A therapy (17), because they also dampen inflammatory cytokine production (20). This adds to previous evidence of a critical role of GSK3 in promoting proinflammatory cytokines and diminishing anti-inflammatory cytokines in innate immunity, whereas in the adaptive immune system GSK3 was thought to mainly influence clonal expansion by direct actions on proliferation and survival and indirectly by modifying the repertoire of cytokines produced to influence differentiation and anergy (42). We now identified GSK3 as a key regulator of Th17 cell differentiation in addition to modulating IL-6 production by innate cells. Consistent with our previous finding that GSK3 promotes STAT3 activation (21, 24), this enables GSK3 to control the fate of CD4+ T cells toward Th17 polarization. This novel discovery reinforces the predominant role of GSK3 in controlling inflammatory responses and renders GSK3 a potential target for therapy of autoimmune diseases involving Th17 cells and inflammation because GSK3 inhibitors dampen both processes. Furthermore, inhibiting GSK3 is feasible for in vivo studies because lithium penetrates all cells and is feasible for therapeutic intervention because lithium is already widely used in humans as a treatment for bipolar disorder, and new selective small-molecule GSK3 inhibitors are under development in many laboratories in academia and the pharmaceutical industry (30).
Supplementary Material
Acknowledgments
We thank Dr. Alessi for providing the GSK3 knock-in mice, Amit R. Ashtekar for infecting mice with F. tularensis LVS, and Ling Song and Robert L. Kress for technical assistance.
This work was supported by grants from the National Institutes of Health (MH090236, MH038752, DE09081, and DK084082), a National Multiple Sclerosis Society Career Transition award (TA3025-A-1), and a Young Investigator award from the National Alliance for Research on Schizophrenia and Depression.
Abbreviations used in this article
- BMDC
bone marrow-derived dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- GSK3
glycogen synthase kinase-3
- LPL
lamina propria lymphocyte
- LVS
live vaccine strain
- MLN
mesenteric lymph node
- PLP
proteolipid protein
- RORγT
retinoid-related orphan receptor γ T
- siRNA
small interfering RNA
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nat. Immunol. 2010;11:36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 6.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 12.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. Anadvanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J. Biol. Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect. Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Beurel E, Jope RS. Glycogen synthase kinase-3 promotes the synergistic action of interferon-gamma on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cell. Signal. 2009;21:978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Wagman AS, Johnson KW, Bussiere DE. Discovery and development of GSK3 inhibitors for the treatment of type 2 diabetes. Curr. Pharm. Des. 2004;10:1105–1137. doi: 10.2174/1381612043452668. [DOI] [PubMed] [Google Scholar]
- 27.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 30.Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J. Med. Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 31.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jope RS. Lithium, the seminal GSK3 inhibitor. In Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors. In: Martinez A, Castro A, Medina M, editors. Hoboken, NJ: John Wiley & Sons; 2006. pp. 223–242. [Google Scholar]
- 33.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 36.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 37.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008;255 Suppl 1:3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 38.De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol. Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 42.Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.