Abstract
The functional and physiological diversity of transmembrane receptors results from factors that influence the pharmacology, signaling, and trafficking of these receptors. Receptor mutations and other modifications may lead to misfolding, intracellular retention, and ineffective signaling of transmembrane receptors. The importance of such mutations is highlighted by the fact that various diseases have been linked to mutations that lead to ineffective signaling of these receptors, resulting from the retention of receptors in intracellular compartments. Studies focused on understanding the regulation of trafficking and cell surface expression of newly synthesized receptors have highlighted molecular chaperones as key regulators of receptor maturation and sorting. In this chapter, we discuss the functions of molecular chaperones in the regulation of seven-transmembrane-containing G-protein-coupled receptor function and trafficking and explore ways in which chaperones can serve as novel therapeutic targets.
I. Introduction
A large family of over 800 genes encodes receptor proteins that are characterized by a signature seven-transmembrane structure. Members of this family include sensory receptors for taste, odorants, and light as well as receptors for many hormones, neurotransmitters, chemokines, and ions. These seven-transmembrane receptors (also known as heptahelical receptors) are commonly referred to as G-protein-coupled receptors (GPCRs) because they mediate their effects through the activation of a variety of heterotrimeric (α, β, γ-subunits) guanine nucleotide-binding G proteins. These GPCRs regulate many physiological processes, and the mechanism by which GPCRs translate extracellular signals into cellular changes has been an area of active research for many years.
Initial theories of GPCR signaling involved agonist binding leading to the activation of the receptor, resulting in dissociation of the G protein into an α subunit and a βγ subunit. Both these subunits have been shown to activate or inhibit various downstream effector molecules. However, further advances in the field of GPCR signaling have demonstrated that the mechanisms by which cell surface receptors orchestrate cellular changes are more complex. The recognition of the importance of GPCR oligomerization, the discovery of regulators of G protein signaling (RGS) proteins, and the identification of accessory/chaperone molecules are just some of the factors that have contributed to the expansion of the role and function of GPCRs.
Not only do the GPCRs regulate a plethora of physiological processes but drugs that target these receptors account for most of the medicines sold worldwide. These drugs target these seven-transmembrane receptors directly or target other proteins that are crucial for signaling through these receptors. This chapter focuses on the functions of molecular chaperones in the regulation of transmembrane receptor function and trafficking and explores ways in which chaperones can serve as novel therapeutic targets.
II. Molecular Chaperones and Accessory Proteins
The molecular chaperone concept was first proposed by John Ellis in 1987; he proposed that the term molecular chaperone be used to describe “a class of cellular proteins whose function is to ensure that the folding of certain other polypeptide chains and their assembly into oligomeric structures occur correctly.”1 There is a commonly held misconception that molecular chaperones are solely involved in ensuring proper protein folding. While many chaperones are involved in stabilizing unfolded protein folding and are involved in protein unfolding and degradation, chaperones also play a crucial role in the assembly of folded subunits into oligomeric structures.
With regard to GPCR function, some GPCRs may require the specific assistance of chaperones for proper folding during maturation. In addition, recent findings have highlighted various cytoplasmic and membrane-associated proteins that interact with GPCRs as they traffic through intracellular compartments and facilitate the cell surface expression of these GPCRs. While many of these chaperone proteins have additional biological roles, it is clear that they are necessary for proper functional expression of the receptors with which they interact.
III. GPCR Maturation and Postendoplasmic Reticulum Trafficking
GPCRs are synthesized by ribosomes attached at the cytosolic face of the endoplasmic reticulum (ER). During biosynthesis, these proteins are targeted by their hydrophobic signal sequences to the translocation complex which facilitates cotranslational entry into the ER lumen. Insertion of transmembrane domains into the membrane is driven by the translocation complex and orientation signals contained in the protein’s polypeptidic chain. This membrane insertion is assisted by molecular chaperones and folding factors.2,3 Most GPCR proteins fold properly with the aid of a conventional chaperone system. This conventional chaperone system comprises classical and lectin chaperones as well as enzymes that catalyze disulfide-bond formation or peptidyl–prolyl cis–trans isomerization.4,5
Once transmembrane proteins have achieved their native conformation, they leave the ER and are transported through the secretory pathway to their subcellular destination. This complex ER machinery constitutes the most important quality-control system for crosschecking newly synthesized proteins. In the event that newly synthesized proteins are defective in their folding, these misfolded polypeptides are exported across the ER membrane into the cytosol and destroyed by the ER-associated degradation pathway (ERAD).6
The general chaperone system that is commonly utilized for membrane proteins in the secretory pathway involves the use of heat-shock proteins (HSPs). HSPs have been implicated as central components of the chaperone-mediated protein folding mechanism. Several physical and chemical conditions that are potentially harmful to cells (such as elevated temperature) result in inappropriate protein folding. This increase in inappropriate protein folding is accompanied by a concomitant increase in the levels and/or activity of HSPs whose job is to deal with misfolded proteins and get the protein folding machinery back on track.7 Many HSPs are constitutively expressed in all cells. There are many families of HSPs and individual members within each family differ in their expression pattern and functions.
The Hsp70 family ER luminal protein BiP (also known as GRP78) is a key regulator of the quality-control system of the ER. BiP, working with Hsp40 family cofactors, facilitates translocation of nascent chains in the ER lumen, participates in protein folding and oligomerization and contributes to the retro-translocation of misfolded proteins to the ER-associated degradation pathway.6 Some GPCRs, such as the luteinizing hormone receptor, have been shown to interact with BiP.8,9 The luteinizing hormone receptor was also found to interact in the ER with GRP94, a member of the Hsp90 family and a known cofactor for BiP.9
Numerous reports have demonstrated the interaction between GPCRs and carbohydrate-binding chaperones.8–10 It is a common feature that many mature GPCRs are N-glycosylated at the N-terminal region and/or at the extracellular loops. These regions are luminal when the immature receptors are present in the ER. Cotranslational addition of sugar chains to asparagine residues by oligosaccharyltransferases provides binding sites for lectin chaperones that are able to bind to carbohydrate molecules. Such lectin chaperones include the proteins calnexin and calreticulin. After the removal of the two terminal glucoses by a glucosidase, the resulting monoglucosylated newly synthesized GPCR proteins interact with lectin chaperones. The interaction between the lectin chaperone and the immature receptor protein is terminated by the cleavage of the last glucose by glucosidase II. Once released, correctly folded glycoproteins can exit the ER. By contrast, incorrectly or incompletely folded glycoproteins are reglucosylated by glucosyltransferase, resulting in another round of association with calnexin and calreticulin. Cycles of glucosylation and deglucosylation continue until the glycoprotein has either reached its proper, folded conformation or is targeted for degradation.
Exit of proteins from the ER occurs at ER-exit sites, where buds are formed and coated with the COPII coat protein. Proteins released from the ER quality-control machinery accumulate in these COPII-coated buds.11 Signals in the cytoplasmically exposed C-terminal tails of transmembrane proteins, such as GPCRs, that are being transported in the secretory pathway are likely to be involved in direct binding with components of COPII.12 These signals comprise diacidic motifs and pairs of aromatic or bulky hydrophobic amino acid residues.11 Cargo receptors for soluble secretory proteins present in ER buds also possess these motifs in their C-terminal tail. Many GPCRs contain these ER-export motifs, indicating that they might interact directly with COPII complex proteins.13
The next step in the maturation of GPCRs involves trafficking to the Golgi apparatus. In mammalian cells, protein traffic moving from ER-exit sites to the Golgi complex passes through the ER–Golgi intermediate compartment. The ER–Golgi intermediate compartment is a site of anterograde and retrograde sorting under the control of COPI coat proteins, Rab and Arf GTPases.14 Vesicles exiting from this compartment are directed either to the Golgi or back to the ER, depending on a series of factors. These factors include the nature of the cargo protein, the Arf GTPase isoform involved in coat recruitment and the Rab effectors.
The ER–Golgi intermediate compartment is known to utilize at least two Rab effector proteins to promote the formation of two separate pools of transport vesicles. It is believed that Rab1 isoforms are involved in the transport from the ER to the ER–Golgi intermediate compartment and transport from the ER–Golgi intermediate compartment to the cis-Golgi complex.15 Conversely, Rab2 is believed to promote the formation of vesicles returning from the ER–Golgi intermediate compartment back to the ER.16
In order to investigate the involvement of these Rab effector proteins in GPCR trafficking through the ER–Golgi intermediate compartment, various studies have investigated the effect of selective small interfering RNA (siRNA)-mediated knockdown of Rab1 and Rab2 and overexpression of dominant-negative Rab1 and Rab2 on GPCR maturation and proper receptor export. Overexpression of dominant-negative Rab1a and siRNA-mediated knockdown of Rab1b perturbed trafficking of both the angiotensin AT1 receptor and the β2-adrenoceptor.17 Such effects appear to be receptor-specific as, in the same report, trafficking of the α2B-adrenoceptor was not affected by these same Rab protein manipulations. Additionally, overexpression of Rab2 mutants and siRNA-mediated knockdown of Rab2 resulted in an inhibition of cell surface expression of both the β2- and α2B-adrenoceptors.18
Transport vesicles that bud from the rough ER carry proteins to the luminal cavity of the Golgi complex. Three-dimensional reconstructions from serial sections of the Golgi complex show a series of flattened membrane sacs. These series of flattened membrane sacs have three defined regions, termed the cis-, medial-, and trans-Golgi. Transport vesicles from the rough ER fuse with the cis-Golgi region of the complex, where these cargo proteins are deposited. Proteins then progress from the cis to the medial to the trans region of the Golgi complex. Within each region are different enzymes (glycosyltransferases) that modify proteins differently depending on their structures and final destinations.19
Once in the Golgi, cargo proteins can be sorted to the endosomal system, the plasma membrane or back to the ER. Retrograde transport to the ER is likely to be involved in retargeting misfolded proteins to the ER-associated degradation pathway.20 Other defective proteins are targeted to lysosomal degradation after sorting to endosomes.20 As is the case with investigating GPCR transport out of the ER, studies examining GPCR trafficking through the Golgi stacks have looked at the effects of Rab effector protein perturbation. The Rab6 GTPase regulates vesicular transport in the Golgi. Perturbing the function of the Rab6 GTPase inhibits the anterograde transport of Drosophila rhodopsin and of mammalian GPCRs.18,21 Additionally, studies of δ-opioid receptor glycosylation demonstrated that O-glycosylation (on serine or threonine residues) and final processing of N-linked oligosaccharides occur in different compartments of the Golgi.22 Studies using a α2B-adrenoceptor mutant in which adjacent tyrosine and serine amino acid residues were substituted provided evidence that the N-terminus of the α2B-adrenoceptor might contain a signal that directs exit of the receptor from the Golgi. When this mutant α2B-adrenoceptor protein was expressed, the mutant receptor was primarily trapped in the Golgi complex.23
IV. Regulated Translocation to Intracellular Compartments and/or the Plasma Membrane
In the most simplified model of GPCR trafficking, GPCRs are expressed on the cell surface following biosynthetic sorting and are then endocytosed in response to activation by agonists. Increasingly, there is evidence that for some GPCRs, this might not always be the case. This is demonstrated by studies performed studying the trafficking of the protease-activated receptor (PAR) family. Irreversible activation of the PAR1 and PAR2 thrombin receptors by cleavage results in internalization and degradation of the receptors in lysosomes. It has been demonstrated that large pools of PAR1 and PAR2 receptors reside in intracellular compartments. Most of these receptors are localized in the Golgi complex, where they are protected from activation by thrombin and subsequent degradation. Upon activation of the cell surface receptors, these intracellular thrombin receptors translocate to the plasma membrane and replenish the pool of plasma membrane receptors that have been degraded following receptor activation. In this way, replenishment of plasma membrane thrombin receptors is correlated with recovery of thrombin responsiveness.24
As is the case with the thrombin receptors, regulated pools of intracellular dopamine D1 receptors exist in tubular renal cells. In these cells, agonist activation of cell surface dopamine receptors results in recruitment of the intracellular receptors to the cell surface.25 It has also been reported that intracellular D1 receptors can be recruited to the plasma membrane by atrial natriuretic peptide-dependent heterologous activation.26 A similar translocation profile has been demonstrated for α1A-adrenoceptors that translocate to the plasma membrane in response to stimulation by neuropeptide Y.26 Additionally, nerve growth factor treatment was shown to lead to the formation of intracellular pools of the δ-opioid receptor in PC12 neurosecretory cells.27 These intracellular δ-opioid receptors exhibited depolarization-dependent translocation to the plasma membrane. A similar intracellular distribution of δ-opioid receptors has been demonstrated in primary cells from the central nervous system of the rat.28 Also, it has been shown that prolonged exposure of neurons to morphine leads to the recruitment of intracellular δ-opioid receptors to the cell surface, both in vitro and in vivo.29,30
The estrogen receptor GPR30 is unique in that it is an intracellular transmembrane estrogen receptor.31 GPR30 is localized in the ER where it has the ability to specifically bind estrogen and estrogen derivatives. Activation of GPR30 with estrogen resulted in downstream signaling effects such as intra-cellular calcium mobilization, indicating that the intracellular receptors are functional. Similarly, studies investigating the function of cannabinoid receptor 1 (CB1) have demonstrated that these receptors are localized at the plasma membrane as well as in intracellular vesicles.32 Additionally, it was demonstrated that intracellular CB1 receptors do not have an endocytic origin. However, these receptors were shown to interact with the adaptor protein AP-3 and traffic to endolysosomes. These CB1 receptors in the endolysosomes associate with heterotrimeric G proteins and have the ability to mediate signaling, illustrating that the intracellular receptors are in fact functional. Hence, proteins that bind and retain these receptors modulate the spatial dynamics of signaling.
V. GPCR Oligomerization
GPCRs physically associate with other cellular proteins, including a large variety of soluble intracellular proteins such as β-arrestins, membrane proteins such as receptor-activity-modifying proteins (RAMPs) as well as other GPCRs. The recognition of GPCR homo- and heteromerization has generated numerous possibilities for expanding the roles and functions of GPCRs. Dimerization of GPCRs has been implicated in modulating a number of functional properties of the receptors, including ligand binding, receptor signaling, and receptor trafficking. Data supporting the association of GPCRs was first presented for muscarinic cholinergic and α2- and β2-adrenergic receptors (ARs).33 However, despite published reports on β2-AR homomerization,34 the concept of GPCR dimerization remained highly controversial until the cloning of the γ-amino-butyric acidB (GABAB) receptor.
After the cloning of the GABABR1 receptor it was quickly discovered that expression of a fully functional receptor in mammalian cells was not possible because the receptor was constitutively trapped in the ER and did not traffic to the cell surface.35 In an attempt to find novel genes related to the GABABR1 gene, two independent groups identified a related protein by homology cloning. This protein exhibited 35% identity to GABABR1, and was named GABABR2.36,37 When expressed in either Xenopus oocytes or HEK293 cells, neither GABABR1 nor GABABR2 receptors were cell surface localized or functional when expressed alone.35,36 However, coexpression of the GABABR1 and R2 receptors in heterologous systems resulted in the expression of these receptors at the cell surface.36
Coexpression of the two receptors also resulted in acquisition of receptor recognition properties characteristic of native GABAB receptors, coupling to K+ currents, and inhibition of adenylyl cyclase.36–40 The GABABR1 and GABABR2 receptors were also shown to physically associate with each other. Yeast two-hybrid screens demonstrated interaction of the C-terminus of GABABR1 with cDNA-encoded GABABR2 fragments.37,38 GST-pull down experiments showed interactions of the C-termini of GABABR1 and GABABR2,38 and these two receptors were co-immunoprecipitated from the rat brain cortex and from heterologous cells expressing both proteins.36,37,40
Further investigation demonstrated that heteromerization of the GABAB receptor appears to promote cell surface localization by masking an ER retention motif, identified as RXR(R), which exists in the C-terminus of GABABR1.41 It is important to note, however, that GABABR2 is not merely a chaperone for GABABR1. In fact, GABABR2 also contributed to signaling and hence is necessary for the formation of fully functional GABAB receptors. In addition, other domains of the two subunits were shown to interact, as C-terminally truncated constructs also form heteromeric complexes, suggesting that this interaction is not due entirely to interactions between the C-terminal domains.42
Unlike the case of the GABAB receptor in which dimerization of the R1 and R2 receptors is required for proper receptor cellular localization and function, opioid receptors subtypes which are properly localized and fully functional when expressed alone have been shown to form both homomers and heteromers.43,44 For example, while both κ-and δ-opioid receptors show high affinity for agonists and antagonists that are subtype-selective, the κ–δ heteromers have no significant affinity for any of the subtype-selective agents. However, the κ–δ-opioid receptor heteromers demonstrate a high affinity for certain partially subtype-selective ligands and display synergistic agonist binding. These opioid receptor heteromers therefore demonstrate potentiated functional responses.43 It is hypothesized that heteromerization results in the formation of novel recognition sites for previously characterized receptors which may provide functionally relevant receptors for endogenous opioid peptides.
In addition to the κ–δ heteromers, δ-opioid receptors also heteromerize with μ-opioid receptors.45 Treatment of cells coexpressing μ- and δ-opioid receptors with very low concentrations of certain δ-opioid agonists significantly potentiates the binding of μ receptor-selective agonists and vice versa. Subsequently, it has been proposed that this μ–δ heteromer interaction may represent the molecular basis for the observed phenomenon of opiate synergy. Opiate synergy is the term used to describe the enhancement of the analgesic effects of one opioid receptor subtype through the treatment with the agonist that is selective for another receptor subtype.
Not only does heteromerization of κ- and δ-opioid receptors lead to the generation of completely distinct pharmacological profiles, heteromerization of these two opioid receptor subtypes modulates receptor trafficking. When these opioid receptors are expressed alone, stimulation of δ-opioid receptors with etorphine results in substantial internalization of these receptors; however, κ receptors do not internalize following stimulation with this agonist.43 However, when these δ and κ receptors are coexpressed and then stimulated with etorphine, internalization of the δ receptor is substantially reduced, indicating that heteromerization of these two opioid receptors significantly changes the trafficking profiles of the receptors.43
In addition to investigating heteromerization between receptor subtypes in the same receptor families, there are reports of GPCR heteromerization between receptors from two distinct subfamilies. For example, it was reported that the β2-adrenergic receptors (β2AR) (which couple to the Gs protein) and κ- and δ-opioid receptors (which couple to Gi proteins) can also form heteromers. This heteromerization was demonstrated by co-immunoprecipitation of independently epitope-tagged receptors. As an important control, it was shown that this co-immunoprecipitation does not occur if the β2AR and opioid receptors are expressed independently and the cells are mixed before extraction and immunoisolation. Heteromerization of these receptor pairs does not alter ligand binding or functional coupling of the receptors; however, the trafficking properties of the receptors was affected by dimerization. When expressed alone, β2ARs undergo robust internalization following agonist stimulation. However, it was demonstrated that when β2AR is coexpressed with κ receptor followed by stimulation with the β-adrenergic agonist isoproterenol the β2AR receptors fail to internalize.46 In contrast, coexpression of δ-opioid and β2ARs leads to agonist-mediated internalization following treatment with either etorphine or isoproterenol.46
In addition to the aforementioned studies looking at β2AR heteromerization, significant effort has been made looking at the ability of β2AR to form homomers. Initial reports of negative cooperativity of ligand binding at β2ARs provided the first indications that β2AR may form homomers.47 Evidence for the existence of β2AR homomers include biochemical data in which differentially epitope-tagged β2AR receptors were coexpressed in the same cell and subsequently co-immunoprecipitated.48 Based on homology to a dimerization motif found in glycophorin A, sequence analysis of β2AR revealed a putative dimerization motif in the sixth transmembrane-spanning segment. Hebert et al. synthesized a peptide corresponding to this motif in the sixth transmembrane-spanning segment of β2AR and found that it was capable of disrupting β2AR dimer formation, as assessed by SDS-PAGE analysis.48 Additionally, researchers found that this peptide diminished β2AR-stimulated adenylyl cyclase activity in a concentration-dependent manner. The functional relevance of β2AR dimerization is further supported by the observation that isoproterenol enhances dimer formation and confers protection from interference by the sixth transmembrane-spanning segment peptide; consistent with the interpretation that agonist occupancy stabilizes β2AR dimer formation.
The conclusion that β2AR oligomerization occurs in living cells is further supported by bioluminescence resonance energy transfer (BRET) studies.49 BRET relies on the transfer of energy from one tagged receptor (the donor) to a differentially tagged receptor (the acceptor). The energy transfer requires that the energy donor receptor and acceptor receptor are in very close proximity. In BRET studies, evidence of two receptors being in close proximity is used as an indication of protein–protein interactions and subsequently receptor oligomerization.
For studies investigating homomerization of the β2AR, fusion proteins of β2AR-luciferase and β2AR-GFP were created and then coexpressed in cells. It was observed that BRET energy transfer could be detected in cells coexpressing the two differentially tagged receptors. In addition, when these cells were activated with the receptor agonist isoproterenol there was a dose-dependent increase in the BRET signal achieved. It was also demonstrated that no BRET signal is detected when the β2AR-luciferase fusion protein is coexpressed with other receptors (including the melatonin 1a receptor-GFP or the chemokine CCR5 receptor-GFP fusion proteins).50 These results are consistent with a model in which the β2AR homomerization is promoted by the receptor agonist.50
But what are the roles of GPCR homomerization in promoting receptor maturation or plasma membrane localization? As discussed earlier, in the case of GABA receptors, heteromerization may help promote exit from the ER by masking retention signals present in the receptors. There is less evidence of specific examples of mechanisms by which homomerization of GPCRs can help promote receptor maturation during biosynthesis. One emerging hypothesis is that homomerization might assist in receptor folding. The membrane-spanning domains of GPCRs contain hydrophobic regions that are particularly prone to nonspecific aggregation. In fact, chaperones are known to associate with newly synthesized polypeptides in order to prevent unproductive interactions with the environment that could result in protein aggregation.51 Thus, one hypothesis is that individual GPCR receptors can potentially serve as folding chaperones for each other. Therefore, the ordered association of two newly synthesized GPCR polypeptides via their hydrophobic transmembrane regions could hide a substantial proportion of the exposed hydrophobic surface and facilitate correct folding. In some cases these hydrophobic transmembrane regions constitute the dimerization interface, supporting such a hypothesis.
This model that GPCRs can act as folding chaperones for each other is supported by the fact that functional GPCR heteromers have been obtained in reconstituted cell models containing receptors that do not encounter each other under normal physiological conditions. In these artificial conditions, in which two distinct GPCR polypeptides are forced to enter simultaneously into the ER, if they display sufficient structure-driven propensity to assemble, they might form heteromers.
Another theory is that homomerization of GPCRs might also contribute to quality control. This theory is based on the fact that dimeric receptors are likely to be structurally symmetric and checking for symmetry between two receptors in a homomer might be one mechanism by which mutant receptor can be identified in the ER. Random mutations affecting the overall structure of one receptor might generate asymmetry within the homomer. If asymmetry is found between receptors in a homomer, this could lead to the recognition of mutant receptors by the ER quality-control system. Once recognized, these mutant receptors can then be retained for disposal via the ER-associated degradation pathway. Consistent with this model, mutant ER-retained GPCRs have been shown to display a dominant-negative effect over wild-type GPCRs in reconstituted cellular models and in heterozygous individuals.52
Studies on receptor homomerization have also provided useful information on how and where dimerization occurs in cells. In a study investigating the biogenesis of serotonin 5-HT2C homomers, confocal microscopy and fluorescence resonance energy transfer (FRET) were used to monitor the proximity of differentially tagged serotonin 5-HT2C receptors in intact living cells.53 These differentially tagged receptors were tracked during biosynthesis in the ER, trafficking through the Golgi complex and subsequent localization at the plasma membrane. The FRET results from these studies provide evidence of oligomerization of these receptors in the ER and the Golgi of living cells. Similarly, a study using photoaffinity labeling to investigate the dimerization of wild-type and mutant dopamine D2 receptors provided evidence for dimerization of these receptors in intracellular compartments.54 These results also highlighted the fact that dimerization is a naturally occurring step in receptor maturation and indicated that, in some cases, dimerization might be a prerequisite for normal receptor trafficking and cell surface localization.
VI. Specific Molecular Chaperones for GPCRs
It has become evident that, in addition to using general chaperones for the folding and sorting of newly synthesized proteins, some proteins may require more specialized assistance and utilize specific chaperones. Some of these specialized chaperones or enzymes directly participate in the folding of their cognate proteins. In the case of GPCRs, a few specialized chaperones have been reported to be involved in GPCR folding. The functions of these chaperones are summarized in Table I. NinaA (neither inactivation nor afterpotential A) is known to be a chaperone for the visual pigment receptor rhodopsin 1 in Drosophila melanogaster. NinaA mediates the cell surface expression of Rhodopsin 1. In vertebrates, the rhodopsin 1 chaperone is RAN-binding protein 2. Both these chaperones are cyclophilin type-II proteins displaying peptidyl–prolyl cis–trans isomerase activity.55,56
TABLE I.
The Functions of Known Molecular Chaperones for GPCRs
| GPCR | Molecular chaperone | Function(s) | Reference(s) |
|---|---|---|---|
| Luteinizing hormone receptor | BiP (GRP78) | Folding | 8,9 |
| GPR94 | Folding | 9 | |
| Rhodopsin I | NinaA (D. melanogaster) | Cell surface targeting | 55 |
| RAN-binding protein 2 (vertebrates) | Cell surface targeting | 56 | |
| Calcitonin receptor-like receptor | RAMP 1, 2, 3 | Cell surface targeting terminal glycosylation and maturation | 63 |
| Olfactory receptors | ODR4 | Folding | 65 |
| Cell surface targeting | |||
| RTPI,2 | Cell surface targeting | 66 | |
| REEP I | Cell surface targeting | 66 | |
| μ–δ-Opioid receptor | RTP4 | Cell surface targeting heteromer protection from ubiquitination/degradation | 67 |
| Vomeronasal receptors | Ml0 | Cell surface targeting | 68 |
| Dopamine D1 receptors | DRiP78 | Cell surface targeting | 70 |
| Angiotensin AT2 receptors | ATBP5O | Cell surface targeting | 73 |
| Thromboxane A2 receptor | RACK 1 | Cell surface targeting | 74 |
| CXCR4 chemokine receptor | RACK1 | cell surface targeting | 74 |
| κ-Opioid receptors | GEC1 | Cell surface targeting | 75 |
| Glycosylation | |||
| μ-Opioid receptor | Ribophorin I | Cell surface targeting | 76 |
| Metabotropic glutamate receptor 5 | Norbin | Cell surface targeting | 77 |
| Serotonin 5-HT1B receptor | p11 | Cell surface targeting | 78 |
| Melanocortin-2 receptor | MRAP | Cell surface targeting | 81,82 |
| Glycosylation | |||
| AdenosineA2a receptor | Usp4 | Cell surface targeting protection from ubiquitination/degradation | 90 |
NinaA was discovered when it was demonstrated that mutation in the gene encoding this protein leads to reduced expression of rhodopsin in R1–6 photo-receptor cells of the Drosophila eye.57 Further investigation indicated that in the absence of NinaA, rhodopsin 1 accumulates in the ER, where it is degraded instead of being transported to rhabdomeres.58 Unlike other cyclophilins which are usually cytosolic proteins, NinaA has a membrane permeating signal sequence and a single transmembrane-spanning domain and therefore functions as an integral membrane protein.55 It has been demonstrated that NinaA forms a stable complex with rhodopsin and, functioning more stoichiometrically than catalytically, ensures proper folding and trafficking of the newly synthesized rhodopsin receptors to the plasma membrane.
Rhodopsin also interacts with human neuron-specific DnaJ-like protein (HSJ1). HSJ1 is a member of the DnaJ and Hsp40 chaperone family.59 In neurons, HSJ1 proteins, which function at the cytosolic face of the ER, facilitate the transfer of newly synthesized proteins onto Hsc70 chaperones. This is followed by ubiquitination of the proteins and subsequent sorting to the proteasome.60 Thus, HSJ1 isoforms probably participate in the ER-associated degradation pathway and protect neurons against cytotoxic protein aggregation. Other specialized chaperones for GPCRs are escort proteins.61 These escort proteins bind newly synthesized proteins in the ER and escort them to the Golgi complex and to the plasma membrane.
One novel group of specialized escort proteins that work with GPCRs is represented by the receptor-activity-modifying protein (RAMP) family of protein.62 RAMPs are type-I single transmembrane domain proteins with a large N-terminal extracellular domain and a short C-terminal domain. RAMPs were initially described as interacting partners for the calcitonin receptor-like receptor (CRLR). In the case of CRLR, RAMPs act as chaperones to assist in the transport of the CRLR receptor to the cell surface.63
RAMP1 was initially identified by expression cloning in Xenopus oocytes for cDNAs encoding the human calcitonin gene related peptide (CGRP) receptor. Coexpression of RAMP1 with the cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel leads to cAMP production following stimulation of CGRP and activation of the CFTR chloride channel. RAMP1 also plays an important role in the trafficking of CRLR receptor to the cell surface. When expressed alone, neither RAMP1 nor CRLR was found to be expressed at the cell surface. However, when the CRLR receptor was coexpressed with the RAMP1 chaperone protein, both proteins showed cell surface localization.63 Such studies demonstrate that RAMPs remain associated with their cognate receptors at the cell surface.
In addition to its role in assisting the transport of the CRLR receptor to the cell surface, RAMP1 also helps define the glycosylation state and recognition properties of the receptor. Using SDS-PAGE analysis it was shown that when expressed alone CRLR is sensitive to endoglycosidase H treatment.63 This result indicates that the CRLR receptors have not been terminally glycosylated. Conversely, CRLR when coexpressed with RAMP1, migrates as a larger molecular weight protein and is resistant to endoglycosidase H treatment. Additionally, when coexpressed with RAMP1, CRLR shows sensitivity to endoglycosidase F (which cleaves all N-linked carbohydrates). These results indicate that while RAMP1 assists in the trafficking of CRLR to the cell surface, the chaperone is also required for terminal glycosylation and maturation of the CRLR receptor through the Golgi complex on its way to the plasma membrane.
Two additional RAMP proteins, RAMP2 and RAMP3 have been identified.63 These three RAMPs have different mRNA expression profiles. Interestingly, when different RAMP proteins act as the chaperone for the CRLR receptor they result in differential ligand binding properties for the receptor. While chaperoning by RAMP1 was shown to increase the CRLR-mediated cAMP production in response to CGRP stimulation, when RAMP2 is used as a chaperone for CRLR the receptor behaves pharmacologically like an adrenomedullin receptor.63
Even though it has been demonstrated that coexpression of CRLR with different RAMP proteins results in differences in the glycosylation state of CRLR, it has been found that the chaperone protein-dependent ligand binding selectivity observed for the CRLR receptor is determined by protein–protein interactions and not by these differences in the glycosylation state of the receptor.64 The realization that CRLR can exhibit two distinct pharmacological profiles depending on which chaperone protein it interacts with expands the already extensive biological diversity of signaling responses that GPCR receptors can generate in cells. These GPCRs can exhibit differential responses to various stimuli based not only on the specific expression pattern of the receptors, but also the specific expression pattern of the cognate accessory proteins.
Another example of GPCRs that require specific chaperones for proper cell surface expression is the group of olfactory or odorant receptors. The necessity of specific chaperones for cell surface expression of olfactory receptors is highlighted by the fact that functional expression of these receptors is essentially impossible in heterologous cell systems where these olfactory neuron-specific chaperones are absent. In fact, when olfactory receptors are expressed in nonolfactory neuronal cells the receptors are retained in the ER and rarely pass into the Golgi complex. When a genetic screen was performed in Caenorhabditis elegans for mutants with olfactory defects the odorant response protein 4 (ODR4) was identified as a chaperone for olfactory receptors.65 ODR4 is expressed specifically in chemosensory neurons where it promotes the folding and cell surface localization of olfactory receptors. In addition, receptor-transporting protein (RTP) 1 and 2 and receptor-expression-enhancing protein 1 (REEP1) were found to also act as chaperones for olfactory receptors and enable functional cell surface targeting of these receptors in fibroblasts.66 Similarly, RTP4 acts as a chaperone for opioid receptors (this is discussed in more detail in Section VIII; Ref. 67).
Other sensory GPCRs are involved in the detection of pheromones. Mammals possess a small sensory organ located near the base of the nasal septum that is involved in the detection of pheromones called the vomeronasal organ. The vomeronasal organ contains specific GPCRs that function as pheromone receptors. As is the case with other GPCRs, these vomeronasal receptors require the association with specific chaperone proteins for proper trafficking to the cell surface. It has been demonstrated that proper cell surface localization of vomeronasal receptors is aided by the action of M10 chaperone.67 M10s belong to the superfamily of major histocompatability complex (MHC) class I molecules and are exclusively expressed in the vomeronasal organ. As is the case with RAMP proteins, results have indicated that in addition to their role as chaperones, M10s might modulate the ligand specificity of vomeronasal receptors and might participate in neuronal plasticity.68
Another area of active research involves understanding the roles of molecular chaperones in modulating the cell surface expression of neurotransmitter receptors. The dopamine receptor-interacting protein of 78 kDa (DRiP78) is a known chaperone for the dopamine receptor. DRiP78 is a putative two transmembrane-spanning domain protein and is ER membrane-associated. When DRiP78 is over expressed or knocked down this results in ER retention of dopamine D1 receptors as well as reduced ligand binding of the receptors and modulation of the receptor glycosylation profile.69 It was hypothesized that DRiP78’s role is not limited to being a chaperone for dopamine receptors and that DRiP78 is capable of functioning as a chaperone for several GPCRs. This hypothesis was developed based on the fact that DRiP78 binds to a Phe-Xaa-Xaa-Xaa-Phe-Xaa-Xaa-Xaa-Phe motif on dopamine receptors and this motif is found in the C-terminus of various GPCRs. This hypothesis has been supported by a study demonstrating a role for DRiP78 in the maturation of the angiotensin II AT1 receptor.70 Expanding its role even further, it has also been demonstrated that DRiP78 specifically interacts with Gγ subunits of heterotrimeric G proteins. This interaction protects the Gγ subunits from degradation until a stable partner (the cognate Gβ subunit) is provided. Such results indicate an additional chaperone role for DRiP78 in the assembly of Gβγ subunits.71
As demonstrated by DRiP78, intracellular membrane proteins are capable of functioning as molecular chaperones and regulating the targeting of GPCR receptors to the cell surface without the chaperone itself being localized at the cell surface. Another example of such chaperone activity is the angiotensin AT2 receptor-binding protein of 50 kDa (ATBP50) which is a membrane-associated Golgi protein. ATBP50 binds to the cytoplasmic C-terminal tail of the angiotensin AT2 receptor and controls the cell surface expression of the receptor. It was demonstrated that inhibition of ATBP50 expression results in retention of the angiotensin AT2 receptor within intracellular compartments.72
Similarly, the receptor for activated C-kinase 1 (RACK1) is an ER chaper-one that regulates the cell surface localization of the thromboxane A2 receptor. RACK1 constitutively binds to the C-terminal tail and also the first intracellular loop of the thromboxane A2 receptor.73 In cells where the levels of RACK1 were reduced by siRNA-mediated knockdown of the chaperone protein, the thromboxane A2 receptor was retained in the ER. Interestingly, reducing the expression level of RACK1 is also able to affect the cell surface distribution of the chemokine receptor CXCR4 but has no effect of the cell surface localization of the β2-adrenoceptor or prostanoid DP receptors, indicating that RACK1 shows specificity for different GPCRs.
Another chaperone protein that demonstrates comparable specificity for GPCRs is GEC1. GEC1 is a member of the microtubule-associated family of proteins.74 It was demonstrated that in the ER and the Golgi, GEC1 interacts with the C-terminus of κ-opioid receptors, but not with that of μ- or δ-opioid receptor subtypes. Additionally, GEC1 expression enhances the level of mature fully glycosylated forms of κ-opioid receptors and facilitates trafficking of the κ-opioid receptor to the cell surface. GEC1 levels seem to be tightly regulated, as indicated by a toxic effect of over expressing GEC1.74
Using a targeted proteomic approach, Ribophorin I was identified as a chaperone for the μ-opioid receptor.75 Overexpression of Ribophorin I was able to rescue the cell surface expression of mutant μ-opioid receptor. The chaperone activity of Ribophorin I is dependent on the glycosylation state of the μ-opioid receptor, as the chaperone is ineffective when overexpressed with glycosylation-deficient mutants. Similarly, the neuron-specific protein Norbin was shown to physically interact with the metabotropic glutamate receptor 5 and increase the cell surface localization of the receptor.76
Additional insights into the function of molecular chaperones come from studies performed characterizing knockout mice. Mice were created that lack the p11 protein.77 P11 (also known as calpactin-I- or annexin-II-light chain) is a member of the S100 EF-hand calcium-dependent signaling-modulator family.78 When p11 was depleted in mice, the animals exhibited a depression-like phenotype. Clinically, such depression-like phenotypes are commonly associated with abnormal serotonin signaling. In fact, the cell surface density and function of the serotonin 5-HT1B receptor is decreased in p11 knockout mice. Additional studies demonstrated that the distribution of p11 mRNA in the brain resembles that of 5-HT1B receptor mRNA. Also, p11 specifically interacts with 5-HT1B and does not interact with other serotonin or dopamine receptors and p11 colocalizes with the receptor at the cell surface of transfected cells.78 Interestingly, p11 levels are increased in the brain of mice treated with anti-depressants and p11 levels are reduced in depressed patients.
VII. The Melanocortin-2 Receptor and MRAP
The melanocortin family of receptors is a group of five structurally related GPCRs that play diverse physiological roles in mammals.79 All the melanocor-tin receptors cause an increase in cAMP levels when stimulated by agonists. However, the receptors differ in their affinity for physiological agonist. Melanocortin-2 is unique because it is the only melanocortin receptor that is selectively regulated by adrenocorticotropin hormone. Melanocortin-2 is also unique in the way that it requires an accessory protein for proper trafficking of the receptor to the cell surface, while the other related melanocortin receptors do not require this chaperone.80 This accessory protein, the melanocortin-2 receptor accessory protein (MRAP) is required not only for the melanocortin-2 receptor to traffic from the ER to the plasma membrane, but is also required for the receptor to undergo proper glycosylation, binding of adrenocorticotropin hormone and stimulation of adenylyl cyclase.80–82
As is the case with odorant receptors, the functional expression of the melanocortin-2 receptor in heterologous systems proved difficult and could be achieved only in cells of adrenocortical origin, highlighting the fact that the melanocortin-2 receptor must be expressed in the presence of a cell-type-specific chaperone protein in order for proper biosynthetic trafficking and localization. Further investigation identified MRAP as the required chaperone protein. MRAP is a small single membrane-spanning protein with a conserved amino terminus. When MRAP was coprecipitated with melanocortin-2 it was discovered that MRAP forms antiparallel homomers.82 Mutational analysis performed on MRAP revealed that the amino-terminal and transmembrane region, and not the carboxyl-terminal domain, is required for MRAP’s function as a molecular chaperone.
In humans, inactivating mutations in the melanocortin-2 receptor lead to glucocorticoid deficiency resulting from adrenocorticotropin hormone resistance. The importance of MRAP’s function is highlighted by the fact that mutations in MRAP can also lead to these disease states. Familial glucocorticoid deficiency is a genetic disease in which the adrenal cortex is resistant to adrenocorticotropin hormone. Inactivating mutations have been identified in the gene that encodes MRAP in patients that suffer from familial glucocorticoid deficiency. This example illustrates, particularly well, the physiopathological relevance of the interaction between a GPCR and a specific chaperone protein.
MRAP’s specificity of action was demonstrated in the study that compared the effects of MRAP activity on the dimerization and trafficking of melanocortin-2 and melanocortin-5 receptors.83 Melanocortin-5 shares 67% homology with the melanocortin-2 receptor at the amino acid level and functions in regulating exocrine gland secretion and behavioral responses following pheromone secretion.84,85 While the melanocortin-2 receptor is activated by adrenocorticotropin hormone, melanocortin-5 responds to melanocyte-stimulating hormone. Unlike melanocortin-2, melanocortin-5 shows cell surface localization when expressed exogenously.81
As expected, coexpression of melanocortin-2 with MRAP revealed that MRAP facilitated the plasma membrane localization of melanocortin-2. In contrast, while melanocortin-5 receptors traffic to the plasma membrane in the absence of MRAP, coexpression of MRAP with melanocortin-5 leads to intracellular retention of the receptors.83 MRAP was shown to form stable complexes with both melanocortin receptors as determined by co-immunoprecipitation experiments. In addition, coexpression of MRAP had no effect of melanocortin-2’s ability to form homomers, but inhibited the formation of melanocortin-5 homomers. One interpretation of these results is that MRAP disrupts a dimerizaton step that is critical for the maturation and trafficking of the melanocortin-5 receptor to the cell surface. In the current model of MRAP’s function, in cells expressing both melanocortin-2 and melanocortin-5 receptors, MRAP will have a dual role in which increased activity of MRAP will favor responsiveness to adrenocorticotropin hormone and decreased responsiveness to melanocyte-stimulating hormone, while decreased MRAP activity will have the opposite effect. This is one way in which a selective chaperone protein can enhance the signaling capabilities of a system comprising of structurally related receptors.
VIII. Opioid Receptors and RTP4
As discussed earlier, the three subtypes of opioid receptors, δ, κ, and μ have the ability to form both homomers and heteromers.86 In addition, there are varied pharmacological profiles for different homomer and heteromer receptor pairs. The clinical importance of heteromerization of opioid receptors is highlighted by the fact that despite μ-opioid receptors mediating, most of the pain-relieving effects of morphine, antagonism of δ-opioid receptors leads to a reduction in the tolerance that develops after chronic morphine administration.87 This observation suggests that there are potential links between opioid receptor dimerization and drug tolerance. Additionally, the analgesic potency of morphine has been linked to the presence of μ–δ-opioid receptor heteromers.88 In the current model for the development of drug tolerance, factors that modulate the ratio of μ-and δ-opioid receptors that exist as homomers and receptors and which exist as μ–δ receptor heteromers serve as critical factors in determining the net effect of receptor stimulation.
Using the μ–δ-opioid receptor heteromer at a model system, it was reported that coexpression of the differentially tagged μ- and δ-opioid receptors leads to increased retention of both receptors in intracellular compartments.89 Further investigation showed that the μ–δ receptor heteromers were accumulating in the Golgi complex and were subsequently targeted to the degradation pathway. In order to identify chaperones that could function at the Golgi and assist in folding and trafficking of these receptor heteromers, these opioid receptors were coexpressed with members of the RTP family of chaperones. As noted earlier, RTP proteins are known to act at the Golgi complex where they assist in the cell surface targeting of odorant and taste receptors.66 Coexpression of RTP4 leads to an increase in the cell surface localization of both μ- and δ-opioid receptors.
RTP4 physically interacts with both opioid receptors and decreases the ubiquitination of the receptor heteromers. Subsequently, RTP4 was shown to be involved in the proper folding of the μ–δ-opioid receptor heteromers as it facilitates the trafficking of the heteromers from the Golgi complex to the cell surface. Furthermore, RTP4 was shown to be associated with the μ–δ-opioid receptor heteromer at the cell surface and could play a role in modulating heteromer-specific pharmacology. It has been proposed that the retention of μ–δ receptor heteromers in the Golgi may be the result of a mechanism in which dimerization of the receptors leads to the masking of motifs needed for recognition of the receptors by accessory proteins. Interaction of RTP4 at this motif could prevent the retention of the heteromers by serving as an adaptor protein for other factors involved in trafficking of the opioid receptors to the plasma membrane.
Similar to the role of RTP4 in decreasing the ubiquitination of opioid receptor heteromers, the ubiquitin-specific protease Usp4 has been shown to regulate the cell surface localization of the adenosine A2a receptor.90 Usp4 was shown to bind to the C-terminus of the adenosine receptor and lead to decrease in the ubiquitination of the receptor, leading to enhanced cell surface expression of the A2a receptor. Additionally, a related ubiquitin-specific protease, Usp14, has been implicated in the deubiquitination of the chemokine receptor CXCR4.91
IX. Pharmacological Chaperones
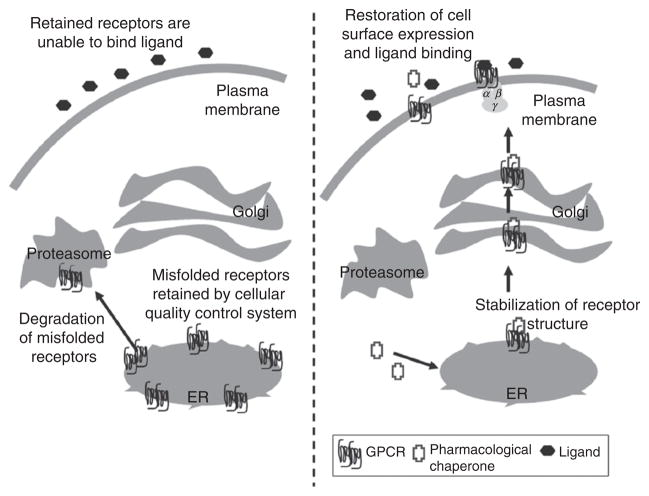
As previously discussed, some receptor proteins do not achieve proper cell surface trafficking when expressed in heterologous systems. This is usually due to the absence of proper endogenous chaperones which lead to the intracellular retention of these receptors by the cell’s quality-control system. One method to overcome this intracellular retention of receptor proteins is through the use of small molecules that act as chaperones and aid in the proper sorting and trafficking of receptors. Such molecules are referred to as pharmacological chaperones or “pharmacoperones.” Pharmacological chaperones are small molecules that have the ability to enter cells, bind specifically to misfolded proteins and correct the folding of these proteins.92,93 The result of the treatment with pharmacological chaperones is that these receptor proteins are able to escape retention by the cellular quality-control system and achieve proper cell surface localization. A proposed model for the action of pharmacological chaperones is illustrated in Fig. 1. In many cases, these pharmacological chaperones were initially identified as peptidomimetic antagonists selected from high-throughput screens.
Fig. 1.
Proposed model for the action of pharmacological chaperones. Left panel: Mutated receptors and wild-type receptors that are misfolded are retained in the ER by the cellular quality-control system and targeted for proteasomal degradation. Subsequently, these receptors are not able to bind ligand and participate in signaling. Right panel: Treatment of cells with cell permeable pharmacological chaperones leads to stabilization of the receptor structure and restoration of the cell surface expression of the receptors. The receptors are therefore able to bind ligands and function in signaling processes.
In addition to having a practical utility in laboratory studies in which the functional expression of receptors in heterologous systems is required, pharmacological chaperones also have potential therapeutic value. As previously discussed, there are diseases that result from the inability of specific GPCRs to fold properly and traffic to the cell surface where they can function effectively (such as melanocortin-2 receptor mutations leading to familial glucocorticoid deficiency). There has been significant interest in identifying small molecules that can bind to disease-causing GPCR mutants that traffic poorly and by acting as pharmacological chaperones, enhance the surface expression and functionality of these receptors. Unlike other protein stabilizing compounds that may only work in vitro (such as polyols and other chemical chaperones) the goal is to develop pharmacological chaperones that can be used in a clinical setting to treat well-defined diseases involving misfolded/retained proteins.
One example of the potential use of pharmacological chaperones for the treatment of a disease resulting from the ineffective sorting of a GPCR is the development of pharmacological chaperones for nephrogenic diabetes insipidus. Nephrogenic diabetes insipidus is a rare X-linked disease characterized by the loss of the antidiuretic response to the hormone arginine-vasopressin, resulting in an inability of the body to concentrate urine. Nephrogenic diabetes insipidus has been linked to a variety of mutations in vasopressin V2 receptors. In fact, the majority of the V2 receptor mutations are mutations that result in the V2 receptors being retained in the ER and degraded.94 Researchers have worked to identify a group of small molecules that are able to act as pharmacological chaperones for the V2 receptor.
Treatment of cells with certain membrane-permeable V2 receptor antagonists that act as pharmacological chaperones leads to the restoration of the cell surface expression of V2 receptors mutants that were previously retained in the ER.95 These results are attributed to binding of the antagonists to the already misfolded V2 receptors in the ER. Binding of these pharmacological chaper-ones results in stabilization of receptor structure and trafficking of V2 receptors to the plasma membrane. Proof-of-concept evidence that such vasopressin receptor-targeted pharmacological chaperones can have beneficial therapeutic effects has been provided from clinical studies involving patients suffering from nephrogenic diabetes insipidus.96
Another disease that is caused from mutations in a specific GPCR resulting in intracellular retention and ineffective traveling of the receptor is retinitis pigmentosa. Retinitis pigmentosa is characterized by progressive photoreceptor degeneration and eventual retinal dysfunction. Retinitis pigmentosa has been linked to a number of mutations in proteins that serve as various components of the visual signaling pathway, including rhodopsin.97 Mutations in the gene encoding rhodopsin are observed in one particular form of retinitis pigmentosa, autosomal dominant retinitis pigmentosa, in which there is a mutated rhodopsin receptor that is retained intracellularly and is unable to bind the ligand, 11-cis-retinal binding. The most common mutation of this type is P23H.98 P23H rhodopsin mutants can be rescued by treatment of cells with an 11-cis-retinal analog, 11-cis-ring-retinal, resulting in restoration of receptor surface expression.99
Hypergonadotropic hypogonadism is a disease characterized by a decrease in gonadal function that manifests in delayed sexual development in patients who suffer from the disease. The clinical phenotypes associated with hypergonadotropic hypogonadism have been linked to mutations within the gonadotropin-releasing hormone receptor gene. Gonadotropin-releasing hormone is secreted by the hypothalamus and induces synthesis and release of follicle stimulating hormone and luteinizing hormone from the pituitary gland. Many of the mutations in the gonadotropin-releasing hormone receptor that have been linked to hypergonadotropic hypogonadism are mutations that result in intracellular retention of the receptor or degradation of the receptor.100
Studies have demonstrated that these mutant gonadotropin-releasing hormone receptors can be made to traffic to the cell surface and function effectively through the use of pharmacological chaperones.101–103 When cells expressing gonadotropin-releasing hormone receptor mutants were treated with the IN3, a membrane-permeable gonadotropin-releasing hormone receptor antagonist the receptors were able to be rescued from intracellular retention, traffic to the plasma membrane and bind ligands.101,102 Such examples highlight the potential utility of small molecule pharmacological chaperones in correcting receptor misfolding. Similarly, treating cells with the opioid antagonist naloxone, the agonist etorphine, and other membrane-permeable hydrophobic ligands enhanced the cell surface expression of μ-opioid receptor mutants.104 These receptor mutants are usually retained in the ER and binding of the ligands resulted in concentration-dependent trafficking of the mutant receptors to the cell surface, indicating that these ligands can act as pharmacological chaperones. These in vitro findings are supported by observations that long-term use of these ligands leads to improved stability, folding efficiency of newly synthesized receptors in vivo.105
This approach of using pharmacological chaperones has the potential to be applied to an array of human diseases that result from ineffective receptor signaling due to protein misfolding. Evidence for the potential use of pharmacological chaperones has been presented for cystic fibrosis, hypercholesterolemia, cataracts and neurodegenerative diseases such as Huntington’s, Alzheimer’s, and Parkinson’s diseases.106–113 Also, while these pharmacological chaperones are being tested for the treatment of mutant receptors that are misfolded, there is increasing evidence that some wild-type GPCRs are also commonly misrouted, presumably as a result of misfolding. This suggests that pharmacological chaperone intervention can potentially be expanded to more general posttranslational control mechanisms and provide another level of potential therapeutic intervention.114
X. Conclusions
With recent advances in techniques to study the pharmacology, signaling, and trafficking of transmembrane receptors we are left with a better understanding of how this tightly regulated network of receptors work. We now have a great appreciation of the role of processes such as receptor oligomerization, receptor desensitization and interaction with accessory proteins and cofactors. Advances in the field of molecular chaperone biology have provided insights into what can happen when receptors are misfolded, retained in intracellular compartments, and degraded. Modulating of protein folding and/or molecular chaperone activity in vitro has provided initial clues to how mutant or otherwise misfolded receptors can be rescued and made to function properly.
As demonstrated by the studies looking at nephrogenic diabetes insipidus, retinitis pigmentosa, and hypergonadotropic hypogonadism, pharmacological chaperones represent the most promising therapeutic approach for the treatment of diseases resulting from protein misfolding. As more receptor-specific molecular chaperones (such as RTP4 for opioid receptor heteromers and MRAP for melanocortin-2 receptors) are identified additional studies will hopefully provide a good framework in which we can develop treatments that specifically modulate the levels/activity of these molecular chaperones to combat the receptor folding or trafficking problems that lead to various disease states.
The discoveries discussed in this chapter raise many new questions and interesting challenges that will certainly be addressed in the near future. This will inevitably expand the potential for the use of pharmacological tools to target these interactions between GPCRs and molecular chaperones and subsequently manipulate the expression, localization, and function of these receptors in order to gain therapeutic benefits. As we develop new therapies, the specificity of these therapies can be increased by using conventional pharmacological approaches to modify the activity of receptors in conjunction with novel pharmacological approaches to control which particular receptors are expressed at the cell surface of specific cells.
Acknowledgments
The authors thank Drs. Raphael Rozenfeld and Ivone Gomes for critically reading the manuscript. These studies were supported by National Institutes of Health Grants DA008863 and DA019521 (to L.A.D.).
References
- 1.Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–9. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 2.Higy M, Junne T, Spiess M. Topogenesis of membrane proteins at the endoplasmic reticulum. Biochemistry. 2004;43:12716–22. doi: 10.1021/bi048368m. [DOI] [PubMed] [Google Scholar]
- 3.Krebs MP, Noorwez SM, Malhotra R, Kaushal S. Quality control of integral membrane proteins. Trends Biochem Sci. 2004;29:648–55. doi: 10.1016/j.tibs.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–91. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 5.Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 6.Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3:246–55. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- 7.Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–96. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 8.Siffroi-Fernandez S, Giraud A, Lanet J, Franc JL. Association of the thyrotropin receptor with calnexin, calreticulin and BiP. Effects on the maturation of the receptor. Eur J Biochem. 2002;269:4930–7. doi: 10.1046/j.1432-1033.2002.03192.x. [DOI] [PubMed] [Google Scholar]
- 9.Mizrachi D, Segaloff DL. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol Endocrinol. 2004;18:1768–77. doi: 10.1210/me.2003-0406. [DOI] [PubMed] [Google Scholar]
- 10.Rozell TG, Davis DP, Chai Y, Segaloff DL. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology. 1998;139:1588–93. doi: 10.1210/endo.139.4.5881. [DOI] [PubMed] [Google Scholar]
- 11.Barlowe C. Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 2003;13:295–300. doi: 10.1016/s0962-8924(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 12.Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–70. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–83. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 15.Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2001;2:268–76. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 16.Tisdale EJ. Rab2 interacts directly with atypical protein kinase C (aPKC) iota/lambda and inhibits aPKCiota/lambda-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J Biol Chem. 2003;278:52524–30. doi: 10.1074/jbc.M309343200. [DOI] [PubMed] [Google Scholar]
- 17.Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem. 2003;278:47062–9. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal. 2007;19:2388–99. doi: 10.1016/j.cellsig.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–81. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 20.Arvan P, Zhao X, Ramos-Castaneda J, Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic. 2002;3:771–80. doi: 10.1034/j.1600-0854.2002.31102.x. [DOI] [PubMed] [Google Scholar]
- 21.Shetty KM, Kurada P, O’Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J Biol Chem. 1998;273:20425–30. doi: 10.1074/jbc.273.32.20425. [DOI] [PubMed] [Google Scholar]
- 22.Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–36. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- 23.Dong C, Wu G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem. 2006;281:38543–54. doi: 10.1074/jbc.M605734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hein L, Ishii K, Coughlin SR, Kobilka BK. Intracellular targeting and trafficking of thrombin receptors. A novel mechanism for resensitization of a G protein-coupled receptor. J Biol Chem. 1994;269:27719–26. [PubMed] [Google Scholar]
- 25.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci U S A. 1998;95:5573–8. doi: 10.1073/pnas.95.10.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holtback U, Brismar H, DiBona GF, Fu M, Greengard P, Aperia A. Receptor recruitment: a mechanism for interactions between G protein-coupled receptors. Proc Natl Acad Sci USA. 1999;96:7271–5. doi: 10.1073/pnas.96.13.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KA, von Zastrow M. Neurotrophin-regulated sorting of opioid receptors in the biosynthetic pathway of neurosecretory cells. J Neurosci. 2003;23:2075–85. doi: 10.1523/JNEUROSCI.23-06-02075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, et al. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- 29.Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. J Mol Neurosci. 2005;25:207–14. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- 31.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intra-cellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 32.Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–22. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggio R, Vogel Z, Wess J. Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “cross-talk” between G-protein-linked receptors. Proc Natl Acad Sci USA. 1993;90:3103–7. doi: 10.1073/pnas.90.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–86. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 35.Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J Biol Chem. 1998;273:26361–7. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- 36.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–9. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 37.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–82. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 38.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–7. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 39.Ng GY, Clark J, Coulombe N, Ethier N, Hebert TE, Sullivan R, et al. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J Biol Chem. 1999;274:7607–10. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- 40.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–7. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 41.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 42.Margeta-Mitrovic M, Jan YN, Jan LY. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc Natl Acad Sci USA. 2001;98:14649–54. doi: 10.1073/pnas.251554498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cvejic S, Devi LA. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–64. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 45.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–8. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Limbird LE, Lefkowitz RJ. Negative cooperativity among beta-adrenergic receptors in frog erythrocyte membranes. J Biol Chem. 1976;251:5007–14. [PubMed] [Google Scholar]
- 48.Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, et al. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J Biol Chem. 1996;271:16384–92. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Piston DW, Johnson CH. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc Natl Acad Sci USA. 1999;96:151–6. doi: 10.1073/pnas.96.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angers S, Salahpour A, Joly E, Hilairet S, Chelsky D, Dennis M, et al. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci USA. 2000;97:3684–9. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 52.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–7. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. Serotonin 5-HT2C receptor homodimer biogenesis in the endoplasmic reticulum: real-time visualization with confocal fluorescence resonance energy transfer. J Biol Chem. 2006;281:27109–16. doi: 10.1074/jbc.M604390200. [DOI] [PubMed] [Google Scholar]
- 54.Lee SP, O’Dowd BF, Ng GY, Varghese G, Akil H, Mansour A, et al. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol Pharmacol. 2000;58:120–8. doi: 10.1124/mol.58.1.120. [DOI] [PubMed] [Google Scholar]
- 55.Baker EK, Colley NJ, Zuker CS. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J. 1994;13:4886–95. doi: 10.1002/j.1460-2075.1994.tb06816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–40. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 57.Shieh BH, Stamnes MA, Seavello S, Harris GL, Zuker CS. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989;338:67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- 58.Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–63. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- 59.Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J Biol Chem. 2003;278:19087–94. doi: 10.1074/jbc.M212349200. [DOI] [PubMed] [Google Scholar]
- 60.Westhoff B, Chapple JP, van der Spuy J, Hohfeld J, Cheetham ME. HSJ1 is a neuronal shuttling factor for the sorting of chaperone clients to the proteasome. Curr Biol. 2005;15:1058–64. doi: 10.1016/j.cub.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 61.Herrmann JM, Malkus P, Schekman R. Out of the ER-outfitters, escorts and guides. Trends Cell Biol. 1999;9:5–7. doi: 10.1016/s0962-8924(98)01414-7. [DOI] [PubMed] [Google Scholar]
- 62.Parameswaran N, Spielman WS. RAMPs: the past, present and future. Trends Biochem Sci. 2006;31:631–8. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 63.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 64.Hilairet S, Foord SM, Marshall FH, Bouvier M. Protein–protein interaction and not glycosylation determines the binding selectivity of heterodimers between the calcitonin receptor-like receptor and the receptor activity-modifying proteins. J Biol Chem. 2001;276:29575–81. doi: 10.1074/jbc.M102722200. [DOI] [PubMed] [Google Scholar]
- 65.Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–66. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 66.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–91. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 67.Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–18. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 68.Olson R, Dulac C, Bjorkman PJ. MHC homologs in the nervous system–they have not lost their groove. Curr Opin Neurobiol. 2006;16:351–7. doi: 10.1016/j.conb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3:492–8. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- 70.Leclerc PC, Auger-Messier M, Lanctot PM, Escher E, Leduc R, Guillemette G. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology. 2002;143:4702–10. doi: 10.1210/en.2002-220679. [DOI] [PubMed] [Google Scholar]
- 71.Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282:13703–15. doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]
- 72.Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25:57–64. doi: 10.1161/01.ATV.0000150662.51436.14. [DOI] [PubMed] [Google Scholar]
- 73.Parent A, Laroche G, Hamelin E, Parent JL. RACK1 regulates the cell surface expression of the G protein-coupled receptor for thromboxane A(2) Traffic. 2008;9:394–407. doi: 10.1111/j.1600-0854.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 74.Chen C, Li JG, Chen Y, Huang P, Wang Y, Liu-Chen LY. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006;281:7983–93. doi: 10.1074/jbc.M509805200. [DOI] [PubMed] [Google Scholar]
- 75.Ge X, Loh HH, Law PY. mu-Opioid receptor cell surface expression is regulated by its direct interaction with ribophorin I. Mol Pharmacol. 2009;75:1307–16. doi: 10.1124/mol.108.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, et al. Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326:1554–7. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 78.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 79.Abdel-Malek ZA. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci. 2001;58:434–41. doi: 10.1007/PL00000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet. 2005;37:166–70. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- 81.Sebag JA, Hinkle PM. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J Biol Chem. 2009;284:610–8. doi: 10.1074/jbc.M804413200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA. 2007;104:20244–9. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sebag JA, Hinkle PM. Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J Biol Chem. 2009;284:22641–8. doi: 10.1074/jbc.M109.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–98. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 85.Morgan C, Cone RD. Melanocortin-5 receptor deficiency in mice blocks a novel pathway influencing pheromone-induced aggression. Behav Genet. 2006;36:291–300. doi: 10.1007/s10519-005-9024-9. [DOI] [PubMed] [Google Scholar]
- 86.Gomes I, Filipovska J, Jordan BA, Devi LA. Oligomerization of opioid receptors. Methods. 2002;27:358–65. doi: 10.1016/s1046-2023(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 87.Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 88.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135–9. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci USA. 2008;105:16045–50. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol. 2006;69:1083–94. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 91.Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK activation. J Biol Chem. 2009;284:5742–52. doi: 10.1074/jbc.M808507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–4. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 93.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 94.Bernier V, Lagace M, Lonergan M, Arthus MF, Bichet DG, Bouvier M. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol. 2004;18:2074–84. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 95.Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–8. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 96.Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–43. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 97.Dejneka NS, Bennett J. Gene therapy and retinitis pigmentosa: advances and future challenges. Bioessays. 2001;23:662–8. doi: 10.1002/bies.1092. [DOI] [PubMed] [Google Scholar]
- 98.Dryja TP, McGee TL, Hahn LB, Cowley GS, Olsson JE, Reichel E, et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323:1302–7. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- 99.Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, et al. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem. 2003;278:14442–50. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maya-Nunez G, Janovick JA, Ulloa-Aguirre A, Soderlund D, Conn PM, Mendez JP. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab. 2002;87:2144–9. doi: 10.1210/jcem.87.5.8386. [DOI] [PubMed] [Google Scholar]
- 101.Leanos-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4825–8. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- 102.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab. 2002;87:3255–62. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 103.Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure–activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther. 2003;305:608–14. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 104.Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY. Rescuing the traffic-deficient mutants of rat mu-opioid receptors with hydrophobic ligands. Mol Pharmacol. 2003;64:32–41. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y, Liu-Chen LY. Chaperone-like effects of cell-permeant ligands on opioid receptors. Front Biosci. 2009;14:634–43. doi: 10.2741/3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dormer RL, Derand R, McNeilly CM, Mettey Y, Bulteau-Pignoux L, Metaye T, et al. Correction of delF508-CFTR activity with benzo(c)quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J Cell Sci. 2001;114:4073–81. doi: 10.1242/jcs.114.22.4073. [DOI] [PubMed] [Google Scholar]
- 107.Benedek GB, Pande J, Thurston GM, Clark JI. Theoretical and experimental basis for the inhibition of cataract. Prog Retin Eye Res. 1999;18:391–402. doi: 10.1016/s1350-9462(98)00023-8. [DOI] [PubMed] [Google Scholar]
- 108.Peng Y, Li C, Chen L, Sebti S, Chen J. Rescue of mutant p53 transcription function by ellipticine. Oncogene. 2003;22:4478–87. doi: 10.1038/sj.onc.1206777. [DOI] [PubMed] [Google Scholar]
- 109.Heiser V, Scherzinger E, Boeddrich A, Nordhoff E, Lurz R, Schugardt N, et al. Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington’s disease therapy. Proc Natl Acad Sci USA. 2000;97:6739–44. doi: 10.1073/pnas.110138997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soto C, Kascsak RJ, Saborio GP, Aucouturier P, Wisniewski T, Prelli F, et al. Reversion of prion protein conformational changes by synthetic beta-sheet breaker peptides. Lancet. 2000;355:192–7. doi: 10.1016/s0140-6736(99)11419-3. [DOI] [PubMed] [Google Scholar]
- 111.Permanne B, Adessi C, Saborio GP, Fraga S, Frossard MJ, Van Dorpe J, et al. Reduction of amyloid load and cerebral damage in a transgenic mouse model of Alzheimer’s disease by treatment with a beta-sheet breaker peptide. FASEB J. 2002;16:860–2. doi: 10.1096/fj.01-0841fje. [DOI] [PubMed] [Google Scholar]
- 112.Forloni G, Terreni L, Bertani I, Fogliarino S, Invernizzi R, Assini A, et al. Protein misfolding in Alzheimer’s and Parkinson’s disease: genetics and molecular mechanisms. Neurobiol Aging. 2002;23:957–76. doi: 10.1016/s0197-4580(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 113.Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci. 2005;6:11–22. doi: 10.1038/nrn1587. [DOI] [PubMed] [Google Scholar]
- 114.Ulloa-Aguirre A, Janovick JA, Miranda AL, Conn PM. G-protein-coupled receptor trafficking: understanding the chemical basis of health and disease. ACS Chem Biol. 2006;1:631–8. doi: 10.1021/cb600360h. [DOI] [PubMed] [Google Scholar]